Introduction
Malignant gliomas, the most common primary tumor
type of the central nervous system, are aggressive, highly
invasive, and neurologically destructive human cancers (1). Despite advances in surgical
techniques, radiotherapy and chemotherapy, the prognoses of
patients with malignant gliomas remain dissatisfactory (2). Establishing the molecular basis of
the tumorigenesis of malignant gliomas is crucial to improving
current therapies and developing novel treatments.
MicroRNAs (miRNAs or miRs) are endogenous, ~19–25
nucleotides long, non-coding small RNA molecules and are emerging
as important post-transcriptional regulators that either inhibit
mRNA translation or direct target mRNA degradation (3). miRs are involved in tumorigenesis and
tumor progression by regulating post-transcriptional gene
expression. Aberrantly expressed miRs have been shown to be
associated with numerous types of cancer. miRs are able to function
as oncogenes as well as tumor suppressors (4). The functional mechanism and
therapeutical potential of miRs specifically to gliomas are
appealing, since there has been very limited progress in the
development of treatments of malignant glioma in the last 25 years
(5). However, studies on miRs in
gliomas remain limited in number and are characterized by different
or paradoxical effects among tissue specimens and studies (6).
miR-27a is part of the miR-23a/27a/24-2 cluster
localized on chromosome 9q22. Several cellular targets of miR-27a
are able to impact cell cycle regulation, proliferation, apoptosis
and differentiation. Overexpression of miR-23a/27a/24-2 sensitized
HEK293T cells to TNF-α cytotoxicity (7,8).
Data indicated that miR-27a induced increased sensitivity of
leukemia (9) and JJN3 cells
(multiple myeloma cell line) (10)
to chemotherapy, as demonstrated by an increase the apoptotic rate.
This suggested that miR-27a may be a valuable target in cancer
therapy. miR-27a has been reported to be significantly upregulated
in several types of human cancer (11–13),
while being downregulated in others (14–16).
The miR-23a/27a/24-2 cluster may exert context-dependent functions
and its molecular functions are urgently required to be elucidated.
Recent studies have demonstrated that miR-27a may act either as an
oncogene or an anti-oncogene by targeting different downstream
targets in a variety of tissues and disease states (15,17).
To the best of our knowledge, the possible role of miR-27a in
malignant glioma has not been investigated.
The application of proteomics to gliomas has
identified lists of key proteins and signaling pathways (18). Among these important proteins, the
protein prohibitin (PHB) was most commonly identified to be
differentially expressed in multiple proteomic studies on glioma
(19–21). Several studies demonstrated that
PHB expression in human gliomas was higher than that in normal
brain tissues (20–22). In fact, it is thought that PHB may
have different roles in tumorigenesis, having either a permissive
action on tumor growth or a tumor suppressor role (23,24).
Of note, PHB has a large highly conserved 3′-untranslated region
(UTR) with several putative miR-binding sites, indicating that miRs
may be heavily involved in its regulation. The potential targeting
association between miR-27a and PHB may have an important role in
the process of cancer progression, tumorigenesis or tumor
suppression. To date, the association of miR-27a and PHB in gliomas
has not been clarified but is worth investigating.
The present study, explored the potential role of
miR-27a in malignant glioma via investigating the effects of
miR-27a the cell growth, apoptosis, cell cycle and invasiveness of
U251 and U87MG glioma cells in vitro. Furthermore, the
targeting effect of miR-27a on PHB in glioma cells was
investigated. The present study provided insight into developing
novel and effective therapeutic strategies for gliomas.
Materials and methods
Cell culture and treatments
The human glioma cell line U251 and the glioblastoma
cell line U87MG were obtained from the Cell Bank of Shanghai
Institutes for Biological Sciences of the Chinese Academy of
Sciences (Shanghai, China). The cells were cultured in DMEM
(Gibco-BRL, Invitrogen Life Technologies, Carlsbad, CA, USA),
supplemented with 10% fetal bovine serum (Gibco-BRL), 1.5 mmol/l
L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin
in a humidified incubator containing 5% CO2 and 95% air
at 37°C.
Oligonucleotides and cell
transfection
Locked Nucleic Acid (LNA)-modified Homo
sapiens (hsa)-miR-27a inhibitor (miR-27a anti-sense
oligonucleotides) and mimics (miR-27a sense oligonucleotides) were
chemically synthesized by Shanghai GenePharma Co., Ltd. (Shanghai,
China). The negative control (NC) was a scrambled oligonucleotide
that has been validated to not produce any identifiable effects on
known miRNA function. The sequences of hsa-miR-27a mimics (MI),
hsa-miR-27a inhibitor (IN), as well as the scrambled sequences MINC
and INNC are shown in Table I. The
cells were transfected with oligonucleotide using Lipofectamine
2000 reagent (Invitrogen Life Technologies) according to the
manufacturer’s instructions.
 | Table ISequences of hsa-miR-27a mimics and
inhibitor. |
Table I
Sequences of hsa-miR-27a mimics and
inhibitor.
| miR | Sequences
|
|---|
| Sense (5′-3′) | Antisense
(5′-3′) |
|---|
| hsa-miR-27a
mimics |
UUCACAGUGGCUAAGUUCCGC |
GGAACUUAGCCACUGUGAAUU |
| Mimics NC |
UUCUCCGAACGUGUCACGUTT |
ACGUGACACGUUCGGAGAATT |
| hsa-miR-27a
inhibitor |
GCGGAACT+T+AGCCACT+GT+GAA | |
| Inhibitors NC |
CAGUACUUUUGUGUAGUACAA | |
Real-time quantification of miR-27a and
PHB mRNA by stem-loop reverse transcription quantitative polymerase
chain reaction (RT-qPCR)
To detect the relative levels of miR-27a in glioma
tissues and PHB mRNA in cell lines, RT-qPCR was performed with a
universal reverse primer and miR-27a-specific primers, which were
designed from the primary precursor molecule sequences of the human
miR database (http://www.mirbase.org). Stem-looped
reverse transcription primers of human miR-27a and PHB primers were
synthesized by Shanghai GenePharma Co., Ltd. Expression of mature
miR-27a was quantified by miR-qRT PCR using the Hairpin-it™ miRNAs
qPCR Quantitation kit (GenePharma Co. Ltd.) and the FTC3000
Real-Time PCR detection system (Funglyn Biotech, Ontario, Canada)
The results of the real-time PCR were analyzed using the
2−ΔΔCt method.
Cell proliferation
The colorimetric Cell Counting kit-8 (CCK-8) assay
(Dojindo, Kumamoto, Japan) was used to measure cell proliferation.
Cells transfected with 10 pmol miR-27a mimics/inhibitor were plated
at a density of 10,000 cells/well in 96-well plates. CCK-8 solution
was added to each well at 0, 24, 48 and 72 h. The optical density
was then determined at 490 nm using a Multiskan Spectrum microplate
reader (Thermo Fisher Scientific, Waltham, MA, USA).
Cell cycle analysis
After each treatment for 48 h, nuclei of cells were
stained with propidium iodide (PI; M3032; Mbchem, Shanghai, China)
and examined by Cytomics FC500 Flow Cytometer (Beckman Coulter,
Pasadena, CA, USA) and DNA histograms were analyzed by MultiCycle
AV for windows software version 300 (Beckman Coulter).
Cell apoptosis assay
Staining with Annexin V conjugated with fluorescein
isothiocyanate (FITC)/propidium iodide (PI; M3021; Mbchem,
Shanghai, China) was performed according to the manufacturer’s
instruction and analyzed using the Cytomics FC500 Flow Cytometer
(Beckman Coulter) to distinguish between live/apoptotic/necrotic
cells.
Cell invasion assay
The invasive capacity of cells was determined using
BD Bio Coat Matrigel invasion chambers (8-μm pores; BD
Biosciences, San Jose, CA, USA). 48 h post-transfection, cells on
the underside of the membrane were counted from four microscopic
fields (magnification, x200; Nikon Eclipse TS100-F; Nikon, Tokyo,
Japan).
Glioma samples and normal brain
tissues
After informed consent from patients diagnosed with
glioma was obtained, human glioma samples were collected from the
Department of Neurosurgery, the First Affiliated Hospital of Xi’an
Jiangtong University (Xi’an, China) in 2012. The eight glioma
samples were diagnosed as World Health Organization grade II or
grade III by pathological diagnosis. Four normal brain tissues were
obtained following informed consent from patients with severe
traumatic brain injury who required post-trauma surgery.
Databases and bioinformatics
In order to define the potential targets of miR-27a,
the miRNA targets predicted by publicly available computational
algorithms were obtained from miRBase Targets (http://www.ebi.ac.uk/enright-srv/microcosm/cgi-bin/targets/v5/search.pl),
TargetScan (http://genes.mit.edu/tscan/targetscanS.html) and
TargetScan Release 6.2 (http://www.targetscan.org/cgi-bin/vert_61/view_gene.cgi?gs=PHB&taxid=9606&members=miR-27abc/27a-3p&showcnc=1&shownc=1)
to obtain the sequences containing a seed region. Furthermore, the
website http://mirecords.biolead.org/prediction_query.php
was used as a multiple miRNA target prediction tool.
Luciferase reporter assay
The full-length 3′-UTR region of the PHB gene (Gene
ID, P35232) was amplified from human genomic DNA including the
predicted target site for miR-27a on PHB, carrying the Xho I and
Not I restriction sites. The PCR product was cloned into the
multiple cloning region, located downstream of the Renilla
luciferase translational stop codon in the psiCHECK-2 vector
(Promega, Madison, WI, USA). 5 ng psiCHECK-2 -PHB mRNA 3′-UTR
luciferase reporter vectors and 5 pmol miR-27a mimics or inhibitor
or a negative control were co-transfected into U251 cells using
Lipofectamine 2000 (Invitrogen Life Technologies) in 24-well
plates. Cells were harvested at 48 h post-transfection and assayed
using the Dual-Luciferase Reporter Assay System (Promega, Madison,
WI, USA), according to the manufacturer’s instructions. Firefly
luciferase activity was normalized to Renilla luciferase
activity.
Western blot analysis
72 or 96 h post-transfection, U251 and U87MG cells
were lysed using Mammalian Protein Extraction Reagent (78503;
Thermo Fisher Scientific). The membranes were incubated at 4°C
overnight with a mouse primary monoclonal antibody against PHB
(ab55618; 1:500 dilution; Abcam, Cambridge, MA, USA) and a mouse
monoclonal anti-β-actin (A5441; 1:10,000 dilution; Sigma-Aldrich,
St. Louis, MO, USA), followed by incubation at room temperature for
2 h with horseradish peroxidase-conjugated goat anti-mouse
secondary Ig antibodies (JIR 115-035-003; 1:8,000 dilution; Jackson
ImmunoResearch, West Grove, PA USA). The immunoreactivity of the
proteins was detected with Super Signal West Pico Chemiluminent
substrates (Thermo Fisher Scientific) and exposed to X-ray film for
autoradiography (Kodak, Rochester, NY, USA). The autoradiograms
were scanned and analyzed with Gel-Pro analyzer software version
4.0 (Media Cybernetics, Rockville, MD USA).
Statistical analysis
Values are expressed as the mean ± standard error of
three or more experiments performed in duplicate. Statistical
analysis was performed by one-way analysis of variance, repeated
measures of general linear models, Fisher’s least significance
difference, Dunnett’s t-test for multiple groups and Student’s
t-test for double groups using SPSS 19.0 (International Business
Machines, Armonk, NY, USA). Differences from the control value were
considered significant when the P-value was <0.05.
Results
Validation of miR-27a mimics/inhibitors
in U251 and U87MG cells
After 24 h of transfection with miR-27a mimics,
miR-27a expression increased in the MI group of U251 and U87MG
cells by 5.85- and 18.43-fold, respectively, as compared with that
in the control and scramble-treated cells [F=39.10 (P<0.001) and
F=51.19 (P<0.001)]. At 48 h post-transfection, miR-27a
expression was also enhanced in U251 and U87MG cells by 18.92- and
4.37-fold, respectively [F=25.89 (P<0.01) and F=12.96
(P<0.01) for 48 h]. 24 h post transfection with miR-27a
inhibitor, miR-27a expression was suppressed in the IN groups of
U251 and U87MG cells by 0.16- and 0.10-fold, respectively [F=16.77
(P<0.01) and F=24.85 (P<0.01)]. At 48 h following
transfection, miR-27a expression was also reduced in U251 and U87MG
cells by 0.28- and 0.29-fold, respectively [F=5.27 (P<0.05) and
F=6.52 (P<0.05)] (Fig. 1).
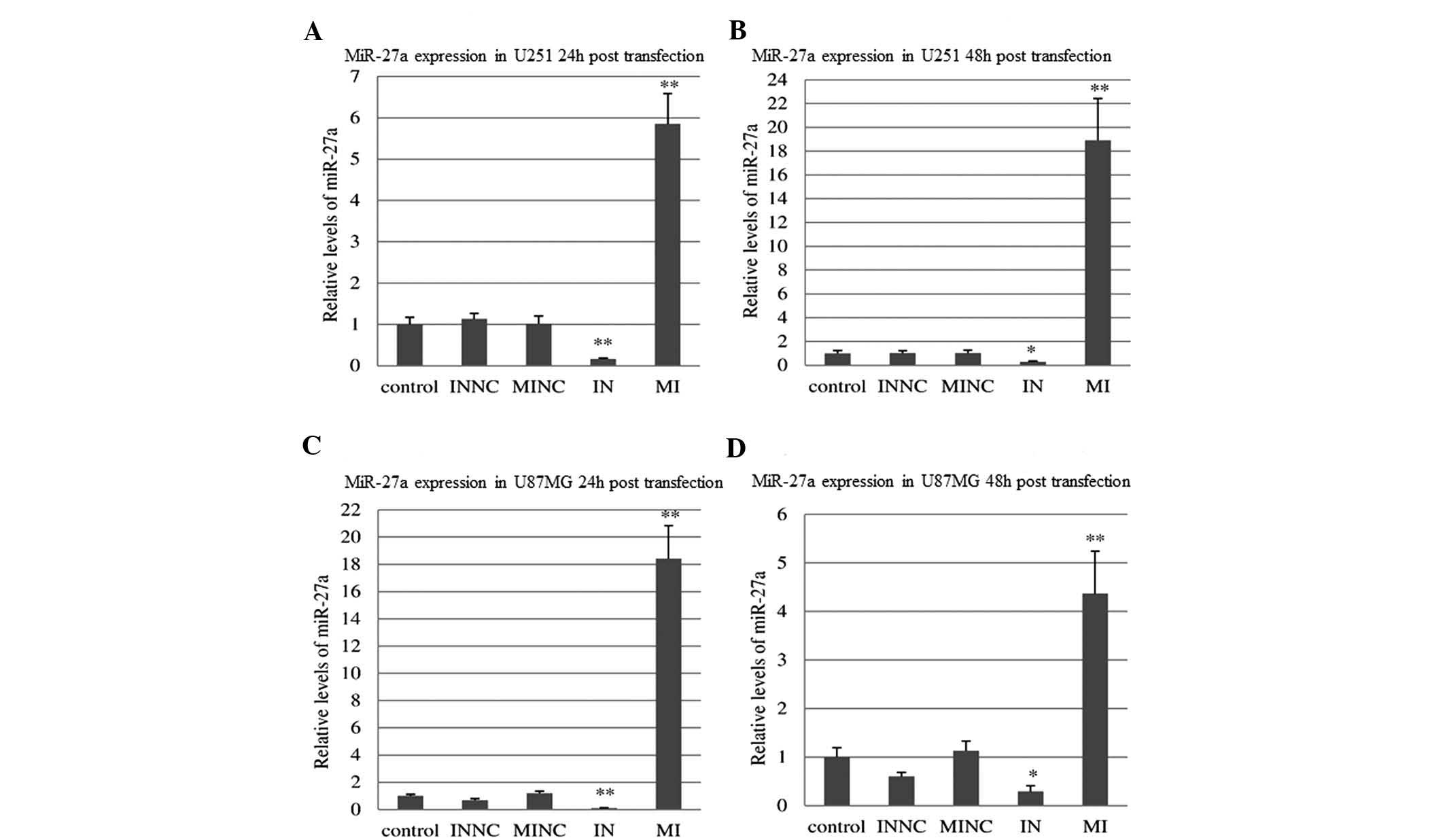 | Figure 1miR-27a was
up-regulated/down-regulated via transfection with miR-27a
mimics/inhibitor into U251 and U87MG cells. (A) Relative levels of
miR-27a mRNA expression in U251 cells were measured 24 h
post-transfection with MI/IN or MINC/INNC. (B) Relative levels of
miR-27a mRNA expression in U251 cells were measured 48 h
post-transfection with MI, IN, MINC or INNC. (C) Relative levels of
miR-27a mRNA expression in U87MG cells were measured 24 h
post-transfection with MI, IN, MINC or INNC. (D) Relative levels of
miR-27a mRNA expression in U87MG cells were measured 48 h
post-transfection with MI, IN, MINC or INNC. Error bars represent
the standard error of triplicate independent experiments.
Statistical analyses were performed using one-way analysis of
variance, Fisher’s least significant difference and Dunnett’s
t-test. *P<0.05, **P<0.01 compared with
control and NC groups. MI, miR-27a mimics; IN, miR-27a inhibitor;
INNC, scrambled inhibitor control; MINC, scrambled mimic
control. |
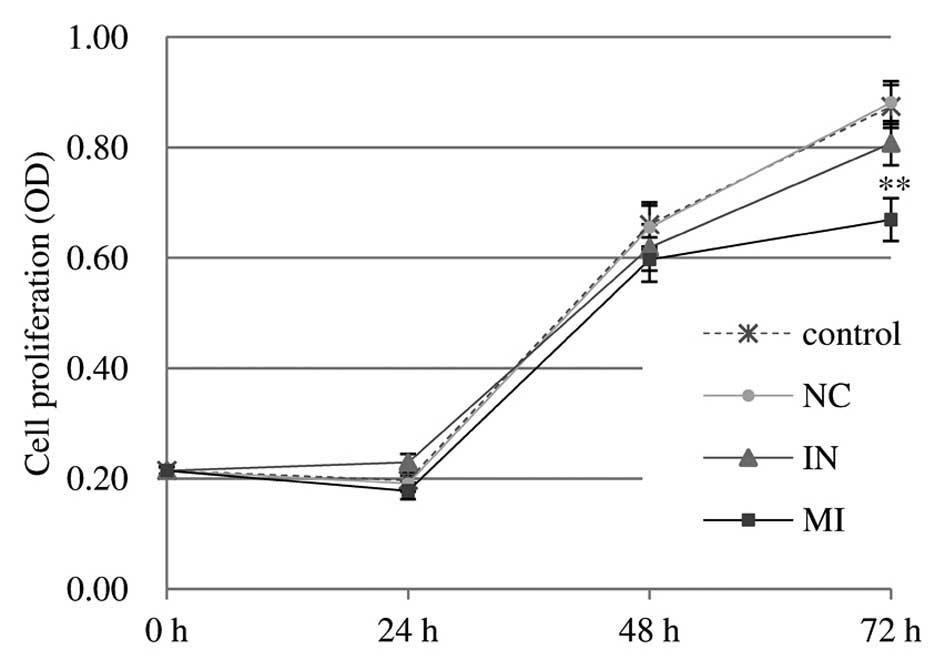
miR-27a mimics reduce the viability of
U251 cells
Compared with the control and scrambled control,
transfection of U251 cells with miR-27a mimics oligonucleotides (10
pmol) impaired the proliferation of U251 cells, as indicated by the
reduced viable cell number at 72 h post-transfection, resulting in
31.78% growth inhibition (P<0.01) (Fig. 2). The results indicated that
miR-27a may function as a tumor suppressor in glioma U251 cells
in vitro. Transfection with inhibitor oligonucleotides did
not show any enhancing or significant inhibitory effects on the
proliferation of U251 cells. By contrast, miR-27a had little or no
effect on the proliferation of U87MG cells (data not shown).
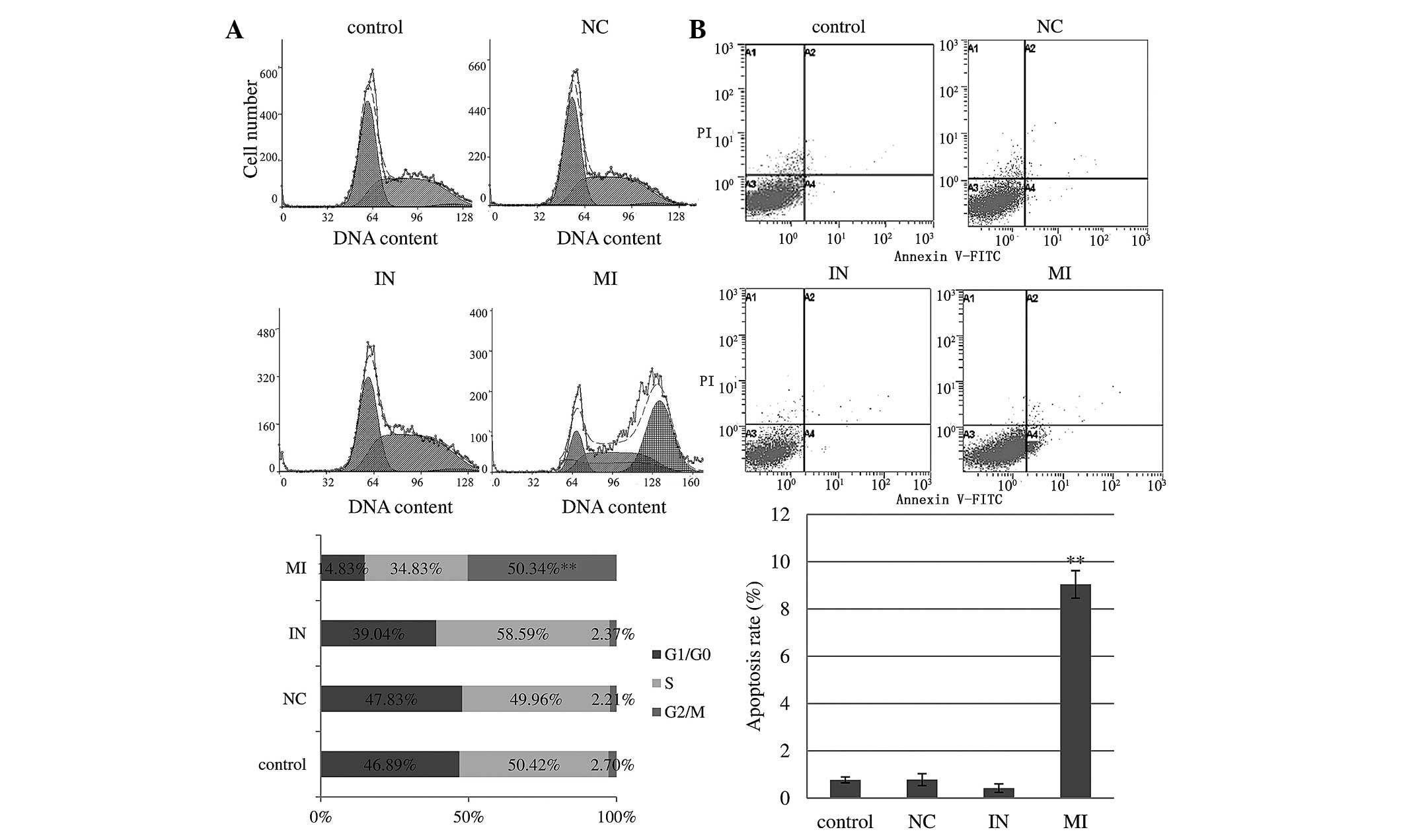
miR-27a mimics cause cell cycle arrest in
U251 cells
As shown in Fig.
3A, 48 h post-transfection with miR-27a mimics, the percentage
of U251 cells in G2/M phase was significantly increased to 50.34%,
compared to only 2.70 and 2.21% for the control and scramble
oligonucleotides (P<0.001). Similarly, the G1/G0 phase
populations in control and scramble-treated cells were 46.89 and
47.83%, respectively. miR-27a overexpression significantly reduced
the G1/G0 phase population to 14.83%. These results suggested that
miR-27a expression induced cell cycle arrest in G2/M phase, a delay
the progression of the cell cycle and inhibition of cell
proliferation. However, transfection with inhibitor
oligonucleotides did not affect the cell cycle in U251 cells.
Little or no effect of miR-27a was found on the cell cycle of U87MG
cells (data not shown).
miR-27a mimics promote apoptosis in U251
cells
Following 48 h of tranfection of miR-27a mimics,
flow cytometric analysis revealed that 8.47% of U251 cells were in
the lower right (LR) quadrant (Annexin
V+/PI−; early apoptotic cells) of the PI vs.
Annexin V dot plot compared with only 0.26, 0.44 and 0.54%
(P<0.001) in the inhibitor, scramble oligonucleotides and
control groups in the LR quadrant, respectively. Furthermore, 0.57%
of cells were in the upper right (UR) quadrant (Annexin
V+/PI+; late apoptotic cells) compared to
only 0.16, 0.33 and 0.24% (P<0.05) in the inhibitor, scramble
oligonucleotides and control groups in the UR quadrant,
respectively (Fig. 3B). The
significantly increased number of early apoptotic and late
apoptotic cells observed in U251 cells confirmed that miR-27a
inhibited glioma cell growth predominantly via inducing early
apoptosis. miR-27a inhibitor transfection decreased the apoptotic
rate of U251 cells, but without statistical significance. Little or
no differences were found in the apoptotic rate of U87MG cell
groups (data not shown).
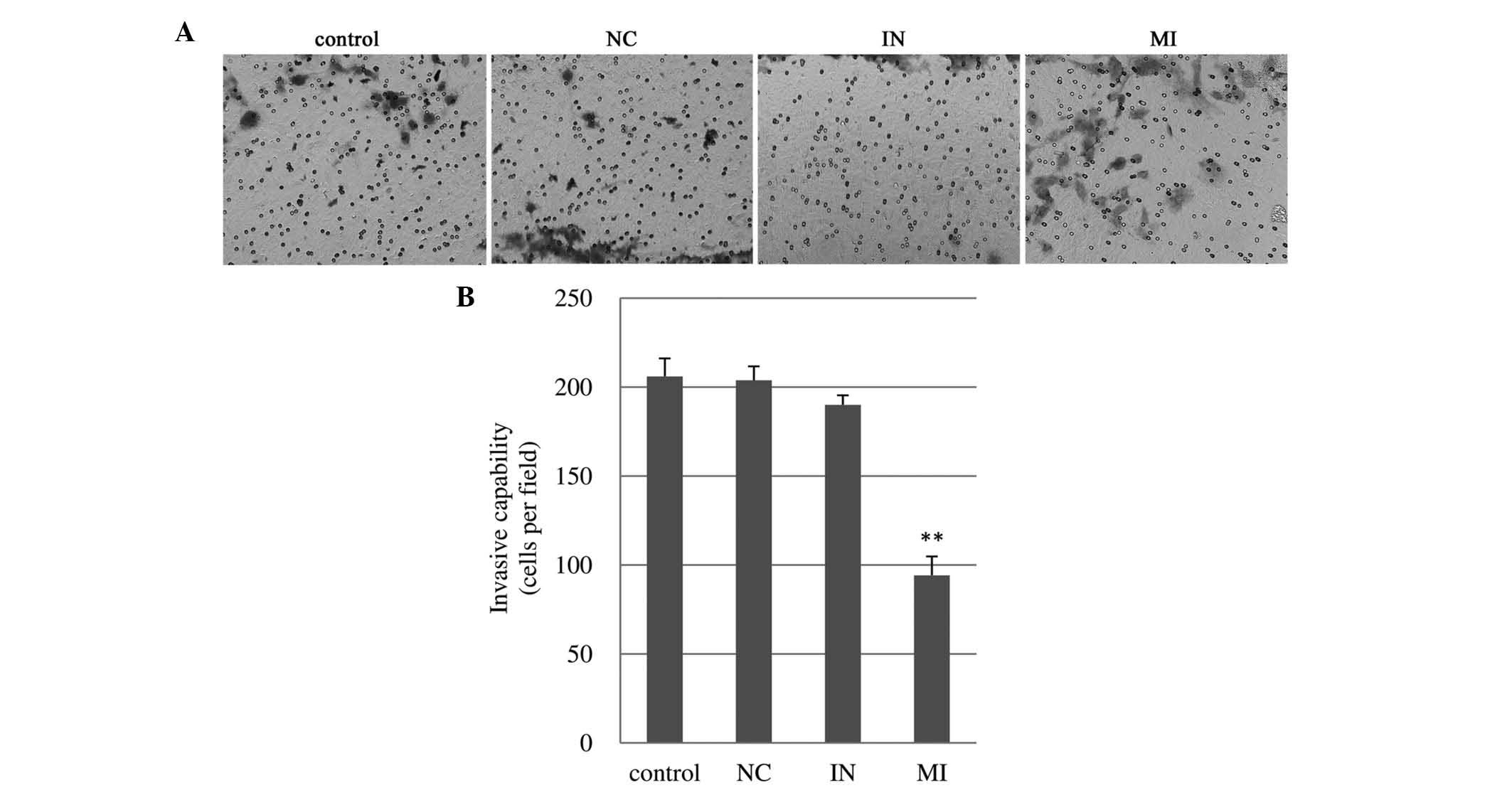
miR-27a mimics impair the invasive
ability of U251 glioma cells
The number of U251 cells invading through the
Matrigel following miR-27a mimics transfection was 9420±10.60
cells, which was half of that in the blank control (206.00±10.10)
and scrambled control (203.80±780) (Fig. 4). However, transfection with
miR-27a inhibitor didn’t affect the invasiveness of U251 cells.
Little or no differences were found in cell invasiveness of U87MG
cells (data not shown).
miR-27a has low expression in glioma
samples
As the effects of miR-27a observed in U251 cells
were not present in U87MG cells, the present study assessed miR-27a
expression in glioma samples diagnosed as grade II and III, but not
grade IV. The results showed that the relative expression levels of
miR-27a in the glioma group were significantly lower than those in
the normal brain group (0.35±0.05 vs. 1.05±0.19; P<0.01).
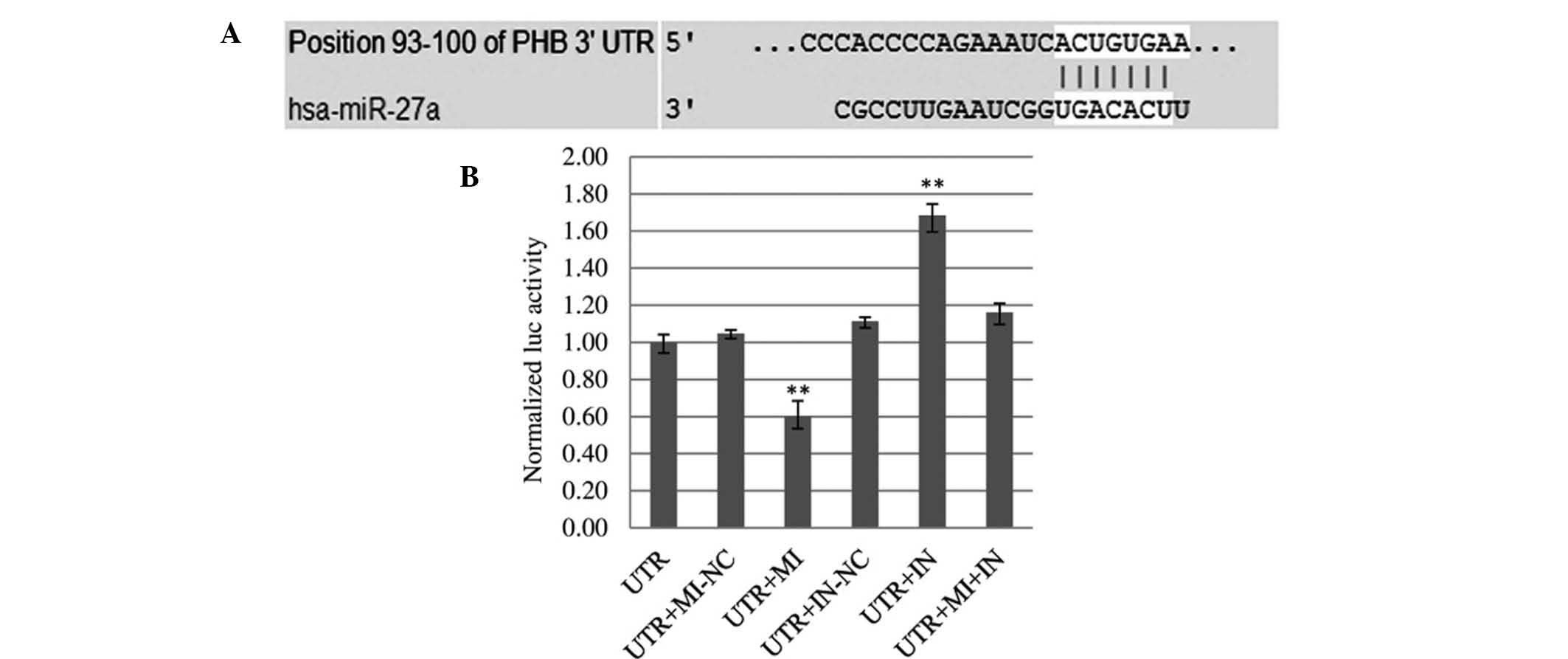
miR-27a directly regulates the expression
of PHB
To explore the mechanism of action of miR-27a in
glioma, online miRNA target prediction tools were used for
bioinformatics analysis, which predicted a potential interaction
between miR-27a and PHB. Using Target Scan Release 6.2, alignment
between the predicted miR-27a target site and binding sequence in
the PHB 3′-UTR was obtained (Fig.
5A). Absolute luciferase reporter assays demonstrated that 48 h
post-co-transfection, overexpression of miR-27a (UTR+MI group)
produced an obvious reduction (42.54%) in luciferase expression in
U251 cells as compared with the control (UTR+MI-NC) (Fig. 5B). This indicated that miR-27a
mimics bound to the PHB mRNA 3′-UTR and inhibited the expression of
renilla. Furthermore, downregulation of miR-27a (UTR+IN group)
resulted in a significant increase (33.90%) in luciferase activity.
When miR-27a mimics and inhibitor were transfected into U251 cells
simultaneously (UTR+MI+IN group), luciferase activity was higher
than that in the UTR group, but lower than that in the UTR+IN
group. This indicated that the miR-27a inhibitor bound not only
intrinsical miR-27a in cells but also exogenous transfected miR-27a
mimics. The luciferase assay confirmed that miR-27a directly
regulates PHB in U251 cells by binding to the predicted target site
in the 3′-UTR of PHB mRNA.
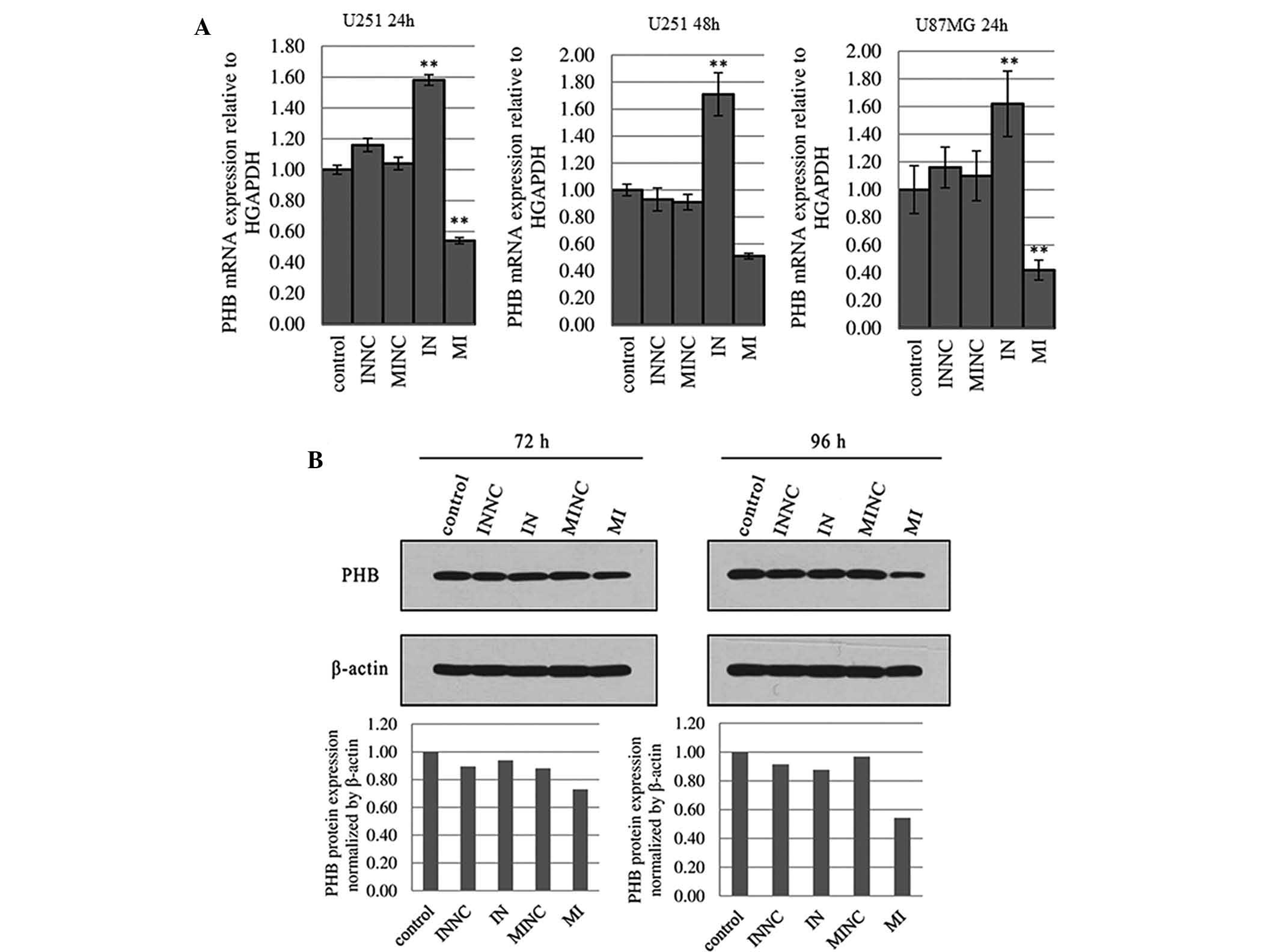
PHB mRNA expression is negatively
correlated with miR-27a levels in U251 cells
When miR-27a was silenced by miR-27a inhibitor for
24 and 48 h in U251 cells, PHB mRNA was subsequently elevated by
36.71 and 83.87%, respectively, compared with the scrambled control
(Fig. 6A). PHB mRNA expression in
U251 cells was decreased by 48.08 and 43.96% following transfection
with miR-27a mimics for 24 and 48 h, respectively (Fig. 6A). The results indicated that
miR-27a regulates endogenous PHB mRNA levels through a mechanism of
mRNA degradation. Similarly, in U87MG cells, miR-27a
inhibitor/mimics were able to up-regulate/down-regulate PHB mRNA
expression 24 h post-transfection (Fig. 6A). However, there was no
correlation between miR-27a expression and PHB mRNA expression in
U87MG cells 48 h post-transfection with miR-27a inhibitor/mimics
(data not shown).
miR-27a negatively regulates PHB protein
expression in U251 cells
Overexpression of miR-27a in U251 cells 72 and 96 h
post-transfection with miR-27a mimics significantly decreased PHB
protein expression levels by 17.18 and 43.90% (P<0.01),
respectively (Fig. 6B). Silencing
of miR-27a expression in U251 cells didn’t increase PHB protein
expression. In U87MG cells, there was no correlation between
miR-27a expression and PHB protein expression at 72 or 96 h
post-transfection (data not shown). Similarly, transfection with
miR-27a mimics or inhibitor for 48 h did not affect the levels of
PHB mRNA in U87MG cells. This may be the reason why up- or
downregulation of miR-27a was not able to affect PHB protein
expression.
Discussion
MiR-27a was shown to impact cell cycle regulation,
proliferation, apoptosis and invasiveness via regulating
post-transcriptional target genes. Previous studies showed that
miR-27a is significantly upregulated in several types of human
cancer, including breast (12),
kidney (11), hepatocellular
(13) and gastric (25) cancer. Increased expression of
miR-27a was shown to initiate tumor development by targeting
certain cellular factors, including FOXO-1 (11), Myt-1 (26), ZBTB10/RINZF (a putative zinc finger
and BTB domain-containing protein) (26,27)
and RYBP/DEDA (an apoptotic facilitator) (27). miR-27a is upregulated in pediatric
B-cell acute lymphoblasic leukemia and acts as a suppressor of the
tumor suppressor FBW7. FBW7 acts as a negative regulator of
proliferation by facilitating proteasome degradation of cyclin E,
c-Myc, c-Jun and Notch 1 (28).
The experimental studies which reported the abovementioned findings
support the oncogenic role of miR-27a in breast (12,26,27),
gastric (29), colon (30) and prostate cancer cells. However,
in certain types of cancer, including acute promyelocytic leukemia
(15), colorectal cancer (16,31),
malignant melanoma (32), oral
squamous cell carcinoma (33) and
prostate cancer (14), expression
of miR-27a was shown to be downregulated. In the present study,
miR-27a expression was significantly downregulated in glioma tissue
diagnosed as grade II–III as compared with that in normal brain
tissue. This indicated that miR-27a is dysregulated in human glioma
and may have a role in tumorigenesis. The number of normal and
tumor samples used in the present study is not large enough to
statistically validate whether miR-27a expression levels are
correlated with the pathological grade and malignancy of glioma,
and therefore, further studies using an expanded sample size are
required.
Through a series of in vitro assays in the
present study, miR-27a was shown to have significant
anti-proliferative effect. The significantly increased number of
early and late apoptotic cells observed in U251 cells confirmed
that miR-27a inhibited glioma cell growth via inducing apoptosis,
as well as arresting the progression of the cell cycle and arising
from a block or delay in the G2/M phase. Furthermore, miR-27a
overexpression impaired the invasive potential of U251 glioma cells
in vitro. All these results indicated that miR-27a may
function as a tumor suppressor in glioma U251 cells. In analogy
with the results of the present study, miR-27a has also been
reported to have anti-tumor activity by certain studies (9,15,17).
Scheibner et al (34)
reported that miR-27a exerted its tumor suppressor-like activity in
acute leukemia cells via regulation of apoptosis. Additionally,
overexpression of the miR-23a/27a/24-2 cluster promoted
caspase-dependent and -independent apoptosis in human embryonic
kidney cells, as well as sensitized HEK293T cells to TNF-alpha
cytotoxicity (7). Recently, Wang
et al (17) reported that
induction of miR-27a strongly decreased the proliferation of human
lung cancer H1299 cells and breast cancer MDA-MB-468 cells in
vitro as well as tumor formation in vivo. These results
indicated that miR-27a may act either as an oncogene or an
anti-oncogene by targeting various cellular proteins in a number of
tissues and disease stages.
The siCHECK™ vectors were designed to provide a
quantitative and rapid approach for identification of the target
genes of miR. These vectors enable the monitoring of changes in
expression of a target gene fused to the reporter gene Renilla
luciferase. The present study showed that miR-27a was able to
directly bind to the 3′-UTR of the PHB gene and induce cleavage of
PHB mRNA, resulting in the loss-of-function of PHB. PHB is an
evolutionarily conserved gene that is ubiquitously expressed and is
localized to the mitochondria, the nucleus and the plasma membrane
(35). The least controversial and
best-characterized function of the mitochondrial PHB is as
chaperones involved in the stabilization and maintenance of
mitochondrial function and protection against senescence (36). The downregulation of PHB through
targeting by miR-27a mimics may have changed the aberrant
mitochondrial morphology and functions, which increased the
sensitivity of U251 cells to apoptotic stimuli. Several studies
have supported the notion that knockdown of PHB facilitates
cellular apoptosis via fragmentation of the mitochondrial network
(37,38). There is controversy among studies
in regard to the function of PHB in cellular differentiation and
apoptosis (39,40). It is associated with the
differential phosphorylation, intracellular concentration of PHB,
the cell type and the tumor stage. Merkwirth et al (39) demonstrated that prohibitins exert
their anti-apoptotic function via OPA1, a dynamin-like guanin
triphosphatase. Recent findings demonstrated an unexpected
indispensable role of PHB in the activation of the
Raf/mitogen-activated protein kinase kinase (MEK)/extracellular
signal-regulated kinase (ERK)1/2 pathway via active Ras as well as
in modulating epithelial cell adhesion and migration (37,41).
Silencing of PHB expression by miR-27a mimics sensitized U251 to
apoptosis and produced a negative impact on cellular invasion via
inhibition of the Ras-Raf-MEK-ERK pathway. Studies performed on
cancer cell lines further demonstrated that the levels of
phospho-PHB in the plasma membrane were correlated with the
invasiveness of human cancer cells (42). Additionally, several studies
demonstrated that the overexpression of PHB inhibited cytochrome
c release by decreasing the mitochondrial membrane potential
and decreasing the level of B-cell lymphoma 2 (Bcl-2) (37,43).
Downregulation of PHB by miR-27a mimics resulted in inhibition of
the Bcl-2/Bcl-2 extra large protein pathway and enhancement of
Bcl-2-associated X protein-Bcl-2 homologous antagonist killer
directly by increasing cytochrome C from the intermitochondrial
space, leading to the induction of downstream activation of cleaved
caspase 3 (37). Furthermore,
studies showed that PHB inhibited apoptosis by downregulating E2F
activity when Rb family members were inactive (44). It is therefore indicated that
downregulation of PHB in U251 mediated by miR-27a mimics induced
apoptosis attributing to the release of free E2F1, which has been
shown to promote apoptosis in several experimental systems reviewed
in Phillips and Vousden (45). In
addition, U251 is a p53-mutant glioma cell line. The dysregulated
expression of PHB was previously reported in multiple proteomic
studies on glioma (20–22). Therefore, in glioma cells, PHB was
not able to function as a regulator at a physiological dose which
keeps the balance between cell hyper-proliferation and apoptosis
through regulating the p53 signaling pathway (46). Studies performed to date have
suggested that PHB functions as a ‘molecular switch’, which
determines cell fate. Targeting PHB may therefore be a useful
therapeutic approach for the treatment of gliomas.
Compared with the MI group, silencing of miR-27a did
not result in any effects opposite of those of miR-27a upregulation
on cellular proliferation, apoptosis, cell cycle and invasiveness
in the present study. The present study demonstrated that, in U251
cells, upregulation of miR-27a expression by mimics not only
induced PHB mRNA degradation, but also inhibited its translation.
miR-27a inhibition ultimately didn’t lead to any alterations in PHB
protein expression, although PHB mRNA expression was negatively
correlated with miR-27a levels. This implied that decreasing PHB
protein expression was a key factor in the anti-oncogenic role of
miR-27a. However, miR-27a can be a downstream transcriptional
target of certain oncogenes, which bind to the miR-27a promoter
region and suppress its expression (17). These two differential pathways can
have an anti-tumorigenic role via overexpression of miR-27a. The
results of the present study implied that upregulation of miR-27a
may exert a distinct anti-tumorigenic effect. The glioblastoma cell
line U87MG with mutant PTEN is classified as grade IV (47), possessing higher malignancy and
complexity in tumorigenesis than U251 cells (http://www.atcc.org/products/all/HTB-14.aspx;http://www.sigmaaldrich.com/catalog/product/sigma/09063001?lang=zh®ion=CN;http://www.cellbank.org.cn/detail_1.asp?id=137&serial=TCHu%2058).
Alterations in miR-27a expression only had a minor influence on PHB
mRNA and protein in U87MG cells. It was difficult to disturb U87MG
cellular processes via transfection with miR-27a mimics or
inhibitor.
In conclusion, miR-27a was downregulated in glioma
diagnosed as grade II-III and acted as a tumor suppressor, which
inhibits proliferation and invasion of glioma U251 cells via
inducing apoptosis and cell cycle arrest. PHB is an endogenous
target of miR-27a in gliomas. Overexpression of miR-27a was shown
to have a significant tumor suppressive action by targeting and
decreasing PHB protein expression. miR-27a targeting of PHB
therefore has the potential to become a novel therapeutic strategy
for human maglinant glioma.
References
|
1
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gabayan AJ, Green SB, Sanan A, et al:
GliaSite brachytherapy for treatment of recurrent malignant
gliomas: a retrospective multi-institutional analysis.
Neurosurgery. 58:701–709. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cho WC: OncomiRs: the discovery and
progress of microRNAs in cancers. Mol Cancer. 6:602007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anderson E, Grant R, Lewis SC and Whittle
IR: Randomized Phase III controlled trials of therapy in malignant
glioma: where are we after 40 years? Br J Neurosurg. 22:339–349.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Auffinger B, Thaci B, Ahmed A, et al:
MicroRNA targeting as a therapeutic strategy against glioma. Curr
Mol Med. 13:535–542. 2013. View Article : Google Scholar
|
|
7
|
Chhabra R, Adlakha YK, Hariharan M, Scaria
V and Saini N: Upregulation of miR-23a-27a-24-2 cluster induces
caspase-dependent and -independent apoptosis in human embryonic
kidney cells. PLoS One. 4:e58482009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chhabra R, Dubey R and Saini N:
Cooperative and individualistic functions of the microRNAs in the
miR-23a~27a~24-2 cluster and its implication in human diseases. Mol
Cancer. 9:2322010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng DD, Zhang H, Zhang P, et al:
Down-regulated miR-331-5p and miR-27a are associated with
chemotherapy resistance and relapse in leukaemia. J Cell Mol Med.
15:2164–2175. 2011. View Article : Google Scholar
|
|
10
|
Ballabio E, Armesto M, Breeze CE, et al:
Bortezomib action in multiple myeloma: microRNA-mediated synergy
(and miR-27a/CDK5 driven sensitivity)? Blood Cancer J. 2:e832012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chow TF, Youssef YM, Lianidou E, et al:
Differential expression profiling of microRNAs and their potential
involvement in renal cell carcinoma pathogenesis. Clin Biochem.
43:150–158. 2010. View Article : Google Scholar
|
|
12
|
Guttilla IK and White BA: Coordinate
regulation of FOXO1 by miR-27a, miR-96 and miR-182 in breast cancer
cells. J Biol Chem. 284:23204–23216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang S, He X, Ding J, et al: Upregulation
of miR-23a approximately 27a approximately 24 decreases
transforming growth factor-beta-induced tumor-suppressive
activities in human hepatocellular carcinoma cells. Int J Cancer.
123:972–978. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prueitt RL, Yi M, Hudson RS, et al:
Expression of microRNAs and protein-coding genes associated with
perineural invasion in prostate cancer. Prostate. 68:1152–1164.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saumet A, Vetter G, Bouttier M, et al:
Transcriptional repression of microRNA genes by PML-RARA increases
expression of key cancer proteins in acute promyelocytic leukemia.
Blood. 113:412–421. 2009. View Article : Google Scholar
|
|
16
|
Xi Y, Shalgi R, Fodstad O, Pilpel Y and Ju
J: Differentially regulated micro-RNAs and actively translated
messenger RNA transcripts by tumor suppressor p53 in colon cancer.
Clin Cancer Res. 12(7 Pt 1): 2014–2024. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang W, Cheng B, Miao L, Mei Y and Wu M:
Mutant p53-R273H gains new function in sustained activation of EGFR
signaling via suppressing miR-27a expression. Cell Death Dis.
4:e5742013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deighton RF, McGregor R, Kemp J, McCulloch
J and Whittle IR: Glioma pathophysiology: insights emerging from
proteomics. Brain Pathol. 20:691–703. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chumbalkar VC, Subhashini C, Dhople VM, et
al: Differential protein expression in human gliomas and molecular
insights. Proteomics. 5:1167–1177. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hiratsuka M, Inoue T, Toda T, et al:
Proteomics-based identification of differentially expressed genes
in human gliomas: down-regulation of SIRT2 gene. Biochem Biophys
Res Commun. 309:558–566. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iwadate Y, Sakaida T, Hiwasa T, et al:
Molecular classification and survival prediction in human gliomas
based on proteome analysis. Cancer Res. 64:2496–2501. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou JQ, Wang JT, Liu QH, Guo Xb, Zhou J
and Song LJ: Proteomic profiling and identification of malignant
grade related proteins in human brain astrocytoma. Chin J Neuromed.
11:780–783. 2012.
|
|
23
|
Mishra S, Murphy LC and Murphy LJ: The
Prohibitins: emerging roles in diverse functions. J Cell Mol Med.
10:353–363. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mishra S, Murphy LC, Nyomba BL and Murphy
LJ: Prohibitin: a potential target for new therapeutics. Trends Mol
Med. 11:192–197. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu T, Tang H, Lang Y, Liu M and Li X:
MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by
targeting prohibitin. Cancer Lett. 273:233–242. 2009. View Article : Google Scholar
|
|
26
|
Mertens-Talcott SU, Chintharlapalli S, Li
X and Safe S: The oncogenic microRNA-27a targets genes that
regulate specificity protein transcription factors and the G2-M
checkpoint in MDA-MB-231 breast cancer cells. Cancer Res.
67:11001–11011. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Scott GK, Mattie MD, Berger CE, Benz SC
and Benz CC: Rapid alteration of microRNA levels by histone
deacetylase inhibition. Cancer Res. 66:1277–1281. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lerner M, Lundgren J, Akhoondi S, et al:
MiRNA-27a controls FBW7/hCDC4-dependent cyclin E degradation and
cell cycle progression. Cell Cycle. 10:2172–2183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Belair C, Darfeuille F and Staedel C:
Helicobacter pylori and gastric cancer: possible role of microRNAs
in this intimate relationship. Clin Microbiol Infect. 15:806–812.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chintharlapalli S, Papineni S, Abdelrahim
M, et al: Oncogenic microRNA-27a is a target for anticancer agent
methyl 2-cyano-3,11-dioxo-18beta-olean-1,12-dien-30-oate in colon
cancer cells. Int J Cancer. 125:1965–1974. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Volinia S, Calin GA, Liu CG, et al: A
microRNA expression signature of human solid tumors defines cancer
gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schultz J, Lorenz P, Gross G, Ibrahim S
and Kunz M: MicroRNA let-7b targets important cell cycle molecules
in malignant melanoma cells and interferes with
anchorage-independent growth. Cell Res. 18:549–557. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kozaki K, Imoto I, Mogi S, Omura K and
Inazawa J: Exploration of tumor-suppressive microRNAs silenced by
DNA hypermethylation in oral cancer. Cancer Res. 68:2094–2105.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Scheibner KA, Teaboldt B, Hauer MC, et al:
MiR-27a functions as a tumor suppressor in acute leukemia by
regulating 14-3-3theta. PLoS One. 7:e508952012. View Article : Google Scholar
|
|
35
|
Zhou TB and Qin YH: Signaling pathways of
prohibitin and its role in diseases. J Recept Signal Transduct Res.
33:28–36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nijtmans LG, de Jong L, Artal Sanz M, et
al: Prohibitins act as a membrane-bound chaperone for the
stabilization of mitochondrial proteins. EMBO J. 19:2444–2451.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chowdhury I, Thompson WE, Welch C, Thomas
K and Matthews R: Prohibitin (PHB) inhibits apoptosis in rat
granulosa cells (GCs) through the extracellular signal-regulated
kinase 1/2 (ERK1/2) and the Bcl family of proteins. Apoptosis.
18:1513–1525. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kasashima K, Ohta E, Kagawa Y and Endo H:
Mitochondrial functions and estrogen receptor-dependent nuclear
translocation of pleiotropic human prohibitin 2. J Biol Chem.
281:36401–36410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Merkwirth C, Dargazanli S, Tatsuta T, et
al: Prohibitins control cell proliferation and apoptosis by
regulating OPA1-dependent cristae morphogenesis in mitochondria.
Genes Dev. 22:476–488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ross JA, Nagy ZS and Kirken RA: The PHB1/2
phosphocomplex is required for mitochondrial homeostasis and
survival of human T cells. J Biol Chem. 283:4699–4713. 2008.
View Article : Google Scholar
|
|
41
|
Rajalingam K, Wunder C, Brinkmann V, et
al: Prohibitin is required for Ras-induced Raf-MEK-ERK activation
and epithelial cell migration. Nat Cell Biol. 7:837–843. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chiu CF, Ho MY, Peng JM, et al: Raf
activation by Ras and promotion of cellular metastasis require
phosphorylation of prohibitin in the raft domain of the plasma
membrane. Oncogene. 32:777–787. 2013. View Article : Google Scholar
|
|
43
|
Muraguchi T, Kawawa A and Kubota S:
Prohibitin protects against hypoxia-induced H9c2 cardiomyocyte cell
death. Biomed Res. 31:113–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fusaro G, Wang S and Chellappan S:
Differential regulation of Rb family proteins and prohibitin during
camptothecin-induced apoptosis. Oncogene. 21:4539–4548. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Phillips AC and Vousden KH: E2F-1 induced
apoptosis. Apoptosis. 6:173–182. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fusaro G, Dasgupta P, Rastogi S, Joshi B
and Chellappan S: Prohibitin induces the transcriptional activity
of p53 and is exported from the nucleus upon apoptotic signaling. J
Biol Chem. 278:47853–47861. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Clark MJ, Homer N, O’Connor BD, et al:
U87MG decoded: the genomic sequence of a cytogenetically aberrant
human cancer cell line. PLoS Genet. 6:e10008322010. View Article : Google Scholar : PubMed/NCBI
|