Introduction
Gastric cancer is among the most common type of
cancer worldwide and is currently the third most common type of
cancer, although the incidence is decreasing (1). Despite novel treatment strategies,
including perioperative chemotherapy and adjuvant chemoradiation
using external radiotherapy, gastric cancer is usually diagnosed at
an advanced stage and the prognosis remains poor (2). Thus, an improved understanding of the
molecular events involved in the development and progression of
gastric cancer may lead to novel treatment methods with improved
efficacy.
Caudal type homeobox transcription factor 2 (CDX2)
is a member of the Cdx gene family and is an intestine-specific
homeobox transcription factor, which is highly expressed in the
intestinal epithelium of adult animals, where it is responsible for
directing the differentiation of intestinal epithelial cells
(3,4). Importantly, CDX2 is associated with
the development of intestinal metaplasia of the stomach and with
gastric carcinogenesis (5,6). Certain studies have demonstrated that
there are significant correlations between CDX2 and intestinal-type
adenocarcinoma (7,8). In addition, a previous biological
study demonstrated that CDX2 may be important in gastric
tumorigenesis (9), whereas another
study suggested that CDX2 is a tumor suppressor (10).
To the best of our knowledge, no comprehensive
studies have been performed to assess the overexpression of CDX2 in
gastric cancer. In our previous study, the overexpression of CDX2
exhibited a significant effect on cell growth and proliferation in
an in vitro cell model of gastric cancer (11). However, the molecular mechanisms
underlying the overexpression of CDX2, which inhibit cell growth
and increase the levels of apoptosis remain to be fully elucidated.
The aim of the present study was to evaluate the effects of the
overepxression of CDX2 on the growth and level of apoptosis in
MGC-803 cells in vivo by administering nude mice with
intratumoral injections of a recombinant CDX2 lentivirus. In
addition, to reveal the possible underlying mechanisms, the effects
of the overexpression of CDX2 on the mRNA and protein expression
levels of B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein
(Bax), survivin, cyclin D1, S-phase kinase-associated protein 2
(Skp2) and c-Myc were examined in MGC-803 cells in vivo.
Materials and methods
Antibodies
Specific rabbit anti-human polyclonal antibodies to
CDX2 (#12306), c-Myc (#5605), Skp2 (#2652), Bax (#5023), Bcl-2
(#2827), cyclin D1 (#2978), survivin (#2808) and GAPDH (#2118) were
provided by Cell Signaling Technology, Inc. (Beverly, MA,
USA). Infrared-labeled secondary goat anti-rabbit antibodies to
IRDye 800 were obtained from Li-Cor Biosciences (Lincoln, NE, USA).
All the above antibodies were used by diluted 1000 times in western
blot analysis.
Cell culture
The MGC-803 human gastric carcinoma cell line and
293T human embryonic kidney cells were provided by the Cell Bank of
Shanghai Institute of Cell Biology, Chinese Academy of Sciences
(Shanghai, China). The cells were maintained at 37°C in an
atmosphere containing 5% CO2 in Dulbecco’s modified
Eagle’s Medium, supplemented with 10% fetal bovine serum, 100U/ml
penicillin and 100 μg/ml streptomycin.
Construction of the CDX2 recombinant
lentiviral vectors
The lentivitus overexpressing the CDX2 gene was
constructed at Shanghai Genechem Co., Ltd. (Shanghai, China). The
lentiviral vector system consisted of a GV208, pHelper 1.0 vector
and pHelper 2.0 vector prior to packaging and was provided by
Shanghai Genechem Co., Ltd. (Shanghai, China). The full length of
the human CDX2 gene (NCBI ID, NM_001265.4), which was indicated by
enhanced green fluorescent protein (GFP), which was encoded into
the GV208 vector. The three vectors were cotransfected into 293T
cells (3×104/ml) in serum-free medium using
Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA,
USA). The medium was replaced with complete medium following 8 h
incubation at 37°C. The high-titer recombinant lentiviral vectors
carrying CDX2 were harvested 48 h following transfection.
Xenograft tumor model
The animals used in the present study were BALB/c
nude male mice (4 weeks old), which were purchased from Guangxi
Animal Center (Nanning, China). The number of nude mice was six in
each group, weighing between 20 and 24 g, and they were fed under
specific pathogen-free conditions. All procedures were in
accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals (National Institutes of Health,
Bethesda, MD, USA). Tumors were established in the mice via a
single subcutaneous injection of 4×107 MGC-803 cells
into the armpit region. The study was approved by the ethics
committee of the First Affiliated Hospital of Guangxi Medical
University, (Guangxi, China).
Treatment of the MGC-803 tumor in nude
mice
When the tumors had reached a diameter of ~5 mm, the
mice were randomized into three groups: Lentivirus (LV)-GFP-CDX2,
LV-GFP-negative control (NC) and phosphate-buffered saline (PBS;
Beyotime Institute of Biotechnology, Shanghai, China). Each group
contained eight mice (n=8). The animals were administered with an
intratumoral injection of either the LV-GFP-CDX2 or LV-GFP-NC at a
titer of 108 transducing units in 100 μl PBS,
while the control group of mice received an equal volume of PBS.
Subsequent to the first injection, the animals were administered
with a similar injection every 2 days. The mouse body weight, the
quantity of water and food intake, vital signs and living status
were assessed daily. The tumor volume was measured and calculated
as follows: The longer diameter, ‘a’, and the shortest diameter,
‘b’, of the tumors were measured using digital calipers, and the
tumor volume (TV) was calculated using the following equation: TV =
a × b2/2. The relative tumor volume (RTV) was calculated
using the formula: RTV = Vt / V0, in which
V0 is the TV on the day when the treatment was
administered, and Vt is the TV of the subsequent
measurement. Following the tumor cell injections (15 days), the
animals were sacrificed by cervical dislocation and the tumors were
then analyzed.
Reverse transcription
semi-quantitative-polymerase chain reaction (RT-sqPCR)
The total RNA was extracted from the tumor tissues
using TRIzol reagent (Sigma-Aldrich, St. Louis, MO, USA), according
to the manufacturer’s instructions. cDNA was generated from a
DNase-1-treated RNA template with 0.2 μg random hexamer
primers (Takara Bio, Inc., Tokyo, Japan) and 200 units RevertAid
H-Minus M-MuLV reverse transcriptase enzyme (Roche, Basel,
Switzerland). The primer sequences used to specifically amplify the
genes of interest are shown in Table
I. The cDNA (2 μl) produced was added to 10 μl
Taq Premix and the upstream and downstream primers (1 μl
each). RT-qPCR was performed as follows: 1 cycle at 94°C for 5 min,
30 cycles at 94°C for 30 sec for denaturation, 56°C for 30 sec for
annealing, 68°C for 45 sec for extension and 1 cycle 5 min at 72°C,
according to the RT-qPCR amplification kit (Takara Bio, Inc.)
manufacturer’s instructions. The amplified PCR products were run on
1.5% agarose gels and visualized under UV light following ethidium
bromide (0.5 μg/ml; Beyotime Institute of Biotechnology)
staining at room temperature (25°C) for 20 min.
 | Table ISequences of the primers used for
reverse transcription semi-quantitative polymerase chain
reaction. |
Table I
Sequences of the primers used for
reverse transcription semi-quantitative polymerase chain
reaction.
| Gene | Primer | Sequence | PCR product
(bp) |
|---|
| CDX2 | Forward | 5′-
CGGCAGCCAAGTGAAAAC-3′ | 217 |
| Reverse |
5′-GATGGTGATGTAGCGACTGTAGTG-3′ |
| Survivin | Forward |
5′-AAATGCACTCCAGCCTCTGT-3′ | 311 |
| Reverse |
5′-TGTCGAGGAAGCTTTCAGGT-3′ |
| Bax | Forward |
5′-CCAAGAAGCTGAGCGAGTGT-3′ | 269 |
| Reverse |
5′-CCGGAGGAAGTCCAATGTC-3′ |
| Bcl-2 | Forward |
5′-GACTTCGCCGAGATGTCCAG-3′ | 259 |
| Reverse |
5′-CATCCCAGCCTCCGTTATCC-3′ |
| Cyclin D1 | Forward |
5′-CCCTCGGTGTCCTACTTCAA-3′ | 237 |
| Reverse |
5′-GGGGATGGTCTCCTTCATCT-3′ |
| Skp2 | Forward |
5′-GCTGCTAAAGGTCTCTGGTGT-3′ | 291 |
| Reverse |
5′-AGGCTTAGATTCTGCAACTTG-3′ |
| C-Myc | Forward |
5′-TTCTCTCCGTCCTCGGATTC-3′ | 282 |
| Reverse |
5′-GTAGTTGTGCTGATGTGTGG-3′ |
| GAPDH | Forward |
5′-ACCACAGTCCATGCCATCAC-3′ | 450 |
| Reverse |
5′-TCACCACCCTGTTGCTGTA-3′ |
Western blot analysis
The tumor tissues were homogenized for tissue lysate
extraction, the tissue lysates were centrifuged and the
supernatants were collected. Equal quantities (150 μg) of
protein were heated to 100°C for 5 min with Laemmli sample buffer
(Beyotime Institute of Biotechnology), then separated on 12%
SDS-PAGE gels (Beyotime Institute of Biotechnology) and transferred
onto polyvinylidene difluoride membranes. The entire process was
performed using Bio-Rad equipment (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) according to the manufacturer’s instructions.
The membrane was probed with the primary antibody (1:1,000) and
incubated overnight at 4°C. The blots were washed three times in
PBS with Tween 20 prior to incubation with species-appropriate,
peroxidase-conjugated secondary antibodies for 1 h. The blots were
then washed again three times in PBS with Tween 20. The net
intensities of the bands were quantified using Odyssey software
version 3.0 (Li-Cor Biosciences, Lincoln, NE, USA).
In situ analysis of MGC-803 tumor cell
apoptosis using a terminal deoxynucleotidyl transferase-mediated
dUTP-biotin nick end labeling (TUNEL) assay
Tissue samples were fixed in 4% buffered
paraformaldehyde at 4℃ for 48 h and then processed for paraffin
embedding. The procedures of paraffin embedding were dehydration
and waxdip. Paraffin-embedded (Beyotime Institute of Biotechnology)
sections were prepared for hematoxylin and eosin (Beyotime
Institute of Biotechnology) staining. The levels of tumor tissue
necrosis were determined by comparing the surface of necrotic areas
with that of the whole tumor. Levels of apoptosis were determined
using a TUNEL assay kit, according to the manufacturer’s
instructions. Briefly, the cells were rinsed with PBS twice for 3
min, prior to the addition of 50 μl TUNEL cocktail on test
sections. Labeling solution (40 μl) was added to control
sections on one slide and PBS was added to the control sections on
other slides, and incubated in a humidified chamber for 60 min at
37ºC in the dark. A sample was considered positive when it
contained 25 positively stained cells in every 100 tumor cells,
which was calculated from five randomly selected fields for each
specimen. The stained tissue sections were visualized by microscopy
(CP-111-2; magnification, x400; Jenco International, Protland, OR,
USA).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean and were analyzed using SPSS version 13.0 (SPSS, Inc.,
Chicago, IL, USA). One-way analysis of variance was used to measure
statistical significance among groups, followed by the
Student-Newman-Keuls test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Construction and identification of
pGCL-GFP-CDX2 lentiviral vectors
The positive clones were confirmed by DNA sequence
analysis (data not shown) and it was demonstrated that the RNA
coding frames and frame sequences were correct and that the
recombinant pGCL-GFP-CDX2 and pGCL-GFP-NC plasmids had been
constructed successfully.
Determination of lentiviral titers
A lentivirus targeting CDX2 and an NC vector
(LV-GFP-CDX2, and LV-GFP-NC, respectively) were produced by
co-transfection with a packaging vector (pHelper1.0) and a
vesicular stomatitis virus glycoprotein expression plasmid
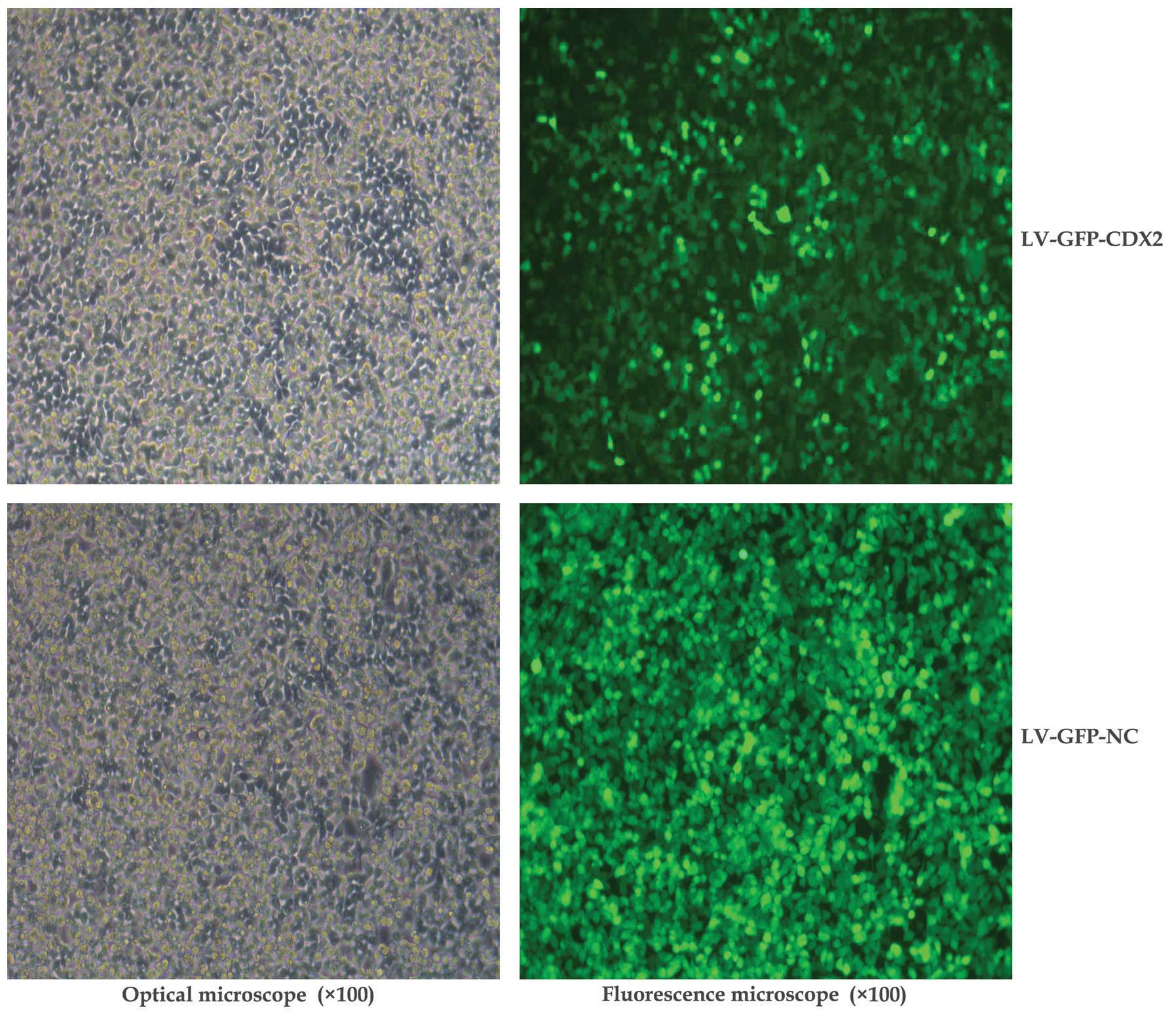
(pHelper2.0) into the 293T cells. As shown in Fig. 1, the GFP-labeling results indicated
that the lentiviral vectors were suitably transfected for use in
the present study.
Overexpression of CDX2 inhibits MGC-803
tumor growth
As shown in Fig.
2A, the tumor growth curves indicated that mice treated with
LV-GFP-CDX2 exhibited significant inhibition of tumor growth when
compared with those treated with the LV-GFP-NC control vector or
PBS (P<0.05). The tumor volumes in the mice in the LV-GFP-CDX2
group were significantly smaller compared with those of the control
groups (P<0.05) at 15 days post-tumor injection, whereas no
difference was identified between the Lv-GFP-NC and PBS groups
(P>0.05; Fig. 2B). These
results indicated that overexpression of CDX2 effectively inhibited
MGC-803 tumor growth in vivo.
 | Figure 2Overexpression of CDX2 inhibits tumor
growth and induces tumor cell apoptosis in LV-GFP-CDX2-treated
mice. (A) Relative tumor volume growth curve revealed significant
growth tendencies in the PBS and LV-GFP-NC groups, while the
MGC-803 tumor growth in the LV-GFP-CDX2 group was markedly
inhibited. (B) Tumor volumes in the LV-GFP-CDX2 groupe were smaller
compared with those in the control group 14 days after tumor
injection (*P<0.05). (C) Tumor cell apoptosis was
assessed using a TUNEL assay and HE staining, revealing that the
MGC-803 tumor cells in the LV-GFP-CDX2 group had higher levels of
apoptosis compared with the LV-GFP-NC and PBS groups
(magnification, x400). CDX2, vaudal type homeobox transcription
factor 2; LV-GFP, lentivirus-green fluoresent protein; PBS,
phosphate-buffered saline; NC, negative control; TV, tumor volume;
RTV, relative TV; TUNEL, terminal deoxynucleotidyl
transferase-mediated dUTP-biotin nick end labeling; HE, hematoxylin
and eosin. |
Overexpression of CDX2 induces MGC-803
tumor cell apoptosis
As shown in Fig.
2C, the percentage of apoptotic tumor cells in the LV-CDX2-GFP
group was 17.32±2.5%, which was significantly higher compared with
that observed in the LV-GFP-NC (7.2±1.7%) and PBS (6.6±1.8%)
groups, demonstrated using the TUNEL method (P<0.05). These
results suggested that overexpression of CDX2 effectively promoted
MGC-803 tumor cell apoptosis in vivo.
mRNA and protein expression levels of
CDX2 are increased in MGC-803 tumor tissues
Densitometric analysis revealed that mRNA and
protein expression levels of CDX2 in the LV-GFP-CDX2 group were
higher compared with those of the two control groups (P<0.05;
Fig. 3A–D). These results
suggested that the nude mouse model overexpressing CDX2 had been
constructed successfully by injection with the CDX2 recombinant
lentiviral vectors.
 | Figure 3Overexpression of CDX2 mRNA and
protein in the LV-GFP-CDX2 group. (A) Reverse transcription
semi-quantitative polymerase chain reaction analysis of CDX2 and
GAPDH in the MGC-803 tumor tissues from the LV-GFP-CDX2, LV-GFP-NC
and PBS groups. M, 500 bp marker. (B) mRNA expression levels of
CDX2 were measured in the three groups, normalized to GAPDH and
presented as the mean ± standard error of the mean (n=8 in each
group). (C) Western blot analysis of the protein expression levels
of CDX2 and GAPDH in the MGC-803 tumor tissues from the three
groups. (D) Protein expression levels of CDX2 were measured in the
three groups, normalized to GAPDH and presented as the mean ±
standard error of the mean (n=8 in each group). Lanes: 1,
LV-GFP-CDX2 group; 2, LV-GFP-NC group; 3, PBS group, GAPDH:
internal control mRNA and protein.*P<0.05 compared
with LV-GFP-NC and PBS group, using analysis of variance and
Student-Newman-Keuls analyses. CDX2, caudal type homeobox
transcription factor 2; LV-GFP, lentivirus-green fluoresent
protein; PBS, phosphate-buffered saline; NC, negative control. |
Overexpression of CDX2 decreases the
expression levels of c-Myc, Skp2, Bcl-2, cyclin D1 and survivin,
and increases the expression of Bax
As shown in Fig. 4A and
B, the densitometric analysis revealed that the mRNA expression
levels of c-Myc, Skp2, Bcl-2, cyclin D1 and survivin in the
LV-GFP-CDX2 group were lower, while the expression of Bax was
higher compared with the LV-GFP-NC and PBS groups (P<0.05). In
addition, as shown in Fig. 5A and
B, the densitometry revealed that the protein expression levels
of c-Myc, Skp2, Bcl-2, cyclin D1 and survivin in the LV-GFP-CDX2
group was lower, that of while Bax was higher compared with the
LV-GFP-and PBS groups (P<0.05). These results suggested that the
overexpression of CDX2 effectively decreased the expression levels
of c-Myc, Skp2, Bcl-2, cyclin D1, survivin, and increased the
expression of Bax in the MGC-803 tumor cells in vivo.
 | Figure 4Overexpression of CDX2 induces
downregulation in the mRNA expression levels of c-Myc, Skp2, Bcl-2,
cyclin D1 and survivin and upregulation in the expression of Bax.
(A) Reverse transcription semi-quantitative polymerase chain
reaction analysis of c-Myc, Skp2, Bcl-2, cyclin D1, survivin, Bax
and GAPDH in the MGC-803 tumor tissues from the LV-GFP-CDX2, 2,
LV-GFP-NC and PBS groups. M, 500 bp marker (B) mRNA expression
levels of c-Myc, Skp2, Bcl-2, cyclinD1, survivin and Bax were
measured in the three groups, normalized to those of GAPDH and
presented as the mean ± standard error of the mean (n=8 in each
group). 1, LV-GFP-CDX2 group; 2, LV-GFP-NC group; 3, PBS group;
GAPDH: internal control. *P<0.05, compared with the
LV-GFP-NC and PBS groups, using analysis of variance and
Student-Newman-Keuls analyses. Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-associated X protein; Skp2, S-phase kinase-associated protein
2; CDX2, caudal type homeobox transcription factor 2; LV-GFP,
lentivirus-green fluoresent protein; PBS, phosphate-buffered
saline. |
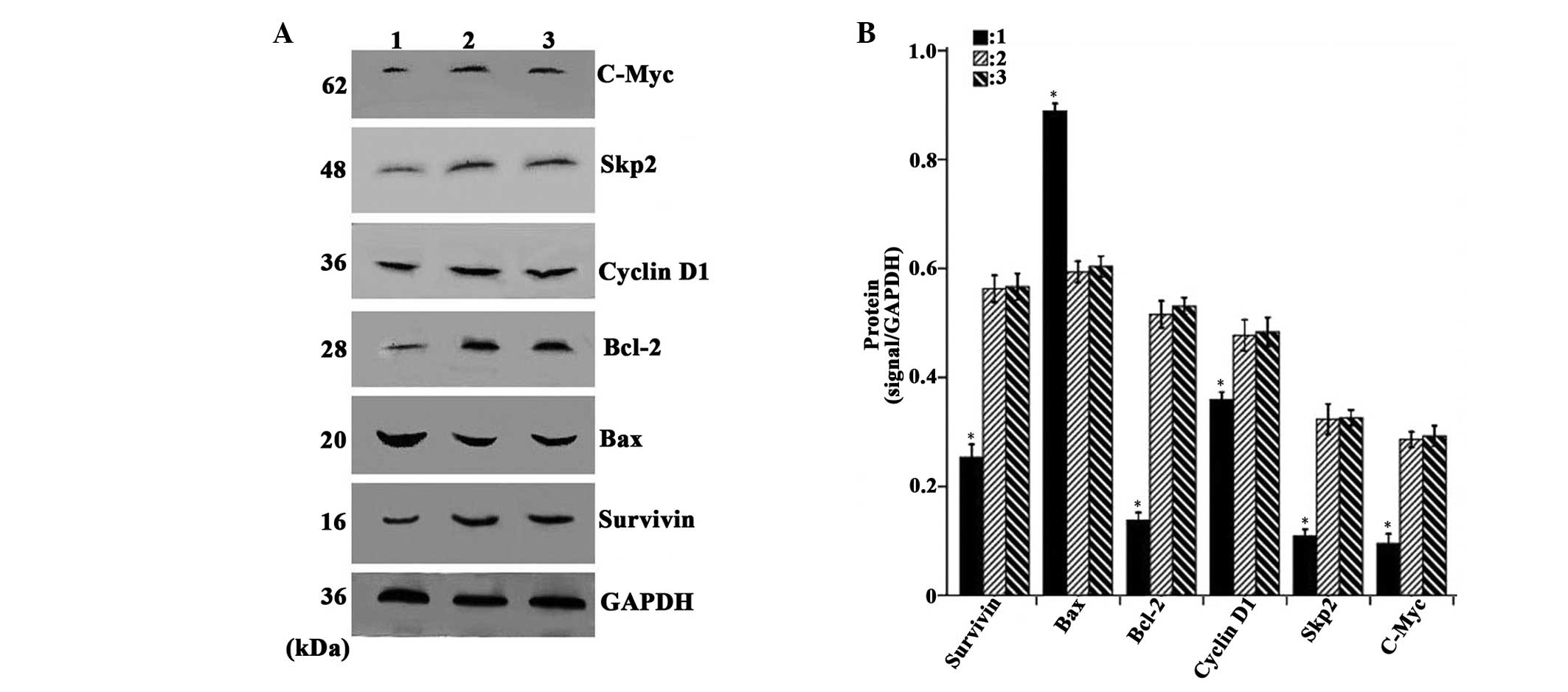 | Figure 5Overexpression of CDX2 induces
downregulation of the protein expression levels of c-Myc, Skp2,
Bcl-2, cyclinD1 and survivin and upregulation of Bax. (A) Western
blot analysis of c-Myc, Skp2, Bcl-2, cyclin D1, survivin, Bax and
GAPDH in the MGC-803 tumor tissue from the LV-GFP-CDX2, LV-GFP-NC
and PBS groups. (B) Protein expression levels of c-Myc, Skp2,
Bcl-2, cyclin D1, survivin and Bax were measured in the three
groups, normalized to those of GAPDH and expressed as the mean ±
standard error of the mean (n=8 in each group). 1, LV-GFP-CDX2
group; 2, LV-GFP-NC group; 3, PBS group; GAPDH: internal control.
*P<0.05, compared with the LV-GFP-NC and PBS group,
using analysis of variance and Student-Newman-Keuls analyses.
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; Skp2,
S-phase kinase-associated protein 2; CDX2, caudal type homeobox
transcription factor 2; LV-GFP, lentivirus-green fluoresenct
protein; PBS, phosphate-buffered saline. |
Discussion
CDX2 is a nuclear transcription factor, which is
important in embryologic development and in the differentiation of
the intestinal tract epithelium (12). It is also highly expressed in
epithelial tumors of the gastrointestinal tract (13) and, for this reason, its role in
tumorigenesis has become an important area of investigation.
Although several lines of evidence have indicated that CDX2 is a
potential tumor suppressor gene in ovarian, gallbladder, colon and
gastric cancer (12,14–17),
the mechanisms associating the overexpression of CDX2 with gastric
cancer remain to be elucidated.
In the present study, a marked antitumoral effect of
the overexpression of CDX2 on MGC-803 cells was observed in
vivo. Tumor growth was suppressed and tumor apoptosis was
increased in nude mice when the CDX2 mRNA and protein were
overexpressed via lentiviral vector-mediated overexpression of
CDX2. These findings were concordant with our previous study, which
observed that the overexpression of CDX2 inhibits the progression
of gastric cancer in vitro (11,18).
Therefore, lentiviral vector-mediated overexpression of CDX2 may be
used as a potent and specific therapeutic tool for the treatment of
gastric cancer. In addition, the present study revealed that
overexpression of CDX2 decreased the expression levels of survivin,
Bcl-2, cyclin D1, Skp2 and c-Myc, and increased the expression of
Bax.
Previous studies have confirmed that the Bax, Bcl-2,
cyclin D1, c-Myc, Skp2 and survivin genes are associated with cell
proliferation, cell apoptosis and tumor development (19–22).
Takahashi et al (23)
suggested that CDX2 inhibited the gene expression of exogenous
nuclear factor (NF)-κB-induced luciferase in a dose-dependent
manner. Furthermore, Yang et al (24) and Saha et al (25) demonstrated that NF-κB induces the
expression of genes involved in cell proliferation (cyclin D1 and
c-Myc) and anti-apoptotic (survivin and Bcl-2), while it inhibits
the expression of the pro-apoptotic gene, Bax. In addition, the
NF-κB signaling pathway regulates the cell cycle by binding of the
NF-κB subunits to the cyclin D1, c-Myc and Skp2 promoters, which
are concomitant with a switch from coactivator to corepressor
recruitment (26). This suggests
that the overexpression of CDX2 may directly or indirectly modulate
the transcriptional activity of downstream genes (Bax, Bcl-2,
cyclin D1, Skp2, c-Myc and survivin) by inhibiting the gene
expression of NF-κB.
Downregulation of the NF-κB signaling pathway
induces downregulation of the anti-apoptotic gene, Bcl-2 and
upregulation of the pro-apoptotic gene, Bax (27). The Bcl-2 family of proteins
represent essential targets in cancer therapy (28). The Bax, Bcl-2 homologous antagonist
killer and Bcl-2 related ovarian killer pro-apoptotic and Bcl-2,
Bcl-extra large and myeloid cell leukemia 1 anti-apoptotic members
of the Bcl-2 family may promote or inhibit apoptosis through the
formation of heterodimers among these proteins (29). Therefore, the ratio between the
pro-apoptotic Bax and anti-apoptotic Bcl-2 proteins is an important
determinant of cell survival and death. In the present study,
upregulation of the pro-apoptotic protein, Bax and downregulation
of the anti-apoptotic protein, Bcl-2 were observed in the
LV-CDX2-GFP group, and gray scale value analysis revealed a
significantly higher Bax/Bcl-2 ratio in the treatment group
compared with the untreated controls. A higher Bax/Bcl-2 ratio has
been reported to be a cause of cell death (30). This suggests that the
overexpression of CDX2 induced apoptosis by altering the Bax/Bcl-2
ratio to suppress gastric cancer growth.
Barré et al (26) observed that NF-κB subunits regulate
the gene expression of the cyclin D1, c-Myc and Skp2, and
downregulation of NF-κB can result in a change in the function of
the NF-κB-binding site, resulting in repression of the cyclin D1,
c-Myc and Skp2 gene promoter. Cyclin D1, c-Myc and Skp2 are cell
cycle regulators, and the cell cycle is arrested through
suppression of the expression of cyclin D1, c-Myc and Skp2
(31-33). Therefore, the overexpression of
CDX2 may also suppress the cell cycle through the indirect
suppression of the expression of cyclin D1, c-Myc and Skp2 through
the NF-κB signaling pathway. In addition, cell immortalization is a
basic step in tumor growth (34).
Therefore, control of the cell cycle may be an important mechanism
in the suppression of tumor growth by CDX2 in gastric cancer.
A previous study demonstrated that survivin is
down-regulated via the NF-κB-mediated signaling pathway, thus
inhibiting the growth of cancer cells (35). Survivin is an important factor in
cell division, and the separation of chromatin in mitosis may be
faulty in cancer cells lacking expression of the survivin gene
(36,37). The cell cycle checkpoint mechanism
activates following mitotic dysfunction, which promotes apoptosis
in abnormal cells (38). The
present study demonstrated that the overexpression of CDX2
significantly inhibited the growth of transplanted tumors and
promoted cell apoptosis, which may be attributed to the indirect
downregulation of survivin by CDX2, by inhibiting the gene
expression of NF-κB.
In conclusion, the CDX2/NF-κB signaling pathway is
an unusually structured network, by which CDX2 inhibits the growth
of MGC-803 cells in vivo. This may explain an important
aspect of the mechanism by which the overexpression of CDX2
contributes to the suppression of gastric cancer cell growth.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 30860273
and 81060201, the Natural Science Foundation of Guangxi (grant nos.
2011GXNSFA018273 and 2013GXNSFAA019163 and the Key Health Science
Project of Guangxi (grant no. Key1298003-2-6).
References
|
1
|
Chong VH, Telisinghe PU, Abdullah MS and
Chong CF: Gastric cancer in Brunei Darussalam: epidemiological
trend over a 27 year period (1986–2012). Asian Pac J Cancer Prev.
15:7281–7285. 2014. View Article : Google Scholar
|
|
2
|
Orditura M, Galizia G, Sforza V, et al:
Treatment of gastric cancer. World J Gastroenterol. 20:1635–1649.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duprey P, Chowdhury K, Dressler GR,
Balling R, Simon D, Guenet JL and Gruss P: A mouse gene homologous
to the Drosophila gene caudal is expressed in epithelial cells from
the embryonic intestine. Genes Dev. 2(12A): 1647–1654. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Drummond F, Putt W, Fox M and Edwards YH:
Cloning and chromosome assignment of the human CDX2 gene. Ann Hum
Genet. 61:393–400. 1997. View Article : Google Scholar
|
|
5
|
Yuasa Y: Control of gut differentiation
and intestinal-type gastric carcinogenesis. Nat Rev Cancer.
3:592–600. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bai YQ, Yamamoto H, Akiyama Y, et al:
Ectopic expression of homeodomain protein CDX2 in intestinal
metaplasia and carcinomas of the stomach. Cancer Lett. 176:47–55.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiao ZY, Ru Y, Sun JT, Gao SG, Wang YF,
Wang LD and Feng XS: Expression of CDX2 and villin in gastric
cardiac intestinal metaplasia and the relation with gastric cardiac
carcinogenesis. Asian Pac J Cancer Prev. 13:247–250. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qin R, Wang NN, Chu J and Wang X:
Expression and significance of homeodomain protein Cdx2 in gastric
carcinoma and precancerous lesions. World J Gastroenterol.
18:3296–3302. 2012.PubMed/NCBI
|
|
9
|
Kang JM, Lee BH, Kim N, Lee HS, Lee HE,
Park JH, Kim JS, Jung HC and Song IS: CDX1 and CDX2 expression in
intestinal metaplasia, dysplasia and gastric cancer. J Korean Med
Sci. 26:647–653. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang JF, Zhang JG, Kuai XL, Zhang H,
Jiang W, Ding WF, Li ZL, Zhu HJ and Mao ZB: Reactivation of the
homeotic tumor suppressor gene CDX2 by
5-aza-2′-deoxycytidine-induced demethylation inhibits cell
proliferation and induces caspase-independent apoptosis in gastric
cancer cells. Exp Ther Med. 5:735–741. 2013.PubMed/NCBI
|
|
11
|
Xie Y, Li L, Wang X, Qin Y, Qian Q, Yuan X
and Xiao Q: Overexpression of Cdx2 inhibits progression of gastric
cancer in vitro. Int J Oncol. 36:509–516. 2010.PubMed/NCBI
|
|
12
|
Li QL, Yang ZL, Liu JQ and Miao XY:
Expression of CDX2 and hepatocyte antigen in benign and malignant
lesions of gallbladder and its correlation with histopathologic
type and clinical outcome. Pathol Oncol Res. 17:561–568. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ikarashi S, Nishikura K, Ajioka Y and
Aoyagi Y: Re-evaluation of phenotypic expression in
undifferentiated-type early gastric adenocarcinomas using mucin
core protein and CDX2. Gastric Cancer. 16:208–219. 2013. View Article : Google Scholar
|
|
14
|
Gross I, Duluc I, Benameur T, Calon A,
Martin E, Brabletz T, Kedinger M, Domon-Dell C and Freund JN: The
intestine-specific homeobox gene Cdx2 decreases mobility and
antagonizes dissemination of colon cancer cells. Oncogene.
27:107–115. 2008. View Article : Google Scholar
|
|
15
|
Park Y, Srivastava A, Kim GH,
Mino-Kenudson M, Deshpande V, Zukerberg LR, Song GA and Lauwers GY:
CDX2 expression in the intestinal-type gastric epithelial
neoplasia: Frequency and significance. Mod Pathol. 23:54–61. 2010.
View Article : Google Scholar
|
|
16
|
Chang YT, Hsu C, Jeng YM, Chang MC, Wei SC
and Wong JM: Expression of the caudal-type homeodomain
transcription factor CDX2 is related to clinical outcome in biliary
tract carcinoma. J Gastroenterol Hepatol. 22:389–394. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang LP, Yu YH, Sheng C and Wang SH:
Up-regulation of cadherin 17 and down-regulation of homeodomain
protein CDX2 correlate with tumor progression and unfavorable
prognosis in epithelial ovarian cancer. Int J Gynecol Cancer.
22:1170–1176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang XT, Wei WY, Kong FB, Lian C, Luo W,
Xiao Q and Xie YB: Prognostic significance of Cdx2
immunohistochemical expression in gastric cancer: A meta-analysis
of published literatures. J Exp Clin Cancer Res. 31:982012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shirali S, Aghaei M, Shabani M, Fathi M,
Sohrabi M and Moeinifard M: Adenosine induces cell cycle arrest and
apoptosis via cyclinD1/Cdk4 and Bcl-2/Bax pathways in human ovarian
cancer cell line OVCAR-3. Tumour Biol. 34:1085–1095. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian YF, Chen TJ, Lin CY, et al: SKP2
overexpression is associated with a poor prognosis of rectal cancer
treated with chemoradio-therapy and represents a therapeutic target
with high potential. Tumour Biol. 34:1107–1117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X, Bi L, Ye Y and Chen J:
Formononetin induces apoptosis in PC-3 prostate cancer cells
through enhancing the Bax/Bcl-2 ratios and regulating the p38/Akt
pathway. Nutr Cancer. 66:656–661. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu X, Yu H, Cai H and Wang Y: Expression
of CD24, p21, p53, and c-myc in alpha-fetoprotein-producing gastric
cancer: Correlation with clinicopathologic characteristics and
survival. J Surg Oncol. 109:859–864. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takahashi K, Hirano F, Matsumoto K, Aso K
and Haneda M: Homeobox gene CDX2 inhibits human pancreatic cancer
cell proliferation by down-regulating cyclin D1 transcriptional
activity. Pancreas. 38:49–57. 2009. View Article : Google Scholar
|
|
24
|
Yang Z, Li C, Wang X, et al: Dauricine
induces apoptosis, inhibits proliferation and invasion through
inhibiting NF-kappaB signaling pathway in colon cancer cells. J
Cell Physiol. 225:266–275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saha A, Blando J, Silver E, Beltran L,
Sessler J and Digiovanni J: 6-Shogaol from dried ginger inhibits
growth of prostate cancer cells both in vitro and in vivo through
inhibition of STAT3 and NF-kappaB Signaling. Cancer Prev Res
(Phila). 7:6272014. View Article : Google Scholar
|
|
26
|
Barré B and Perkins ND: A cell cycle
regulatory network controlling NF-kappaB subunit activity and
function. EMBO J. 26:4841–4855. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kannaiyan R, Hay HS, Rajendran P, et al:
Celastrol inhibits proliferation and induces chemosensitization
through down-regulation of NF-κB and STAT3 regulated gene products
in multiple myeloma cells. Br J Pharmacol. 164:1506–1521. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barrezueta LF, Oshima CT, Lima FO, De
Oliveira Costa H, Gomes TS, Neto RA and De Franco MF: The intrinsic
apoptotic signaling pathway in gastric adenocarcinomas of Brazilian
patients: Immunoexpression of the Bcl-2 family (Bcl-2, Bcl-x, Bak,
Bax, Bad) determined by tissue microarray analysis. Mol Med Rep.
3:261–267. 2010. View Article : Google Scholar
|
|
29
|
Roset R, Ortet L and Gil-Gomez G: Role of
Bcl-2 family members on apoptosis: What we have learned from
knock-out mice. Front Biosci. 12:4722–4730. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang H, Zhao PJ, Su D, Feng J and Ma SL:
Paris saponin I induces apoptosis via increasing the Bax/Bcl-2
ratio and caspase-3 expression in gefitinib-resistant non-small
cell lung cancer in vitro and in vivo. Mol Med Rep. 9:2265–2272.
2014.PubMed/NCBI
|
|
31
|
Kuo HC, Kuo WH, Lee YJ, Lin WL, Chou FP
and Tseng TH: Inhibitory effect of caffeic acid phenethyl ester on
the growth of C6 glioma cells in vitro and in vivo. Cancer Lett.
234:199–208. 2006. View Article : Google Scholar
|
|
32
|
Leu WJ, Chang HS, Chan SH, Hsu JL, Yu CC,
Hsu LC, Chen IS and Guh JH: Reevesioside A, a cardenolide
glycoside, induces anticancer activity against human
hormone-refractory prostate cancers through suppression of c-myc
expression and induction of G1 arrest of the cell cycle. PLoS ONE.
9:e873232014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Castagnino P, Kothapalli D, Hawthorne EA,
Liu SL, Xu T, Rao S, Yung Y and Assoian RK: miR-221/222 compensates
for Skp2-mediated p27 degradation and is a primary target of cell
cycle regulation by prostacyclin and cAMP. PLoS ONE. 8:e561402013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Osawa T, Atsumi Y, Sugihara E, Saya H,
Kanno M, Tashiro F, Masutani M and Yoshioka K: Arf and p53 act as
guardians of a quiescent cellular state by protecting against
immortalization of cells with stable genomes. Biochem Biophys Res
Commun. 432:34–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Choi JK, Kim KH, Park SR and Choi BH:
Granulocyte macrophage colony-stimulating factor shows
anti-apoptotic activity via the PI3K-NF-κB-HIF-1α-survivin pathway
in mouse neural progenitor cells. Mol Neurobiol. 49:724–733. 2014.
View Article : Google Scholar
|
|
36
|
Lee KH, Choi EY, Koh SA, et al:
Down-regulation of survivin suppresses uroplasminogen activator
through transcription factor JunB. Exp Mol Med. 43:501–509. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Szafer-Glusman E, Fuller MT and Giansanti
MG: Role of Survivin in cytokinesis revealed by a
separation-of-function allele. Mol Biol Cell. 22:3779–3790. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Götz J, David D, Hoerndli F, et al:
Functional genomics dissects pathomechanisms in tauopathies:
Mitosis failure and unfolded protein response. Neurodegener Dis.
5:179–181. 2008. View Article : Google Scholar : PubMed/NCBI
|