Introduction
Pancreatic cancer (PC) has one of the highest
mortality rates amongst malignant tumors as well as the poorest
clinical outcome worldwide, with an overall five-year survival rate
of <5% (1). One of the main
reasons for mortality of PC patients is their high potential for
rapid invasion and early metastasis (2). Therefore, PC is usually diagnosed at
advanced, incurable and metastatic stages of the disease; in
addition, only 20% of patients presenting with localized disease
are amenable to surgical resection (3). Furthermore, PC is highly
chemoresistant, which also accounts for the low survival rates of
patients with PC (3). This outcome
suggests that an enhanced understanding of the mechanisms
underlying invasion, metastasis and chemoresistance is required for
the development of novel therapeutic strategies for the successful
treatment of PC.
The ability to evade apoptosis is one of the
hallmarks that characterizes tumour cells (4). The inhibitor of apoptosis protein
(IAP) family is a group of anti-apoptotic factors in the apoptotic
pathway which renders cancer cells insensitive to apoptotic
stimulation (5). To date, eight
human IAP family members have been identified: X-linked inhibitor
of apoptosis (XIAP), cellular IAP 1 (c-IAP-1), c-IAP-2, IAP-like
protein 2, livin, neuronal apoptosis inhibitory protein (NAIP),
survivin and apollon/bruce. XIAP and survivin are two of the most
important members of the IAP family (6); these two factors have been found to
be overexpressed in numerous malignant tumors, including pancreatic
cancer. In addition, XIAP and survivin have been proposed to be
involved in tumor cell proliferation, metastasis and
chemoresistance (7–9). Epithelial-mesenchymal transition
(EMT) is characterized by the loss of cell-to-cell adhesion and a
phenotypic change from an epithelial morphology to a
fibroblast-like motile morphology (10). EMT has been reported to have an
important role in tumor invasion, metastasis and chemoresistance in
diverse solid tumors, including PC (10–12).
However, the association between XIAP, survivin and EMT in PC has
remained to be elucidated.
RNA interference (RNAi) technology has been widely
used in gene function research and cancer gene therapy (13). In the present study, the expression
of XIAP and survivin was stably inhibited using lentivirus-mediated
short hairpin (sh)RNA in the pancreatic cancer cell line Panc-1.
The impact of of XIAP and survivin silencing on proliferation,
invasion, migration, chemosensitivity and EMT was then
evaluated.
Materials and methods
Materials
The Panc-1 pancreatic cancer cell line was purchased
from the Cell Bank of Type Culture Collection (Shanghai Institute
of Cell Biology, Chinese Academy of Sciences, Shanghai, China) and
stored in liquid nitrogen at the Cell Bank of the State Key
Laboratory of Medical Genetics (Changsha, China). When used, the
cells were defrosted and revivified by incubating in a 37°C water
bath for ~1–2 min, agitated for 1–2 min, then centrifuged at 1000
rpm for 5 min; cells were then resuspended in Dulbecco’s modified
Eagle’s medium (DMEM) and fetal bovine serum (FBS), which were
purchased from Invitrogen Life Technologies (Carlsbad, CA, USA),
and 100 μg/ml streptomycin and 100 U/ml penicillin (Gibco
Life Technologies, Carlsbad, CA, USA), then stored at 37°C in a
humidified atmosphere with 5% CO2. XIAP-shRNA lentiviral
vector (LV-X) and survivin-shRNA lentiviral vector (LV-S) were
purchased from Genechem Corp. (Shanghai, China) and constructed as
previously described (8). The
sequences containing nonsense shRNA lentiviral vector (Lv-Xnc and
Lv-Snc) were used as controls. MTT (tetrazolium salt) reagent was
purchased from Sigma-Aldrich (St. Louis, MO, USA). The RevertAid
First Strand cDNA Sysnthesis kit (cat. no. K1622) was purchased
from Fermentas (Waltham, MA, USA). The SYBR TAQ real-time
polymerase chain reaction (PCR) kit was purchased from Takara Bio,
Inc. (Dalian, China). The Caspase-3/7 fluorescent enzyme activity
detection kit was purchased from Promega Corp. (Madison, WI, USA).
Rabbit anti-human polyclonal survivin primary antibodies were
purchased from Novus Biologicals, LLC (NB500-201; Littleton, CO,
USA) and goat anti-human polyclonal XIAP primary antibodies were
products of R&D Systems (AF8221; Minneapolis, MN, USA). Mouse
anti-human monoclonal E-cadherin (sc-21791), mouse anti-human
monoclonal Slug (sc-166476), mouse anti-human monoclonal
phosphatase and tensin homolog (PTEN; sc-7974) and rabbit
anti-human poly-clonal phosphorylated (p)-Akt (Ser473; sc-33437)
primary antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Mouse anti-human β-actin primary antibodies
(A1978) were purchased from Sigma-Aldrich. The corresponding rabbit
anti-mouse (315-065-003), mouse anti-rabbit (211-065-109) and
rabbit anti-goat (305-065-003) secondary antibodies were purchased
from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA,
USA). Matrigel® and Transwell® chambers were
purchased from BD Biosciences, (Franklin Lakes, NJ, USA). Puromycin
and neomycin (G418) were purchased from Invitrogen Life
Technologies.
Construction and infection of shRNA
lentivirus
The construction of shRNA (shRNA) lentivirus
targeting human XIAP and survivin was performed as described
previously (8). Panc-1 cells
(1×105) were seeded onto a six-well plate and
continuously cultured with DMEM (containing 10% FBS) at 37°C and 5%
CO2 for 12 h. Lentiviral infection was performed as
previously described; in brief, the Panc-1 cells were first
transfected with XIAP shRNA lentivirus (LV-X) and selected using
puromycin; then, the stably XIAP shRNA-transfected cell clones were
transfected with survivin shRNA lentivirus (LV-S) and selected
using neomycin. Panc-1 cells transfected with XIAP and survivin
shRNA were named Panc-1-XS, while Panc-1 cells transfected with
nonsense XIAP and survivin shRNA were named Panc-1-XncSnc.
Quantitative PCR (qPCR) and western blot
analysis
Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies) according to the manufacturer’s
instructions. First strand cDNA synthesis was performed using the
RevertAid First Strand cDNA Synthesis kit, as previously described.
qPCR was performed using an ABI PRISM 7900 HT system (Applied
Biosystems, Thermo Fisher Scientific, Waltham, MA, USA). PCR
cycling condition were as follows: 95°C for 30 sec, followed by 40
cycles at 95°C for 5 sec and 60°C for 30 sec. Gene expression was
quantified using the comparative Ct method, by normalizing
Ct-values to a housekeeping gene (β-actin) and calculating the
relative expression values, as previously described (8). Primers used were identical to those
used in a previous study (8). The
total protein concentration was measured using a bicinchoninic acid
protein quantification kit (Sigma-Aldrich, Taufkirchen, Germany). A
total of 20 μg protein was separated on 12% or 15%
SDS-polyacrylamide gel and transferred to polyvinylidene fluoride
membranes. Following blocking with blocking buffer [1X
phosphate-buffered saline (PBS) containing 0.1% Tween-20 (PBST) and
5% nonfat milk] for 45 min, membranes were incubated with anti-XIAP
(1:5,000), anti-survivin (1:2,000), anti-Slug (1:1,000), anti-PTEN
(1:1,000) and anti-p-Akt (1:1,000) primary antibodies overnight at
4°C. Following washing three times with 1X PBST for 10 min,
membranes were incubated with the corresponding rabbit anti-mouse,
mouse anti-rabbit and rabbit anti-goat secondary antibodies
(Jackson ImmunoResearch Laboratories, Inc. West Grove, PA, USA) at
a 1:3,000 dilution ratio for 1h at room temperature. Blots were
washed to remove chemiluminescent substrate, then incubated in
stripping buffer (Biosyntech, Inc., Beijing, China) for 5–15 min at
37°C, followed by washing with 1X PBS for 5 min at room
temperature. Following stripping, the membranes were reprobed with
β-actin primary antibody (1:10,000) overnight at 4°C, followed by
incubation with the corresponding rabbit anti-mouse secondary
antibodies (1:10,000) for 1 h at room temperature. The immune
reaction was visualized using enhanced chemiluminescence substrate
(Western-Star™ Immunodetection System T1046; Life Technologies,
Grand Island, NY, USA) and exposed to an autoradiograph film
(X-OMAT AR, IB1651579; Kodak, Radnor, PA, USA), then developed
using an X-OMAT 1000A developer (Kodak). Bound proteins were
visualized using ECL (Thermo Fisher Scientific) and detected using
BioImaging Systems (BioDoc-It 220; UVP, Inc., Upland CA, USA).
Colony formation assay
5×103 Panc-1-XS, Panc-1-XncSnc and
non-transfected control Panc-1 cells were plated in 10-cm culture
dishes. Following 14–21 days, cells were fixed with methanol and
stained with 0.1% crystal violet, which were purchased from
Chemical Reagent Factory of Hunan Normal University (Hunan, China).
Colonies were counted by visual inspection. Planting efficiency
(PE) and survival fraction (SF) were then calculated as follows:
PE=(number of colonies formed/number of cells seeded)×100%;
SF=number of colonies formed post treatment/(number of cells seeded
× PE). This procedure was performed in triplicate.
MTT assay and cell apoptosis
Panc-1 cells were seeded onto 96-well plates and
continuously cultured for time periods between 1 and 7 days in
order to calculate the IC50 values. After cells
attached, different concentrations of gemcitabine (1,000, 100, 10,
1, 0.1, 0.01 and 0.001 μg/ml) were added followed by
incubation for the indicated times. Cells were then subjected to
the MTT assay. In brief, 20 μl MTT solution (5 mg/ml in PBS)
was added to each well and incubated for 4 h at 37°C. The cells
were lysed in 100 μl dimethyl sulfoxide (Beijing Dingguo
Biotechnology Co., Ltd., Beijing, China) and analyzed on a
ThermoMax microplate reader (Bio-Rad Laboratories Inc.) at a
wavelength of 490 nm. The growth curve was plotted and
corresponding IC50 values were calculated using SPSS
15.0 software (SPSS, Inc., Chicago, IL, USA). Furthermore, in order
to detect caspase-3/7 activity, cells were cultured in six-well
plates and treated with gemcitabine (0.5 mg/l) for 24 h.
Caspase-3/7 activity was measured using a caspase-3/7 fluorescent
enzyme activity detection kit. Caspase-3/-7 activity was evaluated
using the Caspase-Glo®-3/-7 assay (Promega Corp.) according to the
manufacturer’s instructions. Luminescence was detected on a Sirius
Luminometer and its software (version 3.2) (Berthold Inc., Germany)
with 3 sec delay and 10 sec measurement. The caspase-3/-7 activity
was normalized to the number of viable cells as determined by
trypan blue staining Sigma-Aldrich (St. Louis, MO, USA), and the
caspase 3/-7 fold induction by gemcitabine was determined by the
ratio of caspase-3/-7 activity between the treated and control
groups. To observe cell death, cells were cultured in 24-well
plates and treated with gemcitabine (0.5 μg/ml) for 24 h.
Cells were then stained with Hoechst 33342 (5 μg/ml; BD
Pharmingen, San Diego, CA, USA) for 5 min at 37°C. Cells were
washed and resuspended in PBS for morphological observation under a
IX51 fluorescence microscope (Olympus Corp., Tokyo, Japan) with
excitation at 355 nm and emission at 465 nm. A minimum of 400 cells
from six randomly selected fields per dish were counted and each
experiment was performed in triplicate. The apoptotic index was
calculated as previously described (14).
Wound healing assay and invasion
assay
Panc-1 cells (1×105)were seeded onto a
six-well plate and incubated until they reached 95% confluence. A
wound was generated by scratching the surface of the plates with a
pipette tip. Cells were then washed three times with PBS, incubated
in serum-free DMEM for 48 h and then images were captured using an
IX71 inverted microscope (Olympus Corp.). Invasion assays were
performed using 24-well Matrigel®-coated
Transwells®. Panc-1 cells were added to the upper
chamber, which was coated with 75 μl/well
Matrigel® and 0.6 ml 10% FBS-DMEM was added to the lower
chamber. Cells were incubated for 24 h at 37°C and 5%
CO2, and non-invading cells were removed with cotton
swabs. Cells invading to the bottom of the membrane were fixed in
4% paraformaldehyde (Chemical Reagent Factory of Hunan Normal
University) and stained with 0.1% crystal violet for 30 min at
37°C, washed with PBS, and then counted in four different fields of
vision under 40× magnification. Results are presented as the mean
of three independent experiments.
Statistical analysis
All statistical analyses were performed using SPSS
15.0 software. All values are expressed as the mean ± standard
deviation. Student’s t test or one-way analysis of variance were
used to compare differences between groups. P≤0.05 was considered
to indicate a statistically significant difference between
values.
Results
Simultaneous inhibition of XIAP and
survivin expression in Panc-1 cells
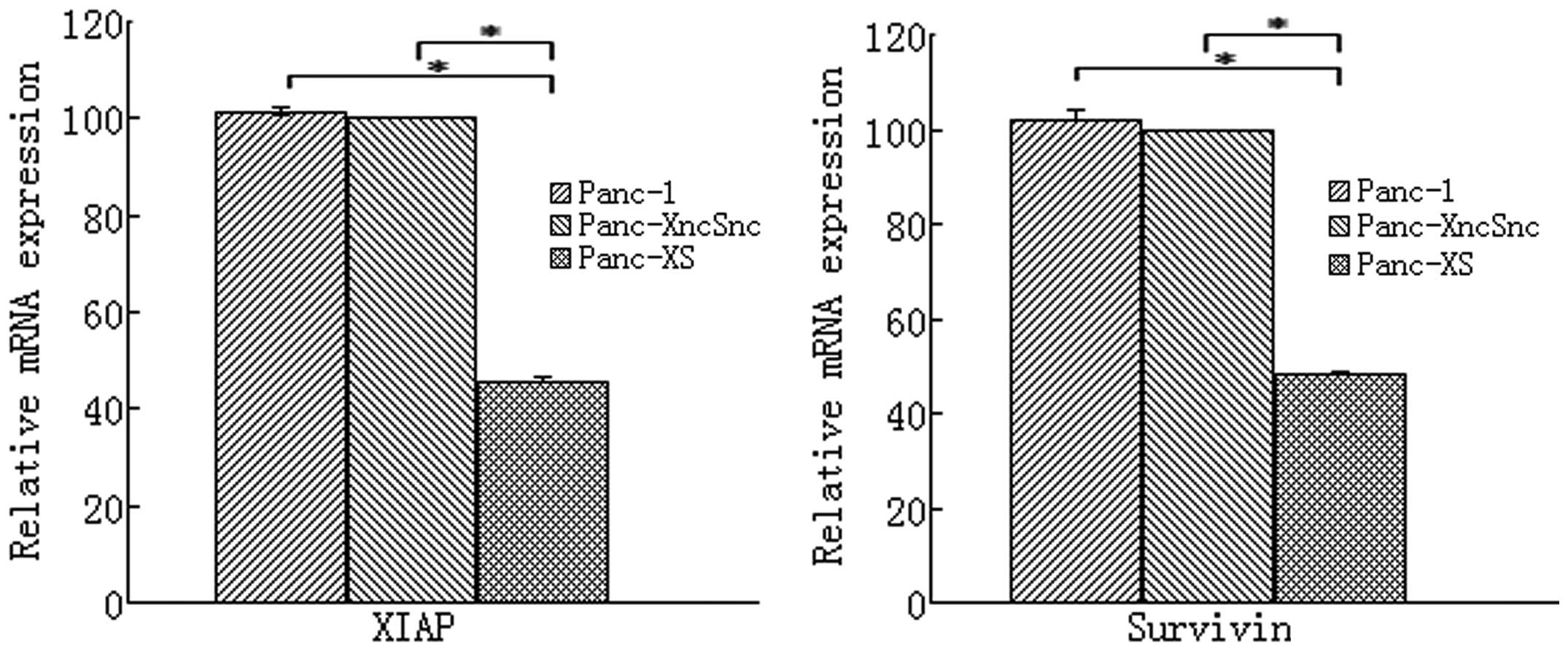
As shown in Fig. 1,
in Panc-1 cells stably expressing XIAP as well as survivin shRNA
(Panc-1-XS), XIAP and survivin mRNA expression was significantly
reduced by 54.62 and 51.99%, respectively, compared with that in
the Panc-1 and Panc-1-XncSnc cells (P<0.05). In addition, XIAP
and survivin protein expression was reduced by 47.19 and 37.29%,
respectively, compared with that in the Panc-1-XncSnc cells
(P<0.05) (Table I; Fig. 2). mRNA and protein expression in
Panc-1-XncSnc cells showed no significant difference compared with
that in the Panc-1 cells (P>0.05).
 | Table IInhibition rate of XIAP/survivin mRNA
and protein expression in Panc-1-XS. |
Table I
Inhibition rate of XIAP/survivin mRNA
and protein expression in Panc-1-XS.
| Expression | Inhibition rate (%)
|
|---|
| XIAP | survivin |
|---|
| mRNA | 54.62±1.45 | 51.99±0.57 |
| Protein | 47.19±3.13 | 37.29±3.25 |
Detection of Panc-1 cell
proliferation
Results showed that cell proliferation of Panc-1-XS
cells was significantly inhibited compared with that of the
Panc-1-XncSnc and Panc-1 cells (P<0.05) (Fig. 3). In addition, the colony formation
rate of Panc-1-XS cells (10.12±1.33%) was significantly reduced
compared with that of the Panc-1-XncSnc cells (96.61±7.89%) and
Panc-1 cells (100.28±8.97%; P<0.05) (Fig. 4). Proliferation of Panc-1-XncSnc
cells showed no significant difference from that of Panc-1 cells
(P>0.05).
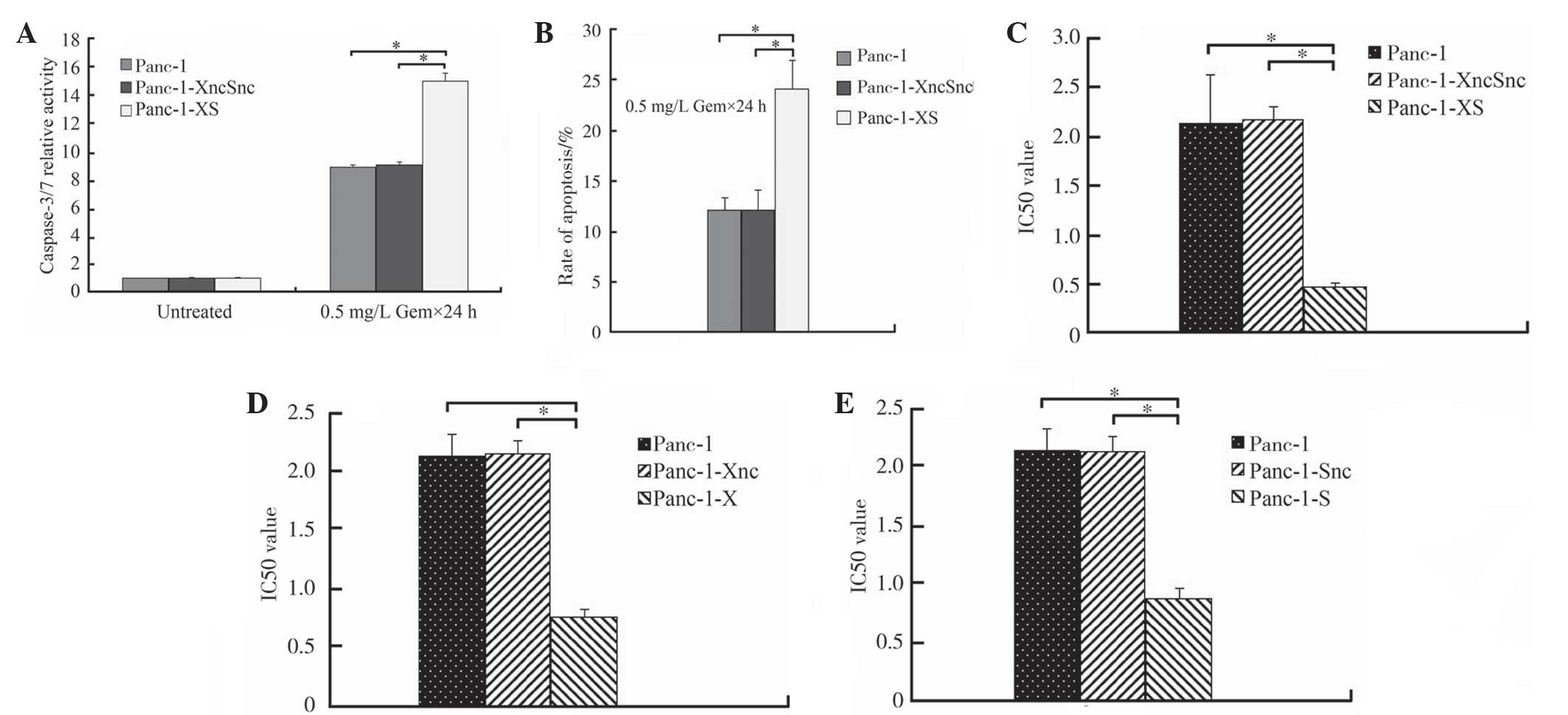
Gemcitabine-induced activation of
caspase-3/7, apoptosis and chemosensitivity are significantly
enhanced in Panc-1-XS cells
Following treatment with various concentrations of
gemcitabine (0.001, 0.01, 0.1, 1, 10, 100 and 1000 mg/ml), the
relative activity of caspase-3/7 in Panc-1-XS cells was
significantly increased to 15.02±0.57 compared with that of Panc-1
cells and Panc-1-XncSnc cells (8.87±0.19 and 9.05±0.23,
respectively; P<0.05; Fig. 5A).
In addition, the rate of apoptosis of Panc-1-XS cells (24.09±2.75%)
was significantly increased compared with that of Panc-1-XncSnc
cells and Panc-1 cells (12.068±1.22% and 12.09±1.97%, respectively;
P<0.05; Fig. 5B). No
significant difference was observed between Panc-1-XncSnc cells and
Panc-1 cells (P>0.05; Fig. 5B).
Furthermore, the IC50-value of Panc-1-XS cells
(0.47±0.07 mg/l) was significantly reduced compared with that of
Panc-1-XncSnc cells and Panc-1 cells (2.18±0.13 and 2.13±0.18 mg/l,
respectively; P<0.05; Fig. 5C).
Further testing showed that the IC50-value of Panc-1-XS
cells was also reduced compared with that of Panc-1-X cells and
Panc-1-S cells (0.76±0.07 mg/l and 0.87±0.09 mg/l, respectively;
P<0.05; Fig. 5D and E).
Cell invasion and migration are
significantly decreased in Panc-1-XS cells
The Transwell® chamber assay showed that
the invasion of Panc-1-XS cells was significantly decreased
compared with that of the Panc-1-XncSnc and Panc-1 cells
(P<0.05) (Fig. 6). In addition,
no significant differences were observed between the invasion of
Panc-1-XncSnc cells and Panc-1 cells (P>0.05). The wound healing
assay demonstrated that the wound healing capacity of Panc-1-XS
cells was significantly decreased compared with that of the
Panc-1-XncSnc and Panc-1 cells (P<0.05) (Fig. 7); however, no significant
difference was observed between the wound healing capacity of
Panc-1-XncSnc cells and Panc-1 cells (P>0.05).
Silencing of XIAP and survivin partially
reverses the EMT, accompanied by increased protein expression of
PTEN and decreased protein expression of p-Akt
In Panc-1-XS cells, protein expression of the
epithelial marker E-cadherin was significantly increased, whereas
the mesenchymal marker Slug was significantly reduced (P<0.05)
(Fig. 8). These data indicated
that simultaneous inhibition of the expression of XIAP and survivin
may partially reverse the EMT in Panc-1 cells. Furthermore, the
expression of PTEN protein was significantly increased in Panc-1-XS
cells, while the p-Akt protein was significantly decreased compared
with that in the Panc-1-XncSnc cells and Panc-1 cells (P<0.05)
(Fig. 9). No significant
differences were observed between the Panc-1-XncSnc and Panc-1
cells (P>0.05).
Discussion
Evasion of apoptosis is a well-known hallmark of
numerous types of cancers (4). The
IAP family has been shown to be particularly important in the
regulation of apoptotic signaling, which results in the failure of
cancer treatment in the clinic (15). XIAP and survivin are two important
IAP family members and are frequently upregulated in numerous types
of human tumor, including PC (15). Previous studies have shown that
XIAP and survivin are highly expressed in PC and were closely
associated with cell proliferation and sensitivity to chemotherapy
with gemcitabine (8,15,16).
Therefore, these two genes represent interesting candidates for a
target-directed molecular-based anti-tumor therapy.
The EMT is characterized by the loss of cell-to-cell
adhesion and a phenotypic change from an epithelial morphology to a
fibroblast-like motile morphology (10). The hallmark of EMT is the loss of
the epithelial adhesion molecule E-cadherin and gain of mesenchymal
cell markers, including Slug and Snail (12). In addition, EMT has been reported
to have an important role in cancer cell invasion and metastasis
(17). Numerous studies have shown
that PC cells which have acquired a mesenchymal phenotype through
EMT are usually gemcitabine-resistant (18). Therefore, EMT has also been
considered as one of the important mechanisms of drug resistance in
PC. However, the molecular mechanisms underlying EMT and
gemcitabine resistance have remained to be elucidated.
RNAi technology is widely used in the study of gene
function and is superior to the majority of alternative gene
knockout technologies. Lentiviral vectors enable the efficient,
stable transfection of target cells and are able to be integrated
into the target cell genome in order to achieve the sustained
expression of carried genes (19).
Lentiviral vector-mediated RNAi technology has been tested for gene
targeting treatment studies in several types of tumor (20–22).
In the present study, lentiviral vectors stably expressing shRNA
targeting the XIAP and survivin genes were constructed and
transfected into Panc-1 cells. Simultaneous silencing of XIAP and
survivin expression significantly inhibited cell proliferation,
increased caspase-3/7 activity and increased sensitivity to
gemcitabine, which was consistent with previous reports using other
tumor cell types (21,23). Of note, a novel phenomenon was
identified in the present study, namely the partial inversion of
the EMT following simultaneous inhibition of the expression of XIAP
and survivin in Panc-1 cells; this process is known as
mesenchymal-epithelial transition (MET). MET was indicated by the
significant upregulation of the protein expression of E-cadherin
and significant downregulation of Slug expression. In addition, MET
was accompanied by significantly reduced cell invasion and
migration. To the best of our knowledge, the present study was the
first to report an association between IAPs, including XIAP and
survivin, and EMT in PC; however, the exact mechanism of this
interaction remains to be elucidated.
As mentioned above, PC cells undergo EMT at early
stages. EMT is a key step in tumor progression as it induces
increased motility, invasion and chemoresistance in PC cells
(11). The results of the present
study demonstrated for the first time, to the best of our
knowledge, that downregulation of XIAP and survivin resulted in the
gain of epithelial markers and the loss of mesenchymal markers,
suggesting that elevated XIAP and survivin expression may induce
EMT and therefore have an important role in the regulation of
motility, invasiveness and metastatic potential in PC cells.
However, the mechanisms underlying the involvement of XIAP and
survivin in EMT require further exploration.
Previous studies have shown that numerous molecular
pathways, including Notch and nuclear factor-κB may have important
roles in the EMT process (18,24).
The PTEN/phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway
regulates oncogene activation through inducing the localization of
Akt to the cell membrane where it activates numerous downstream key
cellular processes, including glucose metabolism, cell
proliferation, apoptosis, transcription and cell migration
(25–27). The PTEN/PI3K/Akt signaling pathway
was found to be deregulated in PC due to the irregular activation
of the PI3K/Akt signaling pathway and downregulation or deletion of
PTEN; this was suggested to have important roles in the occurrence,
development and gemcitabine resistance of PC (28–30).
In the present study, it was demonstrated that Panc-1-XS cells
exhibited a significant upregulation of PTEN and downregulation of
p-Akt protein levels. It was therefore speculated that simultaneous
inhibition of the expression of XIAP and survivin resulted in
upregulated expression of PTEN protein, which led to decreased
p-Akt protein expression and mediated the partial reversion of the
EMT phenotype (MET); therefore, in turn, MET ultimately resulted in
decreased invasion and metastasis as well as increased
chemosensitivity to gemcitabine in PC cells.
In conclusion, the results of the present study
demonstrated that the simultaneous inhibition of XIAP and survivin
expression significantly inhibited proliferation and enhanced
apoptosis in Panc-1 cells. In addition, inhibiting the expression
of XIAP and survivin in Panc-1 cells reversed the EMT and resulted
in increased chemosensitivity as well as reduced cell invasion and
migration. Furthermore, it was hypothesized that the PTEN/PI3K/Akt
pathway may have important roles in this process. The exploration
experiments performed in the present study were a preliminary
approach and a more in-depth investigation is required in order to
confirm these results.
Acknowledgments
The present study was supported by a grant from the
Freedom Exploration Program of Central South University (grant no.
2011QNZT153).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1710. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muniraj T, Jamidar PA and Aslanian HR:
Pancreatic cancer: a comprehensive review and update. Dis Mon.
59:368–402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wei Y, Fan T and Yu M: Inhibitor of
apoptosis proteins and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 40:278–288. 2008. View Article : Google Scholar
|
|
6
|
Silke J and Meier P: Inhibitor of
apoptosis (IAP) proteins-modulators of cell death and inflammation.
Cold Spring Harb Perspect Biol. 5:2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mehrotra S, Languino LR, Raskett CM, et
al: IAP regulation of metastasis. Cancer Cell. 17:53–64. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang C, Tan T, Yi XP, et al:
Lentivirus-mediated shRNA targeting XIAP and survivin inhibit
SW1990 pancreatic cancer cell proliferation in vitro and in vivo.
Mol Med Rep. 4:667–674. 2011.PubMed/NCBI
|
|
9
|
Krepela E, Dankova P, Moravcikova E, et
al: Increased expression of inhibitor of apoptosis proteins,
survivin and XIAP, in non-small cell lung carcinoma. Int J Oncol.
35:1449–1462. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arumugam T, Ramachandran V, Fournier KF,
et al: Epithelial to mesenchymal transition contributes to drug
resistance in pancreatic cancer. Cancer Res. 69:5820–5828. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iwatsuki M, Mimori K, Yokobori T, et al:
Epithelial-mesenchymal transition in cancer development and its
clinical significance. Cancer Sci. 101:293–299. 2010. View Article : Google Scholar
|
|
13
|
Hannon GJ: RNA interference. Nature.
418:244–251. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tonomura H, Takahashi KA, Mazda O, et al:
Glutamine protects articular chondrocytes from heat stress and
NO-induced apoptosis with HSP70 expression. Osteoarthritis
Cartilage. 14:545–553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
LaCasse EC, Mahoney DJ, Cheung HH, et al:
IAP-targeted therapies for cancer. Oncogene. 27:6252–6275. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Urrutia R: The IAP: more international
than ever. Pancreatology. 9:III–IV. 2009.
|
|
17
|
Thiery JP, Acloque H, Huang RYJ and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Li Y, Kong D, et al: Acquisition
of epithelial-mesen-chymal transition phenotype of
gemcitabine-resistant pancreatic cancer cells is linked with
activation of the notch signaling pathway. Cancer Res.
69:2400–2407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deng Y, Wang CC, Choy KW, et al:
Therapeutic potentials of gene silencing by RNA interference:
Principles, challenges, and new strategies. Gene. 538:217–227.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang G, Li J, Zeng Z and Xian L:
Lentivirus-mediated gene therapy by suppressing survivin in BALB/c
nude mice bearing oral squamous cell carcinoma. Cancer Biol Ther.
5:435–440. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kunze D, Kraemer K, Erdmann K, et al:
Simultaneous siRNA-mediated knockdown of antiapoptotic BCL2,
Bcl-xL, XIAP and survivin in bladder cancer cells. Int J Oncol.
41:1271–1277. 2012.PubMed/NCBI
|
|
22
|
Ravet E, Lulka H, Gross F, et al: Using
lentiviral vectors for efficient pancreatic cancer gene therapy.
Cancer Gene Ther. 17:315–324. 2010. View Article : Google Scholar
|
|
23
|
Ruckert F, Samm N, Lehner AK, et al:
Simultaneous gene silencing of Bcl-2, XIAP and Survivin
re-sensitizes pancreatic cancer cells towards apoptosis. Bmc
Cancer. 10:3792010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Min C, Eddy SF, Sherr DH, et al: NF-κB and
epithelial to mesenchymal transition of cancer. J Cell Biochem.
104:733–744. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carnero A, Blanco-Aparicio C, Renner O, et
al: The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic
implications. Curr Cancer Drug Targets. 8:187–198. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang BH and Liu LZ: PI3K/PTEN signaling
in angiogenesis and tumorigenesis. Adv Cancer Res. 102:19–65.
2009.PubMed/NCBI
|
|
27
|
Hafsi S, Pezzino FM, Candido S, et al:
Gene alterations in the PI3K/PTEN/AKT pathway as a mechanism of
drug-resistance (review). Int J Oncol. 40:639–644. 2012.
|
|
28
|
Ma J, Sawai H, Ochi N, et al: PTEN
regulates angiogenesis through PI3K/Akt/VEGF signaling pathway in
human pancreatic cancer cells. Mol Cell Biochem. 331:161–171. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen X, Liao J, Lu Y, et al: Activation of
the PI3K/Akt pathway mediates bone morphogenetic protein 2-induced
invasion of pancreatic cancer cells Panc-1. Pathol Oncol Res.
17:257–261. 2011. View Article : Google Scholar
|
|
30
|
Parsons CM, Muilenburg D, Bowles TL, et
al: The role of Akt activation in the response to chemotherapy in
pancreatic cancer. Anticancer Res. 30:3279–3289. 2010.PubMed/NCBI
|