Introduction
Abnormally pigmented scars are an undesirable
consequence of cutaneous wound healing and are a complication for
which every individual worldwide is at risk. Abnormal pigmentation
renders scars more noticeable, which can have serious and profound
psychological implications (1,2).
The production of pigment is complex and controlled
by several extrinsic and intrinsic factors, and scar repigmentation
patterns cannot be predicted. As there are currently no definitive
treatment options available, this presents a challenge to
physicians. Therefore, the identification of key molecules, which
modulate the mechanism of abnormal pigmentation is of significant
interest.
Previous studies have demonstrated that angiotensin
II (AngII) may be involved in all stages of wound healing (3), including inflammatory cell invasion,
cell proliferation, cell migration, neovascularization and fibrosis
(4). AngII has been observed to
increase vascular permeability, recruit inflammatory cells
(5,6), and promote keratinocyte proliferation
(4,7,8) and
dermal wound closure (9).
Steckelings et al first described the
expression and putative role of AngII in human skin (10), and the regulation of keratinocyte,
dermal myofibroblast, and endothelial cell proliferation have also
been reported (9,11). Steckelings et al (10) reported that human skin expresses
AngII type 1 (AT1) receptors and type 2 (AT2) receptors, which were
suggested to be involved in skin wound healing (3). Steckelings et al (12) later demonstrated that the
expression levels of the AT1 and AT2 receptors was markedly
increased within the epidermal and dermal areas of scars. In
addition, Otake et al reported that inhibition of the AT1
receptor limited murine melanoma growth by reducing tumor volume
and microvessel density, demonstrating the importance of AT1
receptors in melanoma growth (13). A previous study also investigated
the mRNA expression of AT1, but not AT2, in cultured
primary melanocytes (10).
Although several functional roles of AngII have been
suggested, whether AngII induces abnormal pigmentation by
regulating the melanocyte system during wound healing remains to be
elucidated. In the present study, the functional role of AngII in
human melanocytes was investigated, and alterations in human
melanocytes were characterized.
Materials and methods
Compounds and drugs
Rabbit polyclonal anti-AT1 antibodies (sc-1173),
mouse polyclonal anti-tyrosinase antibodies (sc-20035) and
horseradish peroxidase-linked goat anti-rabbit (sc-2004) or goat
anti-mouse (sc-2005) antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). A protein quantification
kit and agarose were purchased from Bio-Rad Laboratories, Inc.
(Hercules, CA, USA). Polymerase chain reaction (PCR) Master Mix was
purchased from Promega (Madison, WI, USA). The commercial sources
of other products were as follows: Gentamicin, phosphate-buffered
saline (PBS), M254 medium and human melanocyte growth supplements
were from Cascade Biologics (Mansfield, UK); fetal bovine serum
(FBS) and RNeasy mini kit were from Qiagen (Valencia, CA, USA);
AngII, the AT1 receptor antagonist, losartan (LOS), ethidium
bromide, NaCl, KH2PO4, CaCl2,
MgSO4, L-3,4-dihydroxyphenylalanine (L-DOPA), glucose,
bovine serum albumin, EDTA, glycine, sodium dodecyl sulfate (SDS)
and Tris were from Sigma-Aldrich (St. Louis, MO, USA).
Melanocyte culture
Normal melanocytes were isolated from the epidermis
of human foreskins obtained from the Department of Urology (General
Hospital of Beijing Military of PLA, Beijing, China). The present
study was approved by the ethics committee of the General Hospital
of Beijing Military of PLA. Written informed consent was obtained
from the patients. The skin grafts were cut into small sections
(5×5 mm) and incubated with trypsin-EDTA (2.5 g/l trypsin, 0.2 g/l
EDTA) at 4°C overnight. Trypsin activity was required to separate
the epidermis from the dermis. The following day, trypsin activity
was neutralized by adding FBS at a 1:1 ratio, and replacing it with
PBS solution. The epidermis was separated from the dermis using
sterile forceps. Thorough pipetting was performed to separate the
cells, resulting in the formation of and cell-rich suspensions. The
solid tissue waste was removed and the suspension was centrifuged
at 1,000 x g for 5 min. The melanocytes were selectively grown in
defined M254 medium supplemented with human melanocyte growth
supplements. The number of cells was adjusted to 2.5×104
cells/cm2 and the cultures were maintained at 37°C in a
humidified 95% air and 5% CO2 atmosphere. The medium was
replaced at 2–3-day intervals. The cultures were routinely examined
for contamination and cell outgrowth. The cells were then split, at
confluence, by 5 min trypsin (2.5 g/l) treatment at room
temperature. The cells were subcultured once per week and
experiments were performed using cells between passages two and
four.
Treatment with AngII alone or in
combination with AT1 receptor antagonists
The confluent cells were seeded at sub-confluent
densities (2×105 cells) in 6-well plates and were grown
for 4 days, until confluent. The cells were subsequently treated
with different concentrations of AngII (0.01, 0.1, 1, 10 and 100
nM) for 24 h at 37°C. In certain experiments, the cells were
exposed to 1,000 nM LOS, an AT1 receptor antagonist, in
addition to the AngII, and/or were exposed to LOS for 30 min prior
to AngII stimulation. The culture medium was removed and the cells
were washed twice with PBS prior to adding fresh assay medium
supplemented with 0.1% FBS for 24 h. Following culture, a melanin
content assay was performed, and tyrosinase activity and cell
proliferation were measured. The cell homogenates and supernatants
were collected for RNA extraction using an RNeasy mini kit (Bio-Rad
Technologies, Inc.) for protein quantification, based on the
Bradford method (14).
Tyrosinase activity assay
The melanocytes were treated with AngII and/or LOS
for 24 h and were subsequently washed with ice-cold 1X PBS. Lysis
buffer, containing 150 μl 1% Triton X-100 in 0.1 M phosphate
buffer, was added to each 6-well plate. The cells were scraped and
transferred to a 1.5 ml tube, lysed using between three and five
freeze-thaw cycles in liquid nitrogen, and centrifuged at 5,000 × g
for 5–10 min at 4°C. The samples (300–500 μg/80 μl)
were transferred into a new 96-well plate on ice. L-DOPA (20
μl; 5 mM) was added to each well, the plate was incubated at
37°C for 1 h and the absorbance was measured at 475 nm using a
spectrophotometer (DU-70; Beckman Coulter, Brea, CA, USA).
Melanin content assay
The melanin content was determined, as described
previously (15). Briefly, the
cells were lysed with 200 μl 1M NaOH and pipetted repeatedly
to homogenize the samples. The cell extract was subsequently
transferred into 96-well plates, and the relative melanin content
was determined by measuring the absorbance at 405 nm using an
enzyme-linked immunosorbent assay (ELISA) plate reader (Synergy
H1MF; BioTek, Winooski, VT, USA).
Tetrazolium assay
A
3-(4,5-dimethylthiazol-2-yl)-2,5-diphe-nyltetrazolium bromide (MTT)
yellow tetrazole assay was performed to investigate cell
proliferation (14). Following the
treatment with Ang II and/or LOS, 100 μl aliquots of the
cells were harvested, as detailed above, and plated in
flat-bottomed 96-well plates (2.5×104
cells/cm2). The cells were allowed to attach and grow
overnight at 37°C. The MTT assay was performed, according to the
manufacturer’s instructions. The formazan precipitates were
quantified by measuring the absorbance at 562 nm using an ELISA
plate reader.
RT-qPCR
The total RNA extraction and RT reaction were
performed, as described previously (16), and the mRNA expression levels of
AT1 and tyrosinase were evaluated using qPCR. The total RNA
was extracted 24 h after drug treatment using a TRIzol kit
(Invitrogen Life Technologies, Carlsbad, CA, USA) and
reverse-transcribed using an RT kit (ReverTra Ace® qPCR
RT kit; Toyobo, Osaka, Japan). Semi-qPCR was performed using
primers (Table I) for the AT1
receptor and tyrosinase (Beijing Dingguo Biological Technology Co.,
Ltd., Beijing, China). The qPCR was performed using a C100 Thermal
Cycler (Bio-Rad Laboratories, Inc.) and a touchdown protocol, as
described previously (17), using
the following program: 1 cycle at 94°C for 2 min, 12 cycles at 92°C
for 20 sec, 68°C for 30 sec and 70°C for 45 sec (with a decrease of
1°C per cycle) and 22 cycles at 92°C for 20 sec, 55°C for 30 sec
and 70°C for 45 sec. The PCR products were separated by
electrophoresis using 2% agarose gels and visualized by ethidium
bromide (0.5 μg/ml) staining for 5 min at room temperature.
The PCR band intensity was determined using Quantity One software
(v4.62; Bio-Rad Laboratories, Inc.), and was expressed as the
relative intensity against that of β-actin.
 | Table IPrimers used in the reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primers used in the reverse
transcription-quantitative polymerase chain reaction.
| Primer | Sequence
(5′–3′) | Size (bp) |
|---|
| Angiotensin II type
1 |
| Forward |
ATTGCCAACAGCCTATCT | 270 |
| Reverse |
CCATCCTCCTGGTCCTTA | |
| Tyrosinase |
| Forward |
ACGATGTGGACGAGTGT | 133 |
| Reverse |
CAGAGGCAGGTGAAGGT | |
| β-actin |
| Forward |
ATCATGTTTGAGACCTTCAACA | 318 |
| Reverse |
CATCTCTTGCTCGAAGTCCA | |
Western blotting
The protein expression levels of AT1 and tyrosinase
were assessed by western blotting. The human foreskin were cut into
small sections and incubated with trypsin-EDTA (2.5 g/l trypsin,
0.2 g/l EDTA) at 4°C overnight. Trypsin activity was required to
separate the epidermis from the dermis. Subsequently, the
menlanocytes were isolated and cultured, and treated with AngII
alone or in combination with AT1 receptor antagonists. The
menlanocytes were then collected in lysis buffer and centrifuged
for 30 min at 15,000 × g at 4°C. The supernatant was collected and
the protein concentration was determined using the bicinchoninic
acid method (Bio-Rad Laboratories, Inc.). The proteins (20
μg) were denatured with SDS sample buffer, boiled for 5 min
and separated onto 10–12% polyacrylamide gels (Novex, San Diego,
CA, USA). Following electrophoresis, the proteins were transferred
into 1X transfer buffer, containing 25 mM Tris, 192 mM glycine and
0.1% SDS and 20% methanol (pH 8.4), onto a 0.45 μm
Immobilon-P polyvinyl difluoride membrane (Millipore, Temecula, CA,
USA) in a Mini Protean II transfer cell (Bio-Rad Laboratories,
Inc.) set at a constant voltage of 120 mV for 2 h. The membranes
were blocked in 5% non-fat dry milk in Tris-buffered saline (TBS)
for at least 1 h at room temperature. The blots were subsequently
incubated overnight at 4°C with either rabbit anti-AT1 or mouse
anti-tyrosinase (1:1,000 dilution). The membranes were washed three
times with TBS containing 1% Triton X-100 (TBS-T), incubated with
horseradish peroxidase-linked goat anti-rabbit or goat anti-mouse
antibodies (1:2,000 dilution) for 2 h at room temperature and were
ten washed four times with TBS-T. The immunoreactive bands were
visualized by exposing the membrane blots to an enhanced
chemiluminescence substrate (Thermo Fisher Scientific, Waltham, MA,
USA), and the proteins of interest were visualized on X-ray film
(BT Film; Carestream Health, Xiamen, China). Three independent
experiments were performed in triplicate.
Statistical analysis
Statistical analyses were performed using SPSS 14.0
(SPSS, Inc., Chicago, IL, USA). The data are expressed as the mean
± standard error of the mean. Statistical analysis between groups
was performed using analysis of variance. P<0.05 was considered
to indicate a statistically significant difference.
Results
AngII regulation of tyrosinase activity,
melanin content and cell proliferation via the AT1 receptor
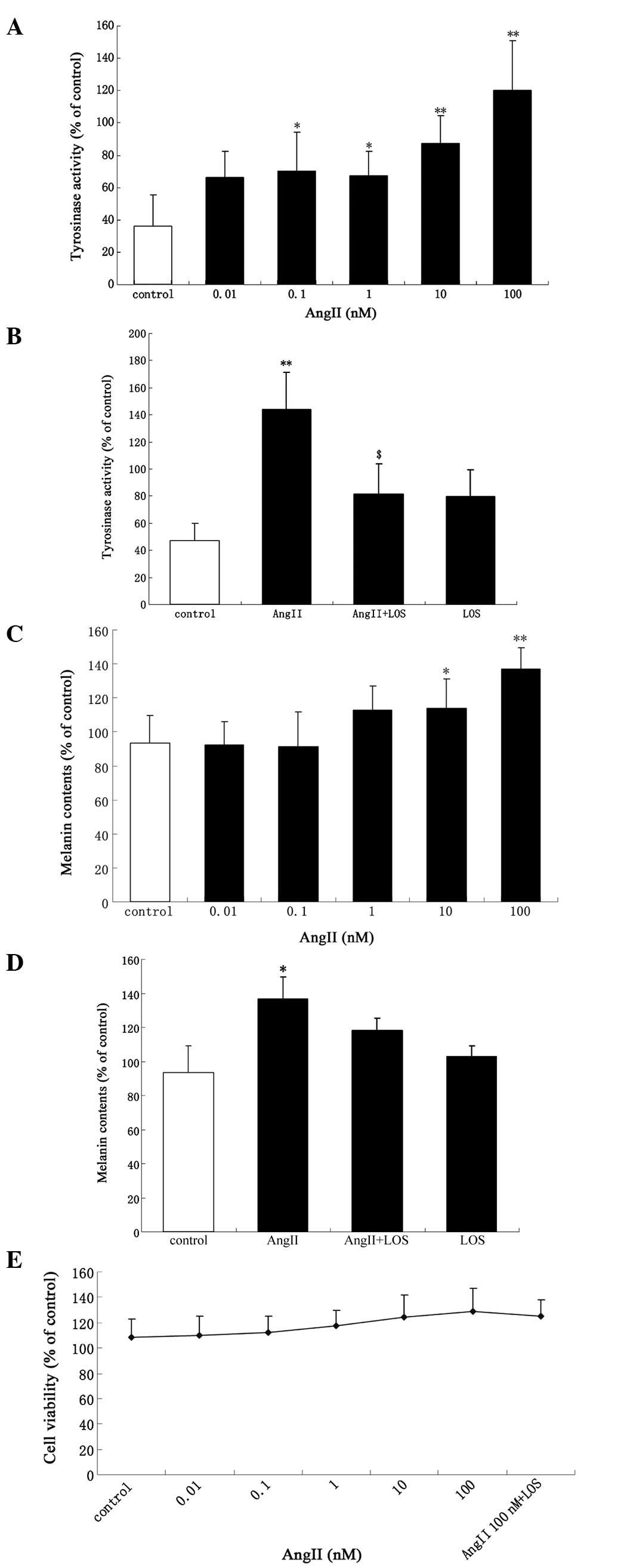
Tyrosinase activity and melanin content increased
significantly in a dose-dependent manner following treatment with
AngII (Fig. 1A and C). There were
significant increases in the activity of tyrosinase and melanin
content following incubation of the cells with increasing
concentrations of AngII (0.1–100 nM) and AngII (10–100 nM) for 24
h, respectively. The maximal increase was observed with 100 nM
AngII (P<0.01). An increase of ~17% (114±16.89; P<0.05) in
tyrosinase activity was observed following treatment with 100 nM
AngII (Fig. 1A). Treatment with
<0.01 nM AngII had no effect on tyrosinase activity compared
with the control (Fig. 1A). At
0.01–1 nM AngII, no difference in melanin content was observed
compared with the control (Fig.
1C). The tyrosinase activity and melanin content were inhibited
following incubation with 100 nM AngII alone for 24 h or following
exposure to 1 μM LOS, a selective AT1 receptor antagonist,
for 30 min prior to AngII treatment (Fig. 1B and D). There was a marginal, but
non-significant increase in cell proliferation (P>0.05; Fig. 1E).
AngII regulation of the mRNA expression
of AT1
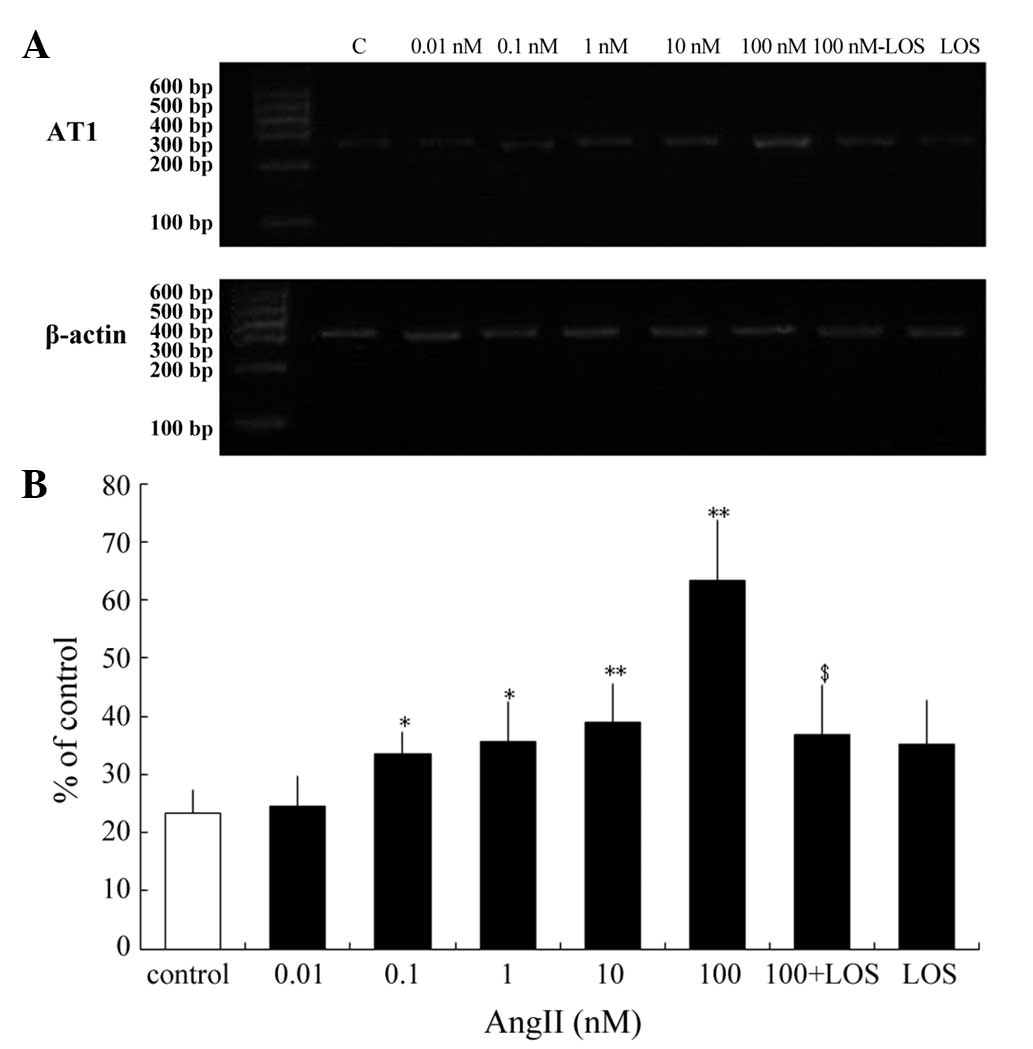
Previous studies have reported that AngII regulates
the expression levels of the AngII receptor subtypes in non-ocular
tissues and in human retinal pigment epithelium tissues (18,19).
Steckelings et al examined cultured primary melanocytes and
examined the mRNA expression of AT1, but not AT2 (10). The present study investigated
whether AngII modulates the expression of the AT1 receptor
in cultured human melanocytes. To determine the effective range of
AngII, the cells were treated with 0.01–100 nM AngII for 24 h, and
the AngII modulation of the expression of AT1 was assessed.
At 0.1–100 nM, AngII increased the mRNA expression of AT1.
The maximal increase was observed at 100 nM AngII, increasing the
mRNA expression of AT1 2.7-fold (63.21±10.59%; P<0.01).
Treatment with 10 nM AngII increased the mRNA expression of
AT1 1.67-fold (38.94±6.54%; P<0.01), whereas 0.1 and 1 nM
AngII increased the mRNA expression levels of AT1 1.44-and
1.53-fold (33.47±3.91 and 35.54±6.821%) respectively (P<0.05).
However, treatment with 0.01 nM AngII had no effect on the mRNA
expression of AT1 compared with the control (Fig. 2). Pretreatment with LOS prevented
the maximal upregulation of the mRNA expression of AT1 by
100 nM AngII (Fig. 2). Therefore,
AngII regulated the mRNA expression of AT1 in human
melanocytes and LOS inhibited these effects.
Regulation of the mRNA and protein
expression levels of tyrosinase by Ang II via the AT1 receptor
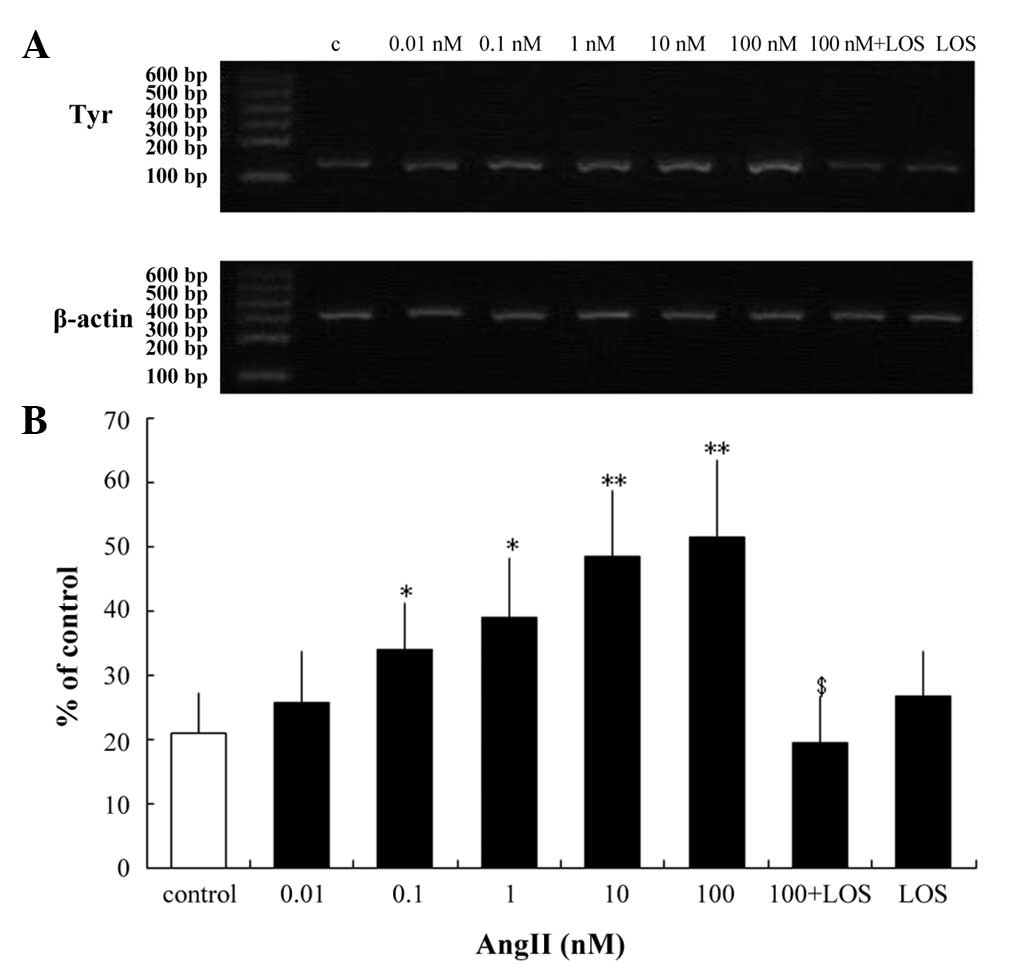
Tyrosinase is the key regulatory enzyme in melanin
biosynthesis (20). The present
study examined whether AngII alters the mRNA and protein expression
levels of tyrosinase in human melanocytes. RT-qPCR analysis of
tyrosinase revealed no changes in transcription following 24 h
incubation with 0.01 nM AngII (Fig.
3). However, significant increases in transcription were
observed following 24 h incubation with higher concentrations of
AngII (0.1–100 nM; Fig. 3).
Following incubation with 0.1 and 1 nM AngII, there
was an increase of 38.562 and 46.259% (P<0.05), respectively)
and a 57% increase (P<0.01) following treatment with 10 nM AngII
(Fig. 3). A maximal increase of
59.5% (P<0.01) was observed following treatment with 100 nM
AngII (Fig. 3). However,
pretreatment with LOS eradicated this effect (Fig. 3B). Western blotting was performed
to compare the effect of AngII on the ratio of secreted tyrosinase
protein. Following 24 h incubation with 100 nM AngII, there was a
significant increase in tyrosinase in the melanocytes (Fig. 4). Pretreatment with LOS prevented
the AngII-induced increase in protein expression levels of
tyrosinase and AT1 (Fig.
4), suggesting that the AT1 receptor mediates the
AngII-induced increase of tyrosinase in human melanocytes and that
it is coupled to tyrosinase signaling.
Discussion
Despite extensive investigations on pigment cells
and wound healing, current understanding of scar repigmentation
following cutaneous injury remains limited, and the mechanisms
associating AngII with abnormal pigmentation following wound
healing remains to be elucidated. Notably, the localization of
AngII and its receptors in the skin (10,11,21)
has suggested the possibility of a prominent pathological role for
AngII, as demonstrated by previous studies investigating wounding
healing in skin (11,22).
The present study aimed to characterize the
expression and function of AngII receptors in human melanocytes and
investigate the contribution of AngII to abnormal pigmentation.
AngII regulated the expression of the AT1 receptor in human
melanocytes, confirming that these receptors are functional in this
cell type. In addition, it was demonstrated that AngII alters
normal human melanocyte physiology, leading to a potentially
favorable upregulation of tyrosinase. A previous study examining
the localization of the AT1 and AT2 receptors in
whole human skin using immunohistochemistry, revealed the
expression of AT1 receptors in melanocytes, but not
AT2 receptors (10). The
present study utilized molecular biology techniques, directly
applied to isolated human melanocytes. Using this approach, it was
confirmed that AT1 receptors are expressed in human
melanocytes as mRNA and protein, consistent with Steckelings et
al (10). However, the
relative expression levels of these receptors in melanocytes remain
to be elucidated. Based on the fact that AT1 receptors
confer an active physiological role to AngII, their regulation
becomes essential in determining the action of AngII. Investigating
the regulation of the expression of these receptors may assist in
understanding how they are involved in either physiological or
pathological events. In the present study, the melanocytes
expressed markedly low levels of AT1 receptor subtypes in
the complete absence of AngII or in the presence of 0.01 nM AngII.
Treatment with 0.1 nM AngII increased the expression of the
AT1 receptor. By contrast, higher concentrations of AngII
(1–100 nM) increased the expression levels of the AT1
receptor in a dose-dependent manner. These effects were AngII
receptor-mediated as the AngII receptor antagonist, LOS, eliminated
the effects of AngII on the transcriptional regulation of the
AT1 receptors in the melanocytes. This is supported by
evidence that the AT1/AT2 receptors are involved in
pathological events in other tissue types (23–25).
A previous study determined that AT1 receptors are expressed
in human skin (10), and the
present study confirmed that AngII regulated the AT1
receptors at the transcriptional level in melanocytes. Whether
these receptors are functional in this cell type was also
investigated. It has been reported that the AT1 receptor
belongs to the G protein-coupled, seven-transmembrane receptor
family (24,26). The present study demonstrated that
stimulation of the AT1 receptor by AngII is transduced into
an intracellular signal, increasing the levels of tyrosinase. This
result provides evidence that the AT1 receptor expressed in
human melanocytes is functionally active and may be efficiently
coupled to the tyrosinase transduction pathway, however,
upregulation of the AT1 receptor transduction pathway
remains to be investigated. Melanocytes are central in the
pathogenesis of abnormal pigmentation and, although the cellular
mechanism underlying abnormal pigmentation remain to be elucidated,
evidence suggests that tyrosinase may be involved (19,27).
Previous studies have demonstrated that physical stimuli regulate
the activity of tyrosinase (28).
The present study investigated whether AngII affects the regulation
of tyrosinase and observed an increase in the mRNA and protein
expression levels of tyrosinase. In addition, the AT1
antagonist, LOS, eliminated the effect of AngII on tyrosinase
activity and melanin content, indicating that AngII induced the
pigmentation changes by enhancing tyrosinase activity via the
AT1 receptor. The melanocytes were treated with AngII for
only 24 h, therefore the possibility that longer exposure may
induce an increase in tyrosinase cannot be ruled out.
The levels of tyrosinase protein were increased
following exposure to 100 nM AngII, via the AT1 receptors,
suggesting a synergistic effect of the AT1 receptor on
tyrosinase upregulation in human melanocytes. This suggested that
certain effects induced by AngII may be specific to the type of
cell targeted. The present study demonstrated that AngII receptor
inhibitors may prevent these changes.
Notably, the results of the present study suggested
a potential role for AngII in tyrosinase regulation through the
AT1 receptors, and supported an association between AngII,
tyrosinase and AT1 receptor activation, which may be
involved in abnormal pigmentation by upregulating tyrosinase in
melanocytes.
The identification of functional AT1
receptors in human melanocytes has provided novel insights into
melanocyte physiology. The nature of these receptors, their
additional functional activities and their regulatory mechanism
require further investigation to assess the role of AngII in
cutaneous pathophysiology.
Acknowledgments
The authors would like to thank the staff at the
Department of Dermatology, General Hospital of Beijing Military of
PLA (Beijing, China) for their assistance during the preparation of
this manuscript. The authors would also like to thank Professor
Junhong Ao for their technical assistance and Professor Wenling
Wang for their critical reading of the manuscript. This study was
supported by grants from the Foundation of Capital Medical
Development and Research (no. 2007–3027) and the Second Five-Year
Plan of Military Medical Science and Technology Research Foundation
(no. CWS11J218).
References
|
1
|
Wisely JA, Hoyle E, Tarrier N and Edwards
J: Where to start? Attempting to meet the psychological needs of
burned patients. Burns. 33:736–746. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zeitlin RE: Long-term psychosocial
sequelae of paediatric burns. Burns. 23:467–472. 1997. View Article : Google Scholar
|
|
3
|
Singer AJ and Clark RA: Cutaneous wound
healing. N Engl J Med. 341:738–746. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rodgers K, Xiong S, Felix J, Roda N,
Espinoza T, Maldonado S and Dizerega G: Development of angiotensin
(1–7) as an agent to accelerate dermal repair. Wound Repair Regen.
9:238–247. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim JA, Berliner JA and Nadler JL:
Angiotensin II increases monocyte binding to endothelial cells.
Biochem Biophys Res Commun. 226:862–868. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reddy HK, Sigusch H, Zhou G, Tyagi SC,
Janicki JS and Weber KT: Coronary vascular hyperpermeability and
angiotensin II. J Lab Clin Med. 126:307–315. 1995.PubMed/NCBI
|
|
7
|
Steckelings UM, Artuc M, Paul M, Stoll M
and Henz BM: Angiotensin II stimulates proliferation of primary
human keratinocytes via a non-AT1, non-AT2 angiotensin receptor.
Biochem Biophys Res Commun. 229:329–333. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takeda H, Katagata Y, Hozumi Y and Kondo
S: Effects of angiotensin II receptor signaling during skin wound
healing. Am J Pathol. 165:1653–1662. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rodgers K, Abiko M, Girgis W, St Amand K,
Campeau J and diZerega G: Acceleration of dermal tissue repair by
angiotensin II. Wound Repair Regen. 5:175–183. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Steckelings UM, Wollschläger T, Peters J,
Henz BM, Hermes B and Artuc M: Human skin: source of and target
organ for angiotensin II. Exp Dermatol. 13:148–154. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nozawa Y, Matsuura N, Miyake H, Yamada S
and Kimura R: Effects of TH-142177 on angiotensin II-induced
proliferation, migration and intracellular signaling in vascular
smooth muscle cells and on neointimal thickening after balloon
injury. Life Sci. 64:2061–2070. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Steckelings UM, Henz BM, Wiehstutz S,
Unger T and Artuc M: Differential expression of angiotensin
receptors in human cutaneous wound healing. Br J Dermatol.
153:887–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Otake AH, Mattar AL, Freitas HC, et al:
Inhibition of angiotensin II receptor 1 limits tumor-associated
angiogenesis and attenuates growth of murine melanoma. Cancer
Chemother Pharmacol. 66:79–87. 2010. View Article : Google Scholar
|
|
14
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lei TC, Virador VM, Vieira WD and Hearing
VJ: A melanocyte-keratinocyte coculture model to assess regulators
of pigmentation in vitro. Anal Biochem. 305:260–268. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Virador VM, Kobayashi N, Matsunaga J and
Hearing VJ: A standardized protocol for assessing regulators of
pigmentation. Anal Biochem. 270:207–219. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marin-Castaño ME, Elliot SJ, Potier M, et
al: Regulation of estrogen receptors and MMP-2 expression by
estrogens in human retinal pigment epithelium. Invest Ophthalmol
Vis Sci. 44:50–59. 2003. View Article : Google Scholar
|
|
18
|
Peng Y, Kang Q, Cheng H, et al:
Transcriptional characterization of bone morphogenetic proteins
(BMPs)-mediated osteogenic signaling. J Cell Biochem. 90:1149–1165.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Gasparo M, Catt KJ, Inagami T, Wright
JW and Unger T: International union of pharmacology. XXIII The
angiotensin II receptors. Pharmacol Rev. 52:415–472.
2000.PubMed/NCBI
|
|
20
|
Striker GE, Praddaude F, Alcazar O,
Cousins SW and Marin-Castaño ME: Regulation of angiotensin II
receptors and extracellular matrix turnover in human retinal
pigment epithelium: roleof angiotensin II. Am J Physiol Cell
Physiol. 295:C1633–C1646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Olivares C and Solano F: New insights into
the active site structure and catalytic mechanism of tyrosinase and
its related proteins. Pigment Cell Melanoma Res. 22:750–760. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yahata Y, Shirakata Y, Tokumaru S, et al:
A novel function of angiotensin II in skin wound healing. Induction
of fibroblast and keratinocyte migration by angiotensin II via
heparin-binding epidermal growth factor (EGF)-like growth
factor-mediated EGF receptor transactivation. J Biol Chem.
281:13209–13216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang W, Yu LF, Zhong J, et al:
Angiotensin II type 1 receptor expression in human gastric cancer
and induces MMP2 and MMP9 expression in MKN-28 cells. Dig Dis Sci.
53:163–168. 2008. View Article : Google Scholar
|
|
24
|
Sarlos S, Rizkalla B, Moravski CJ, Cao Z,
Cooper ME and Wilkinson-Berka JL: Retinal angiogenesis is mediated
by an interaction between the angiotensin type 2 receptor, VEGF and
angiopoietin. Am J Pathol. 163:879–887. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wolf G: Angiotensin II and tubular
development. Nephrol Dial Transplant. 17(Suppl 9): 48–51. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kaschina E and Unger T: Angiotensin
AT1/AT2 receptors: regulation, signalling and function. Blood
Press. 12:70–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iozumi K, Hoganson GE, Pennella R, Everett
MA and Fuller BB: Role of tyrosinase as the determinant of
pigmentation in cultured human melanocytes. J Invest Dermatol.
100:806–811. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamaguchi Y and Hearing VJ: Physiological
factors that regulate skin pigmentation. Biofactors. 35:193–199.
2009. View
Article : Google Scholar : PubMed/NCBI
|