Introduction
Glioblastoma multiforme (GBM) is one of the most
malignant types of primary tumors in the central nervous system,
the prognosis of which is poor (1). The growth of GBM is dependent on
angiogenesis (2); therefore,
antiangiogenic therapy has been considered to be a promising
strategy for the inhibition of tumor progression (3). However, there is increasing evidence
that traditional antiangiogenic therapy may elicit a greater
malignancy in tumors, which was suggested to occur due to
vasculogenic mimicry (VM) (4,5). VM
is defined as the formation of blood-conducting channels by highly
malignant tumor cells (6). A
previous study suggested that the presence of VM was a prognostic
factor for postoperative survival in GBM patients (7). Consequently, the elucidation of an
effective approach for inhibiting VM formation has been the focus
of an increasing number of studies (8,9).
The mechanism of VM formation remains to be fully
elucidated; however, it has been suggested that cancer stem-like
cells (SLCs) may have an important role in this process (10). Cancer SLCs were reported to have
the capacity for self-renewal, extensive proliferation,
multi-lineage differentiation and tumor initiation (11); these characteristics may also be
defined as plasticity. The existence of SLCs in the majority of
cancers had been considered to be a possible explanation for the
resistance of tumors to traditional radiation and chemotherapy
(12,13). Therefore, it was suggested that
cancers may be effectively treated through eradicating small
subpopulations of SLCs (14).
Differentiation-inducing treatment has been
considered to be a promising strategy for the elimination of
embryonic cancer SLCs, as it may decrease the ability of these cell
to differentiate into multiple linages. A potent
differentiation-inducing treatment using all-trans retinoic acid
(ATRA) was reported to reduce the tumorigenicity of glioma SLCs,
thus exhibiting antitumor effects (15). However, the underlying antitumor
mechanism of ATRA remains to be elucidated. It was hypothesized
that the antitumor effects of ATRA may be associated with the
inhibition of VM formation inhibition. Therefore, the aim of the
present study was to investigate the influence of ATRA on the VM
formation ability of SLCs derived from the U87 glioblastoma cell
line.
Materials and methods
Culture of U87
The U87 human glioblastoma cell line (World Health
Organization grading guidelines, grade IV) was purchased from the
American Type Culture Collection (Manassas, VA, USA) and was
maintained in high-glucose Dulbecco’s modified Eagle’s medium
(DMEM; Invitrogen Life Technologies, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (FBS; Invitrogen Life
Technologies) in 5% CO2 at 37°C. The U87 cell line was
used for subsequent experiments up to passage 5.
U87 SLCs culture and identification
U87 cells were seeded into flasks at a density of
2×105/ml. The serum-free culture medium (stem cell
medium) consisted of DMEM/F12 (Invitrogen Life Technologies)
supplemented with 20 ng/ml epidermal growth factor (EGF;
Sigma-Aldrich, Lyon, France), 20 ng/ml basic fibroblast growth
factor (bFGF; Sigma-Aldrich) and B27 (1:50; Invitrogen Life
Technologies SAS, Saint Aubin, France). Cultures were incubated in
5% CO2 at 37°C and half of the medium was replaced with
DMEM every 2 days. When the cultured stem cell spheroids became
dark in the center with a diameter of 70–100 μm, they were
dissociated enzymatically using 0.25% Trypsin-EDTA (Invitrogen Life
Technologies) into single cells and plated at a density of
5×103/cm2 in the presence of stem cell
medium. Cells were fixed in 4% neutral-buffered formalin (Shanghai
Haoran Biotechnology Co., Ltd., Shanghai, China) for 30 min, then
blocked with phosphate-buffered saline (PBS)/3% goat serum
(Shanghai Haoran Biotechnology Co., Ltd.) for 20 min. Cells were
then immunostained overnight at 4°C with the following primary
antibodies: Mouse monoclonal immunoglobulin (Ig)G anti-nestin (cat.
no. sc-377380; 1:100; Santa Cruz Biotechnology, Inc., Heidelberg,
Germany) and rabbit polyclonal IgG anti-CD133 (cat. no. PAB12663;
1:100; Abnova, Taipei, Taiwan). Following washing with DMEM three
times, cells were incubated with DyLight™ 594-conjugated goat
anti-rabbit IgG (cat. no. 35561; 1:100) or fluorescein
isothiocyanate-conjugated goat anti-mouse IgG secondary antibodies
(cat. no. A-11001; 1:100; Invitrogen Life Technologies SAS),
accordingly, for 1 h at 37°C. Cells were then washed and their
nuclei were stained with Hoechst 33342 (Shanghai Haoran
Biotechnology Co., Ltd.) for 10 min. Cultures were examined under a
Leica DMI4000B fluorescent microscope (Leica Microsystems, Wetzlar,
Germany).
Differentiation assay
U87 SLCs spheres were plated (~8–10 spheres per
well) onto sterile a 24-well glass slide coated with
poly-L-ornithine (Sigma-Aldrich) in neurosphere medium without EGF
and bFGF. The medium was supplemented with 10% FBS (blank control
group, BC), FBS combined with 10 nmol/l dimethyl sulfoxide (DMSO;
negative control group, NC) or FBS combined with ATRA (dissolved in
DMSO; Shanghai Haoran Biotechnology Co., Ltd.) at various
concentrations (1, 10 and 100 nmol/l). A non-treated group (NT) of
U87 SLCs spheres without any treatment (serum-free) was also
established. Spheres were fixed with 4% paraformaldehyde (Shanghai
Haoran Biotechnology Co., Ltd.) for 15 min, permeabilized with
PBS/0.3% Triton X-100 (Shanghai Haoran Biotechnology Co., Ltd.) and
blocked with PBS/3% goat serum for 20 min The spheres were then
identified using immunofluorescent staining, as described above,
using the following primary antibodies: Chick polyclonal IgG
anti-glial fibrillary acidic protein (GFAP; cat. no. ab4674; 1:100;
Abcam, Cambridge, MA, USA), rabbit polyclonal IgG anti-β-tubulin
III (cat. no. ab18207; 1:100; Abcam) and rabbit polyclonal IgG
anti-galactosylceramidase (Galc; cat. no. ab8375; 1:100; EMD
Millipore, Billerica, MA, USA). Spheres were then incubated with
DyLight™ 488-conjugated goat anti-chick IgG (cat. no. 103-485-155;
1:100; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA,
USA) or DyLight™ 594-conjugated goat anti-rabbit IgG (cat. no.
35561; 1:100; Invitrogen Life Technologies SAS) secondary
antibodies, accordingly. Following staining with Hoechst 33342, the
differentiation rate of U87 SLCs was evaluated by comparing the
area of cells stained by Hoechst vs. the area of cells stained by
GFAP.
Proliferation assay
The proliferation ability of cells was evaluated
using a cell counting kit (CCK)-8 test (Shanghai Haoran
Biotechnology Co., Ltd.). In brief, differentiated cells were
plated into single cell suspensions at a density of
1×103 cells/well into wells of a 96-well plate. Each
well was then treated with 10 μl CCK-8 every day for 7 days.
Cultures were incubated in 5% CO2 at 37°C for 4 h
following daily CCK-8 administration. Supernatants were obtained by
centrifuging at 1,000 × g for 20 min. Subsequently, the absorbance
of the supernatant from each well was detected at 450 nm using a
microplate reader (model 550; Bio-Rad Laboratories, Inc., Hercules,
USA).
Cell invasion assay
The invasive ability of differentiated cells derived
from U87 SLCs spheres was investigated using an invasion assay. The
assay was performed using Transwell® cell culture
inserts (Invitrogen Life Technologies) according to the
manufacturer’s instructions. Cells were then allowed to invade for
24 h. The rate of cell invasion was determined by calculating the
mean number of invasive cells from five randomly selected fields of
vision for each well (Leica DMI3000B; Leica Microsystems).
In vitro tube formation assay
A tube formation assay was established, as
previously described (16). In
brief, wells of a 24-well tissue culture plate were coated with
Matrigel® Basement Membrane Matrix (0.1 ml/well; 356234;
BD Bioscience, Franklin Lakes, NJ, USA), which was allowed to
polymerase at 37°C for 1 h. Differentiated cells were resuspended
and seeded onto the Matrigel® at a density of
2.5×105/ml, then incubated without serum in 5%
CO2 at 37°C for 24 h. Images of the cultures were
captured using a Leica inverted microscope (Leica DMI3000B; Leica
Microsystems). VM formation ability was determined by counting the
total length of tubes per field in five randomly selected fields of
vision (magnification, ×100) using Leica Application Suite v3.60
(Leica Microsystems).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells from each group
using TRIzol® (Invitrogen Life Technologies SAS) and
then verified by electrophoresis using the Sebia Hydrasys 2 agarose
gel electrophoresis system (Sebia Inc., Norcross, GA, USA). RNA was
then reverse transcribed using the SuperScript™ III First Strand
Synthesis System for RT-PCR (Invitrogen Life Technologies). PCR was
performed in a 50 μl reaction volume containing 1 μl
complementary DNA, 6X reaction buffer, 0.75 mM MgCl2,
0.04 mM deoxynucleotide mix, 0.2 pmol/μl forward primer, 0.2
pmol/μl reverse primer and 1 Unit Taq polymerase (Qiagen
Multiplex PCR kit, cat. no. 206143; Qiagen, Inc., Valencia, CA,
USA) for 35 cycles of 30 sec at 94°C, 58°C and 72°C using a Life
ECO Thermal Cycler (BYQ6078; Bioer Technology Co., Ltd., Hangzhou,
China). The primers used for amplification were as follows:
Vascular endothelial growth factor (VEGF) sense,
5′-CAGCTACTGCCATCCAATC-3′ and antisense, 5′-CAAATGCTTTCTCCGCTCTG-3′
(313 bp); and GAPDH sense, 5′-TGCCAGTGGTAATACGATT-3′ and antisense,
5′-TAGGAATACTGCCATCACAA-3′ (458 bp). GAPDH served as an internal
control. RT-qPCR products were electrophoretically analyzed in 1%
agarose and visualized with ethidium bromide staining (Shanghai
Haoran Biotechnology Co., Ltd.).
Enzyme-linked immunosorbent assay
(ELISA)
VEGF secreted into culture supernatant by treated
cells was detected using a HU VEGF ELISA kit (cat. no. KHG0111;
Invitrogen Life Technologies). In brief, cells from each group were
seeded into 96-well plates at a density of 1×104
cells/well and were cultured for 24 h. Cultures in serum-free
medium were incubated in 5% CO2 at 37°C for 24 h. The
culture medium was then collected and assayed according to the
manufacturer’s instructions. Absorbance detection was performed at
450 nm using a microplate reader (model 550; Bio-Rad Laboratories,
Inc.).
Statistical analysis
Values are presented as the mean ± standard
deviation of three independent experiments. All statistical
analyzes were performed using SPSS 13.0 software (SPSS Inc.,
Chicago, IL, USA). One-way analysis of variance and least
significant difference tests were used to analyze the differences
between groups. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
U87 spheres show properties of SLCs
CD133 and nestin were reported to be markers of most
tumor SLCs (17). Therefore, in
the present study, it was determined whether these markers were
expressed in U87 tumor spheres. As shown in Fig. 1, the majority of U87 cells in the
spheres were positive for CD133 and nestin.
ATRA increases the differentiation
efficacy of U87 spheres
In order to determine whether ATRA enhanced the
multi-lineage differentiation of U87 spheres, the expression of
GFAP, β-tubulin III and Galc was detected using immunofluorescent
staining. As shown in Fig. 2,
cells in all treatment groups (including the blank and negative
control groups) began spreading around, forming small
protuberances, connecting with neighboring cells and disrupting the
morphology of the spheres; however, these morphological
characteristics were not observed in the NT group. By detecting the
ratio of GFAP-positive cells to total number of cells, it was
demonstrated that 1 nmol/l ATRA (35.33%), 10 nmol/l ATRA (49.50%)
and 100 nmol/l ATRA (70.17%) exhibited a significantly more potent
ability to promote differentiation compared with that of the BC
group (25.83%; P=0.08, P<0.001 and P<0.001, respectively)
(Fig. 2). By contrast, the NC
group (24.33%) had no significant impact on promoting
differentiation compared with that of the BC group (P=0.623). In
addition, the NT group (9.67%) had a significantly weaker ability
to promote differentiation compared with that of the BC group
(P<0.001). The ratio of β-tubulin III-positive cells showed that
1 nmol/l ATRA (15.50%), 10 nmol/l ATRA (28.83%) and 100 nmol/l ATRA
(44.17%) had a significantly more potent effect on promoting
differentiation compared with that of the BC group (5.00%; all
P<0.001) (Fig. 2). By contrast,
the NT (2.17%) and NC (5.17%) groups showed no significant impact
on promoting differentiation compared with that of the BC group
(P=0.218 and 0.940, respectively). Furthermore, the ratio of
Galc-positive cells showed that 1 nmol/l ATRA (11.33%), 10 nmol/l
ATRA (27.17%) and 100 nmol/l ATRA (47.17%) significantly promoted
differentiation in U87 SLCs compared with that of BC group (5.67%;
P=0.026, P<0.001 and P<0.001, respectively). By contrast, the
NT (1.50%) and NC (4.83%) groups exhibited no significant effect on
differentiation compared with that of the BC group (P=0.086 and
0.715, respectively) (Fig. 2).
 | Figure 2Differentiation of U87 glioblastoma
spheres following incubation with different concentrations of ATRA.
Immunofluorescence staining was used to determine the expression of
(A–F) β-tubulin III, (G–L) Galc and (M–R) GFAP. (S–X) Hoechst 33342
staining of U87 cell nuclei. Spheres were divided into different
treatment groups as follows: (A, G, M and S) NT group (serum free);
(B, H, N and T) BC group; (C, I, O and U) NC group; (D, J, P and V)
1 nmol/l ATRA group; (E, K, Q and W) 10 nmol/l ATRA group; and (F,
L, R and X) 100 nmol/l ATRA group (scale bar, 200 μm). The
differentiation rate of spheres was determined by the area of
positive-stained cells vs. the total cells stained.
*P<0.05 vs. GFAP in BC group, #P<0.05
vs. β-tubulin III in BC group and &P<0.05 vs.
Galc in BC group. ATRA, all-trans retinoic acid; Galc,
galactosylceramidase; GFAP, glial fibrillary acidic protein; NT,
non-treated; BC, blank control; NC, negative control. |
ATRA decreases the proliferation of
differentiated U87 SLCs
A CCK-8 assay was performed in order to determine
the influence of ATRA on the proliferation of differentiated U87
SLCs. Proliferation was evaluated by determining the optical
density (OD) of each well of ATRA-treated, control and NT cells
(Fig. 3). The results showed that
cells treated with 10 nmol/l ATRA had a significantly reduced OD
compared with that of the BC group on the 3rd day (0.118±0.008;
P=0.008), 4th day (0.171±0.010; P=0.030), 5th day (0.246±0.018;
P=0.001), 6th day (0.387±0.031; P<0.001) and 7th day
(0.658±0.041; P=0.003) of CCK-8 administration. In addition, cells
treated with 100 nmol/l ATRA had a significant decreased OD
compared with that of the BC group on the 3rd day (0.091±0.008;
P<0.001), 4th day (0.142±0.007; P=0.001), 5th day (0.190±0.003;
P<0.001), 6th day (0.353±0.008; P<0.001) and 7th day
(0.495±0.017; P<0.001). However, the OD of the 10 nmol/l ATRA or
100 nmol/l ATRA groups showed no significant differences on the 1st
and 2nd day of the CCK-8 assay compared with that of the BC group.
Furthermore, the OD of the NC and 1 nmol/ATRA groups showed no
significant difference compared with that of the BC group on any
day of the CCK-8 assay (Fig.
3).
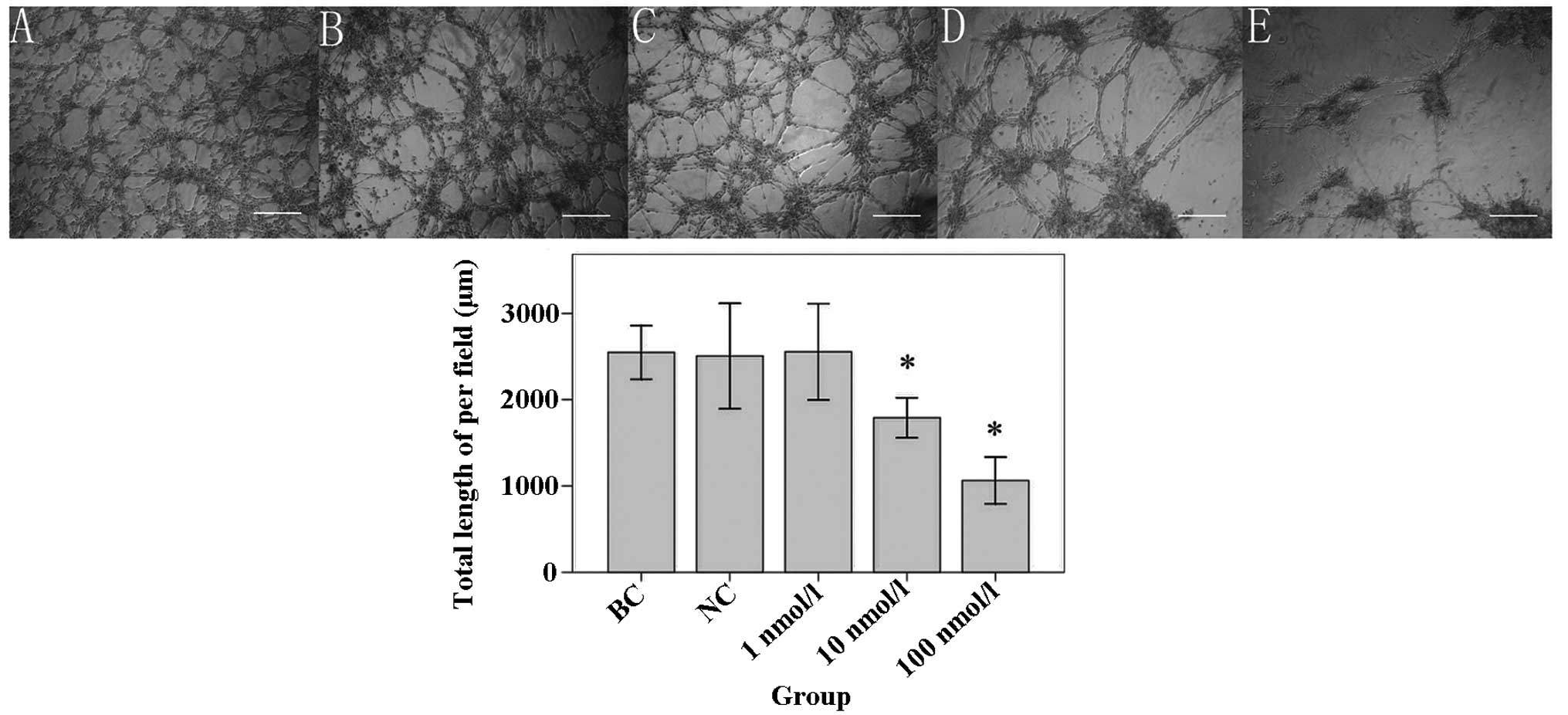
ATRA reduces the invasive and tube
formation abilities of U87 SLCs
The results of the Transwell® invasion
analysis (Fig. 4) showed that the
number of invading cells in the 10 nmol/l ATRA group (44.33±4.16)
and the 100 nmol/l ATRA group (23.33±2.52) were significantly
reduced compared with that of the BC group (72.33±5.03; all
P<0.001). However, the number of invading cells in NC group
(70.00±3.61) or 1 nmol/l ATRA group (72.00±5.57) was not
significantly different compared with that of the BC group (P=0.523
and 0.926, respectively). In addition, the tube formation ability
of U87 SLCs was evaluated using a Matrigel® assay
(Fig. 5). The results showed that
the total length of VM tubes following treatment with 10 nmol/l
ATRA (1790.00±93.34 μm) or 100 nmol/l ATRA (1063.33±108.93
μm) was significantly shorter compared with that of the BC
group (2547.00±124.85 μm; both P<0.001). By contrast, the
total length of the VM tubes in the NC (2505.67±245.98 μm)
or 1 nmol/l ATRA (2555.00±224.39 μm) groups were not
significantly altered compared with that in BC group (P=0.774 or
0.956, respectively).
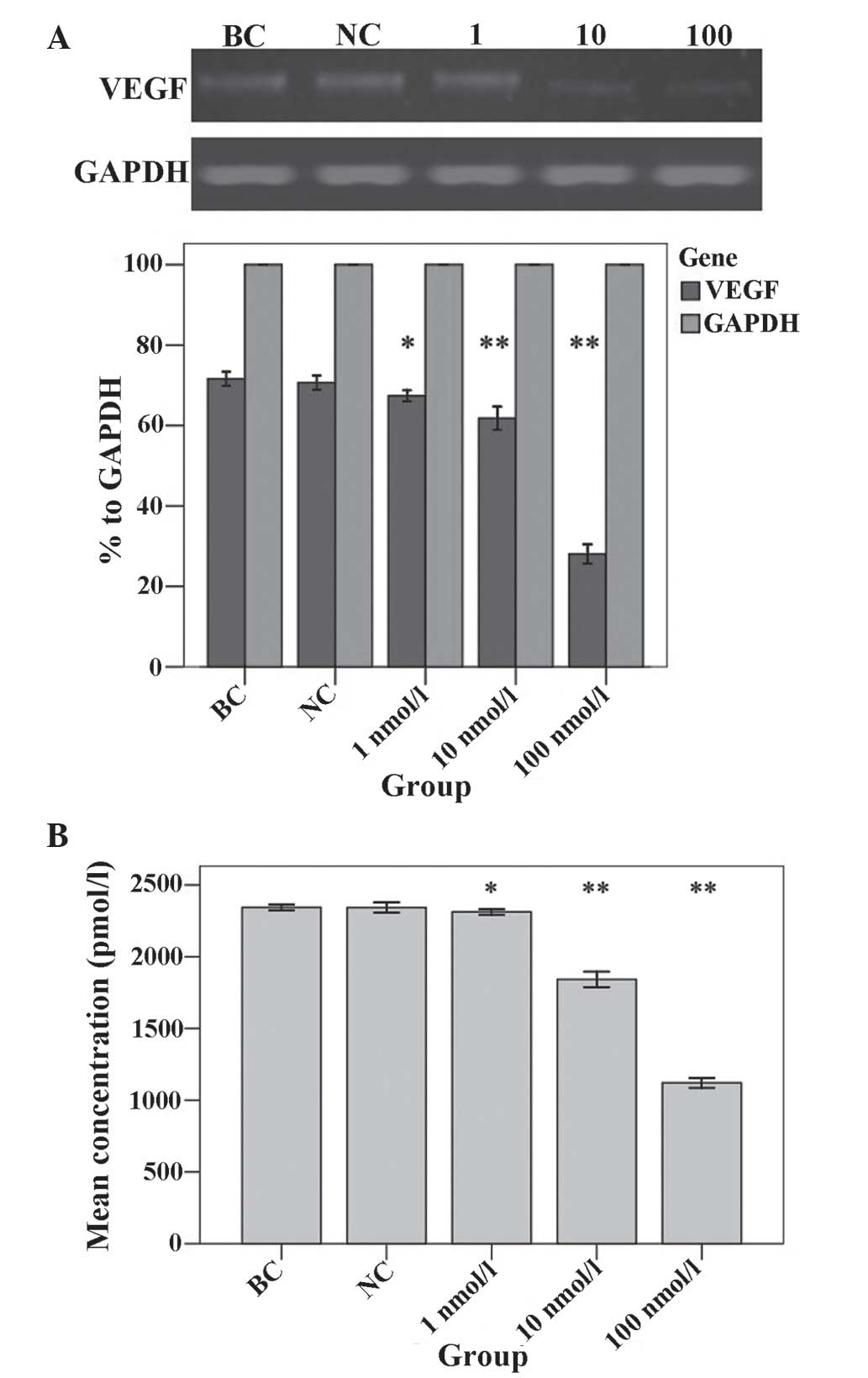
VEGF expression is downregulated in
ATRA-treated U87 SLCs
VEGF expression was detected in U87 SLCs using
RT-qPCR (Fig. 6A) and ELISA
(Fig. 6B) analysis. The results of
the RT-qPCR analysis were normalized to that of GAPDH. These
results showed that the expression levels of the VEGF transcript
were significantly reduced in groups treated with 1 nmol/l ATRA
(67.40%), 10 nmol/l ATRA (61.79%) and 100 nmol/l ATRA (28.04%)
compared with those of the BC group (71.61%; P=0.028, P<0.001
and P<0.001, respectively) (Fig
6A). By contrast, the NC group (70.67%) expressed comparable
levels of VEGF transcript to those of the BC group (P=0.878). The
VEGF protein concentration in the supernatant of the groups treated
with 1 nmol/l ATRA (2312.67±7.64 pmol/l), 10 nmol/l ATRA
(1841.67±21.55 pmol/l) and 100 nmol/l ATRA (1120.33±14.01 pmol/l)
were all significantly reduced compared with that of the BC group
(2343.67±7.51 pmol/l; P=0.022, P<0.001 and P<0.001,
respectively) (Fig. 6B). By
contrast, the NC group (2343.00±14.42 pmol/l) expressed comparable
protein levels of VEGF to those of the BC group (P=0.955).
Discussion
The results of the present study extended the
findings of previous studies and verified the hypothesis that ATRA
induces the differentiation of GBM cells. Of note, to the best of
our knowledge, the present study was the first to report the
influence of ATRA on the VM formation ability of U87 glioblastoma
cells. In addition, it was demonstrated that ATRA was able to
impair the plasticity of U87 SLCs, especially at higher
concentration, which was reflected in the increased differentiation
as well as the decreased proliferation, invasiveness, tube
formation and VM-associated cytokine secretion in U87 SLCs.
Cancer SLCs have been identified in numerous primary
culture GBM cells and tumor cell lines originating from GBM
(18,19). Tumor spheres which have been
derived from cultured cells in serum-free medium in the presence of
bFGF and EGF, were found to be rich in cancer SLCs (20). In addition, CD133 and nestin have
been confirmed to be molecular markers, which can be used for the
identification of SLCs (17).
Furthermore, SLCs have been reported to have the multi-lineage
differentiation abilities. Xiao et al (21) reported that 9L glioma cell spheres
derived from mice were able to differentiate into cells positive
for GFAP, neuron-specific enolase and Galc, which are
representative markers of neuronal, astroglial and oligodendroglial
cells; however, in humans, such markers differentiated from SLCs
were GFAP, β-tubulin III and Galc (22). In the present study, U87 spheres
were found to be positive for CD133 and nestin; in addition, the
cultured tumor spheres were able to differentiate into cells
positive for GFAP, β-tubulin III and Galc, therefore suggesting the
successful induction of U87 SLCs.
Traditional anticancer therapies primarily focused
on removing differentiated cancer cells, regardless of SLCs, which
are thought to be responsible for tumor progression and relapse
(23). In addition, increasing
evidence has suggested that cancer SLCs may be incorporated into
tumor vascularization, as it has been demonstrated that SLCs were
able to give rise to endothelial cells and vascular smooth
muscle-like cells (24,25). This therefore suggested that SLCs
may function as normal vascular mural cells originated from
vascular progenitor cells. Furthermore, El Hallani et al
(26) reported that SLCs derived
from primary GBM were able to form VM-tubes, without the assistance
of endothelial cells. Therefore, it is necessary to develop novel
strategies targeted at the elimination of SLCs in order to
effectively treat cancers and prevent reoccurrences.
A previous study showed that ATRA impaired
tumor-induced endothelial-dependent vessel formation in
vitro and in vivo (15). In the present study, the number of
U87 cells expressing GFAP, β-tubulin III and Galc was significantly
increased following the administration of ATRA, confirming the
potent differentiation-inducing ability of ATRA in malignant glioma
cells. In addition, differentiated cells derived from U87 SLCs
demonstrated significantly depleted tube formation abilities
following treatment with ATRA compared with those of the BC group.
According to Ma et al (27), the VM formation ability of SLCs may
also be evaluated using other approaches, such as cell
invasiveness. In the present study, cell invasion analysis was also
performed, the results of which showed that the invasive ability of
U87 SLCs was negatively correlated with the increasing
concentrations of ATRA. In addition, a negative correlation was
observed between the proliferation of differentiated U87 SLCs and
the increasing concentrations of ATRA. This therefore indicated
that ATRA significantly impaired the proliferating ability of SLCs
in a dose-dependent manner. Overall, it was inferred that ATRA may
have an important role in eradicating cancer SLCs, thus may provide
an effective therapeutic strategy for the inhibition of
endothelial-dependent and endothelial-independent tumor
vascularization.
A previous study suggested that SLCs contributed to
tumor neovascularization via three possible mechanisms: The
production of proangiogenic factors, transdifferentiation and the
formation of VM tubes (28). In
addition, studies have shown that the expression of certain
proangiogenic factors, including VEGF and bFGF, represented the
angiogenic ability of tumor cells as well as correlated with the VM
formation ability of tumor cells (29,30).
It has been reported that downregulation of VEGF disrupted
vasculogenic-like networks formed by osteosarcoma cells in
three-dimensional culture (31).
The results of the present study demonstrated that the expression
of VEGF in U87 SLCs was significantly decreased following treatment
with ATRA, indicating the anti-VM effect of ATRA. However, there
was no significant difference between the results of the 1 nmol/l
ATRA group and the BC group in the cell invasion, proliferation and
tube formation analyzes, although a significant difference was
observed between these two groups in the RT-qPCR and ELISA
analyzes. It was therefore suggested that the levels of secreted
VEGF may be an indicator of the number of U87 SLCs.
Two major limitations were not addressed in the
present study. Firstly, although the U87 cell line has been widely
used (32,33), it is still controversial as to
whether it is representative of highly malignant GBM. Lee et
al (34) reported that U87
cells may not represent primary tumors genetically and
epigenetically in primary GBM patients, unless the cells were
cultured in serum-free medium. Under this consideration, the U87
SLCs used in the present study were cultured in serum-free medium;
however, further experiments on primary cell lines are still
required. Secondly, convincing in vivo studies have not yet
been performed. Unlike that found in animals with leukemia, it was
reported that ATRA displayed a limited efficacy on the progression
of solid tumors (35,36). This may be due to the additional
metabolism in vivo, which may limit the effectiveness of the
administered ATRA dosage in a short period of time and ultimately
terminate the effects of ATRA (15). Therefore, further studies are
required in order investigate how the ATRA concentration may be
maintained in vivo. Charoenputtakhun et al (37) produced ATRA-loaded lipid
nanoparticles for transdermal drug delivery, which obtained
promising results. In addition, Hattori et al (38) found that ATRA-coated nanoparticles
produced an improved curative effect on wound healing compared with
that of unpacked ATRA. However, the performance of ATRA-coated
nanoparticles requires confirmation in GBM in vivo.
In conclusion, the results of the present study
demonstrated that ATRA exhibited potent differentiation-promoting
effects on U87 SLCs. In addition, ATRA reduced the proliferation
and invasiveness of U87 SLCs as well as decreased tube formation
and VEGF secretion. Furthermore, the VM formation ability of U87
SLCs was found to be negatively correlated with the differentiation
of these cells. These results therefore indicated that ATRA may
serve as a promising agent for the treatment of GBM, the mechanism
of which proceeds via the inhibition of VM formation.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81302177 and
81272806).
References
|
1
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: a clinical review. JAMA. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hardee ME and Zagzag D: Mechanisms of
glioma-associated neovascularization. Am J Pathol. 181:1126–1141.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vredenburgh JJ, Desjardins A, Herndon JE
II, et al: Phase II trial of bevacizumab and irinotecan in
recurrent malignant glioma. Clin Cancer Res. 13:1253–1259. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bergers G and Hanahan D: Modes of
resistance to anti-angiogenic therapy. Nat Rev Cancer. 8:592–603.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Soda Y, Myskiw C, Rommel A and Verma IM:
Mechanisms of neovascularization and resistance to anti-angiogenic
therapies in glioblastoma multiforme. J Mol Med. 91:439–448. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maniotis AJ, Folberg R, Hess A, et al:
Vascular channel formation by human melanoma cells in vivo and in
vitro: vasculogenic mimicry. Am J Pathol. 155:739–752. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang SY, Ke YQ, Lu GH, et al: Vasculogenic
mimicry is a prognostic factor for postoperative survival in
patients with glioblastoma. J Neurooncol. 112:339–345. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen LX, He YJ, Zhao SZ, et al: Inhibition
of tumor growth and vasculogenic mimicry by curcumin through
down-regulation of the EphA2/PI3K/MMP pathway in a murine choroidal
melanoma model. Cancer Biol Ther. 11:229–235. 2011. View Article : Google Scholar
|
|
9
|
Hu A, Huang JJ, Jin XJ, et al: Curcumin
suppresses invasiveness and vasculogenic mimicry of squamous cell
carcinoma of the larynx through the inhibition of JAK-2/STAT-3
signaling pathway. Am J Cancer Res. 5:278–288. 2014.
|
|
10
|
Liu TJ, Sun BC, Zhao XL, et al: CD133+
cells with cancer stem cell characteristics associates with
vasculogenic mimicry in triple-negative breast cancer. Oncogene.
32:544–553. 2013. View Article : Google Scholar
|
|
11
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Charles N, Ozawa T, Squatrito M, et al:
Perivascular nitric oxide activates notch signaling and promotes
stem-like character in PDGF-induced glioma cells. Cell Stem Cell.
6:141–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eyler CE and Rich JN: Survival of the
fittest: cancer stem cells in therapeutic resistance and
angiogenesis. J Clin Oncol. 26:2839–2845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao Z, Shang B, Zhang G, et al: Tumor
cell-mediated neovascularization and lymphan-giogenesis contrive
tumor progression and cancer metastasis. Biochim Biophys Acta.
1836:273–286. 2013.PubMed/NCBI
|
|
15
|
Campos B, Wan F, Farhadi M, et al:
Differentiation therapy exerts antitumor effects on stem-like
glioma cells. Clin Cancer Res. 16:2715–2728. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ling G, Wang S, Song Z, et al:
Transforming growth factor-β is required for vasculogenic mimicry
formation in glioma cell line U251MG. Cancer Biol Ther. 12:978–988.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang M, Song T, Yang L, et al: Nestin and
CD133: valuable stem cell-specific markers for determining clinical
outcome of glioma patients. J Exp Clin Cancer Res. 27:852008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh SK, Hawkins C, Clarke ID, et al:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singh SK, Clarke ID, Terasaki M, et al:
Identification of a cancer stem cell in human brain tumors. Cancer
Res. 63:5821–5828. 2003.PubMed/NCBI
|
|
20
|
Galli R, Binda E, Orfanelli U, et al:
Isolation and characterization of tumorigenic, stem-like neural
precursors from human glioblastoma. Cancer Res. 64:7011–7021. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiao ZY, Tang H, Xu ZM, et al: An
experimental study of dendritic cells transfected with cancer
stem-like cells RNA against 9L brain tumors. Cancer Biol Ther.
11:974–980. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu Y, Richard JP, Wang SD, et al:
Regulation of glioblastoma multiforme stem-like cells by inhibitor
of DNA binding proteins and oligodendroglial lineage-associated
transcription factors. Cancer Sci. 103:1028–1037. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gupta PB, Chaffer CL and Weinberg RA:
Cancer stem cells: mirage or reality? Nat Med. 15:1010–1012. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang R, Chadalavada K, Wilshire J, et al:
Glioblastoma stem-like cells give rise to tumor endothelium.
Nature. 468:829–833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ricci-Vitiani L, Pallini R, Biffoni M, et
al: Tumor vascularization via endothelial differentiation of
glioblastoma stem-like cells. Nature. 468:824–828. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
El Hallani S, Boisselier B, Peglion F, et
al: A new alternative mechanism in glioblastoma vascularization:
Tubular vasculogenic mimicry. Brain. 133:973–982. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma JL, Han SX, Zhu Q, et al: Role of Twist
in vasculogenic mimicry formation in hypoxic hepatocellular
carcinoma cells in vitro. Biochem Biophys Res Commun. 408:686–691.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ping YF and Bian XW: Concise review:
Contribution of cancer stem cells to neovascularization. Stem
Cells. 29:888–894. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang JY, Sun T, Zhao XL, et al: Functional
significance of VEGF-a in human ovarian carcinoma: role in
vasculogenic mimicry. Cancer Biol Ther. 7:758–766. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen Y, Jing Z, Luo C, et al: Vasculogenic
mimicry-potential target for glioblastoma therapy: an in vitro and
in vivo study. Med Oncol. 29:324–331. 2012. View Article : Google Scholar
|
|
31
|
Mei J, Gao Y, Zhang L, et al: VEGF-siRNA
silencing induces apoptosis, inhibits proliferation and suppresses
vasculogenic mimicry in osteosarcoma in vitro. Exp Oncol. 30:29–34.
2008.PubMed/NCBI
|
|
32
|
Shi Z, Lou M, Zhao Y, et al: Effect of
all-trans retinoic acid on the differentiation of U87 glioma
stem/progenitor cells. Cell Mol Neurobiol. 33:943–951. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Karsy M, Albert L, Tobias ME, et al:
All-trans retinoic acid modulates cancer stem cells of glioblastoma
multiforme in an MAPK-dependent manner. Anticancer Res.
30:4915–4920. 2010.PubMed/NCBI
|
|
34
|
Lee J, Kotliarova S, Kotliarov Y, et al:
Tumor stem cells derived from glioblastomas cultured in bFGF and
EGF more closely mirror the phenotype and genotype of primary
tumors than do serum-cultured cell lines. Cancer Cell. 9:391–403.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kaba SE, Kyritsis AP, Conrad C, et al: The
treatment of recurrent cerebral gliomas with all-trans-retinoic
acid tretinoin. J Neurooncol. 34:145–151. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Phuphanich S, Scott C, Fischbach AJ, et
al: All-trans-retinoic acid: a phase II Radiation Therapy Oncology
Group study (RTOG 91-13) in patients with recurrent malignant
astrocytoma. J Neurooncol. 34:193–200. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Charoenputtakhun P, Opanasopit P,
Rojanarata T and Ngawhirunpat T: All-trans retinoic acid-loaded
lipid nanoparticles as a transdermal drug delivery carrier. Pharm
Dev Technol. 19:164–172. 2014. View Article : Google Scholar
|
|
38
|
Hattori M, Shimizu K, Katsumura K, et al:
Effects of all-trans retinoic acid nanoparticles on corneal
epithelial wound healing. Graefes Arch Clin Exp Ophthalmol.
250:557–563. 2012. View Article : Google Scholar
|