Introduction
Spindle cell oncocytoma (SCO) of the adenohypophysis
is a rare benign tumor in the sellar region, accounting for
0.1–0.4% of all sellar tumors (1,2). SCO
was first identified as a distinct entity by the World Health
Organization (WHO) classification of tumors of the central nervous
system (CNS) in 2007 (3). To date,
only 24 cases have been reported in the literature (1,2,4–18).
Due to its rarity, little information is available regarding the
imaging features and surgical characteristics of SCO (2). As SCO shares a similar clinical
presentation and imaging features with nonfunctional pituitary
adenoma, it is often misdiagnosed as a nonfunctional pituitary
adenoma (7). However, SCO tumors
have a greater blood supply than pituitary adenomas, thereby
increasing difficulties associated with surgical removal of the
tumor (15). Preoperative
misdiagnosis as pituitary adenoma may result in an underestimation
of the surgical difficulty. In the present study, two cases of SCO
were reported and 24 cases of SCO in the literature were reviewed.
The imaging features, intraoperative findings, immunohistochemical
features and prognosis of SCO were summarized. The present study
provided important clinical information for the correct
preoperative diagnosis and intraoperative removal of SCO tumors.
Written informed consent was obtained from all of the patients.
Case report
Case 1
A 35-year-old female was admitted to the First
Hospital of Jilin University (Changchun, China), who had been
presenting with amenorrhea and lactation for two years. On
examination, her visual acuity was 0.8 in the left eye and 0.5 in
the right eye. Dark spots were observed in the left inferior
temporal quadrant and decreased light sensitivity was observed in
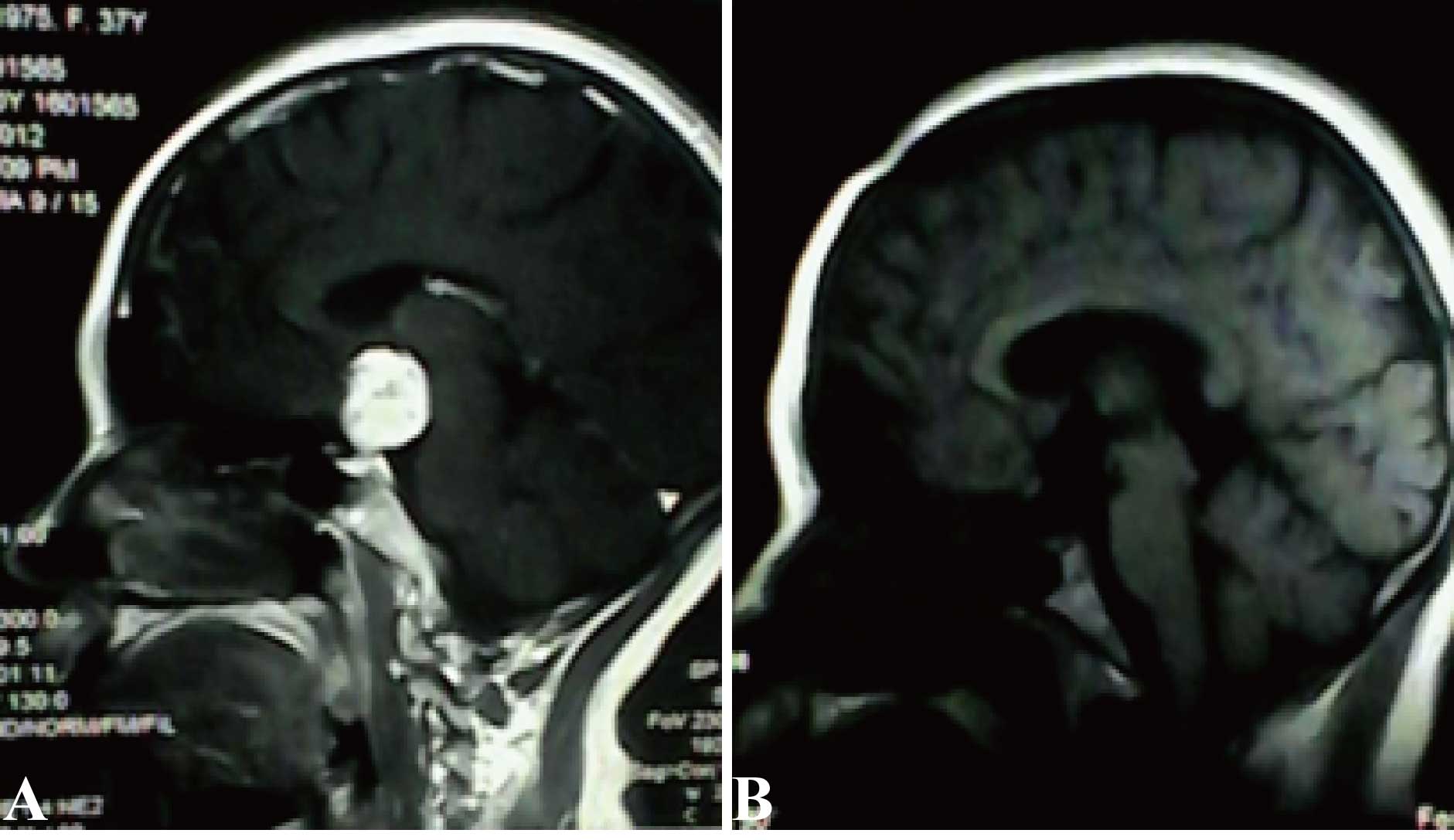
the right nasal quadrant. Cranial magnetic resonance imaging (MRI;
3.0 Tesla Trio MRI Scanner; Siemens AG, Erfurt, Germany) revealed a
suprasellar round mass of 2.5×3.0×1.0 cm, with equal T1 and T2
signals. This mass exhibited marked homogeneous enhancement
(Fig. 1A). Laboratory assessments
used to examine pituitary disorders revealed an elevated level of
prolactin (34.62 ng/ml; normal range, 1.4–24.0 ng/ml), a decreased
level of luteinizing hormone (LH; 1.410 IU/l; normal range,
2.12–10.891 IU/l) and normal levels of follicle-stimulating hormone
(FSH), corticotropin and thyrotropin.
The patient was diagnosed as having a pituitary
prolactin adenoma. The tumor was resected through the second gap
using a right expanded frontotemporal craniotomy. The tumor was
gray with blood vessels on the surface, was firm without clear
encapsulation and had an appearance similar to that of normal brain
tissue with a rich blood supply. The pituitary stalk was adherent
to the tumor and was pushed inferiorly by the tumor. The stalk was
partially preserved following careful dissection. The tumor was
removed section by section until complete removal of the tumor was
achieved by visualization under a microscope. Persistent diabetes
insipidus occurred following surgery and it was gradually relieved
following oral administration of desmopressin acetate for 2.5
months. An enhanced MRI performed at seven days after surgery
confirmed the complete removal of the tumor. No recurrence had
occurred by the time-point of the 21-month follow-up examination
(Fig. 1B).
Postoperative hematoxylin and eosin
(H&E)-stained sections revealed that the tumor was composed of
spindled and epithelioid cells arranged in nests and sheets. The
cells had an abundant eosinophilic cytoplasm. Mild to moderate
nuclear atypia was identified; however, mitosis was not observed.
Nuclear pleomorphism was observed in certain cells and a double
nucleus was occasionally present. Infiltration of scattered mature
lymphocytes was observed in the extracellular matrix (Fig. 2A and B). The tumor was
immunonegative for GFAP and Syn, whilst it was immunopositive for
vimentin, EMA, S-100 and TTF-1 (Fig.
2C-F). The mindbomb E3 ubiquitin protein ligase 1 (MIB-1)
labeling index was ~3%. The tumor was pathologically diagnosed as
SCO.
Case 2
A 62-year-old female was admitted to the First
Hospital of Jilin University due to a sellar mass identified during
a routine physical examination. She presented no clear symptoms or
signs. Cranial MRI revealed a suprasellar mass of 2.3×1.7×2.0 cm,
with long T1 and short T2 signals (Fig. 3A). This mass exhibited marked
homogeneous enhancement. The pituitary gland was flattened due to
tumor compression and the pituitary stalk was not clearly observed.
The optic chiasm was elevated; however, the cavernous sinus was not
invaded by the tumor. Laboratory assessments used to examine
pituitary disorders revealed a decreased level of LH (0.390 IU/l;
normal range, 2.12–10.891 IU/l), but normal levels of pituitary
hormones, including FSH, prolactin, corticotropin and
thyrotropin.
The patient was diagnosed as having a nonfunctional
adenoma. The tumor was resected between the first and second gap
using a right transpterional craniotomy. The tumor was light yellow
and slightly soft, and it had a brain stem-like appearance with a
rich blood supply. The tumor was removed section by section. Care
was taken to preserve the membrane-like pituitary stalk
dorsolateral to the tumor until the tumor was completely removed.
Transient diabetes insipidus occurred immediately following
surgery; however, it was effectively treated following two weeks of
oral administration of desmopressin acetate. An enhanced MRI
performed at three days after surgery confirmed the complete
removal of the tumor. No recurrence had occurred by the time-point
of the 15-month follow-up examination (Fig. 3B).
Post-operative H&E-stained sections demonstrated
that the tumor was composed of spindled and epithelioid cells
arranged in intersecting fascicles. The cells had an abundant
eosinophilic cytoplasm with round or oval nuclei and inconspicuous
nucleoli. Mild to moderate nuclear atypia was identified; however;
mitosis was not observed. Nuclear pleomorphism was observed in
certain cells. Infiltration of a few mature lymphocytes and local
interstitial mucoid degeneration was observed (Fig. 4A and B). The tumor was
immunonegative for GFAP, creatine kinase, Syn and B-cell lymphoma
2; however, it was immunopositive for vimentin, EMA, S-100 and
TTF-1 (Fig. 4C-F). The MIB-1
labeling index was ~1.5%. The tumor was pathologically diagnosed as
SCO.
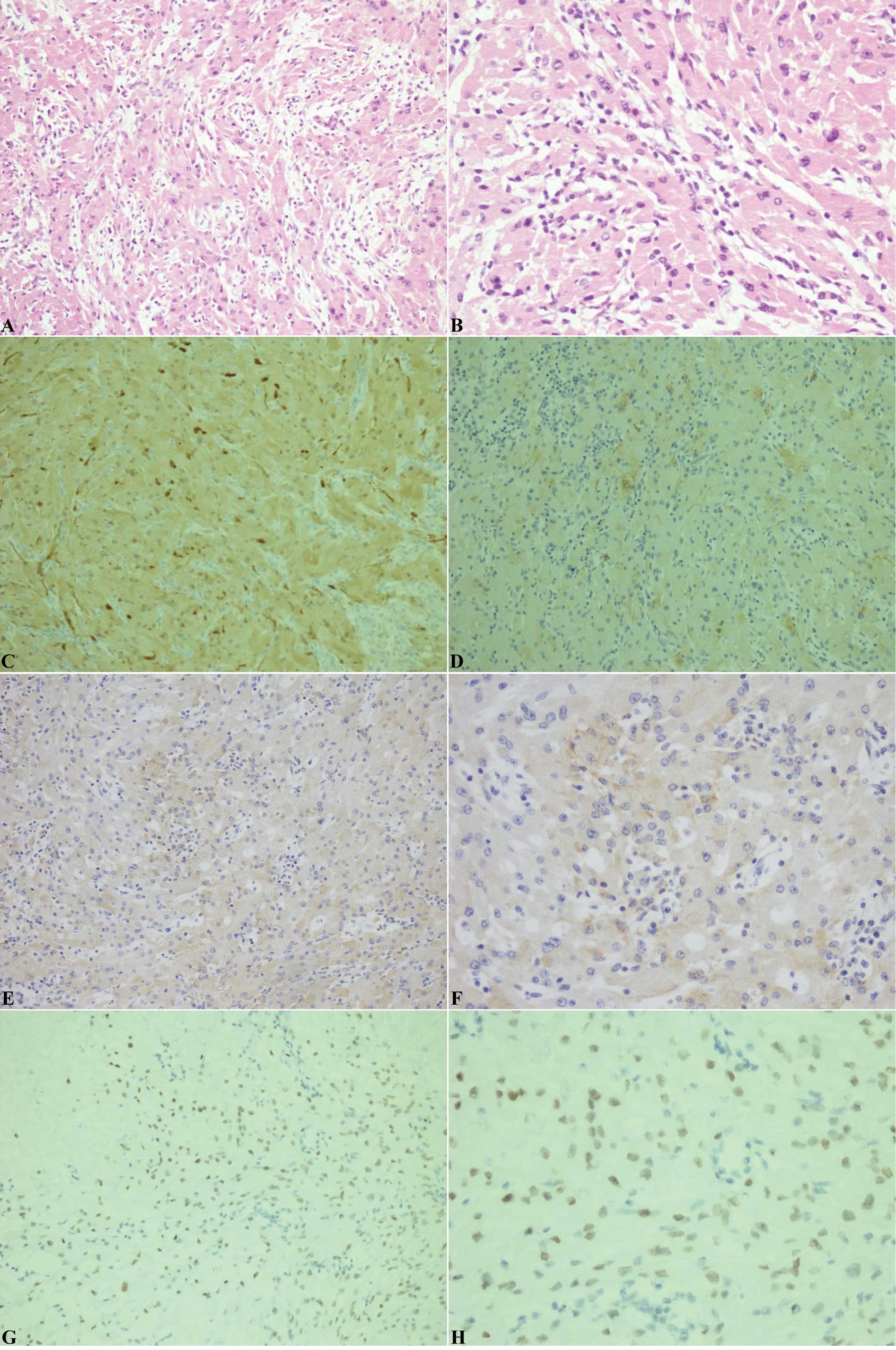 | Figure 4Histological and immunohistochemical
staining of the spindle cell oncocytoma in case 2. (A and B)
Hematoxylin and eosin staining revealing that the tumor was
composed of spindled and epithelioid cells arranged in intersecting
fascicles. The cells had an abundant eosinophilic cytoplasm. Mild
to moderate nuclear atypia was identified, but mitosis was not
observed. Nuclear pleomorphism was found in certain cells.
Infiltration of a few mature lymphocytes and local interstitial
mucoid degeneration was observed. A, magnification, ×20; B,
magnification, ×40. Immunohistochemical staining for (C) S-100, (D)
epithelial membrane antigen, (E and F) vimentin and (G and H)
thyroid transcription factor-1. C-E and G, magnification, ×20; F
and H, magnification, ×40. |
Discussion
SCO was first reported by Roncaroli et al
(1) in 2002 in five patients and
it was later identified as a novel entity by the WHO classification
of tumors of the CNS in 2007 (3).
Histologically, SCO cells are mainly composed of a bundle of
spindle cells with an eosinophilic and granular cytoplasm. They are
immunopositive for vimentin, EMA, S-100 and galectin-3, but
immunonegative for pituitary hormones, chromogranin and Syn. SCO is
similar to nonfunctional pituitary adenoma and accounts for
0.1–0.4% of all sellar tumors (1,2). In
a retrospective study of 2,000 cases of pituitary tumors, only two
cases were diagnosed as being SCO (2). Due to its rarity, only 16 studies
were available describing 24 cases of SCO using a PUBMED search
(http://www.ncbi.nlm.nih.gov/pubmed)
for studies published between 2002 and 2013. The clinical
characteristics, intraoperative findings as well as the imaging and
immunohistochemical features of the 24 published cases, and the two
cases reported in the present study were assessed. SCO commonly
occurred in middle-aged and elderly males or females. Of the 24
cases published in the literature, 10 patients were male and 14
were female. No gender preference for SCO is therefore present. The
average age of the SCO patients was 56.4 years (range, 24–76
years). In the present two cases, the patients were females aged 35
and 62 years old, respectively.
Similar to nonfunctional pituitary adenoma, the most
common clinical manifestations of SCO are visual impairment and
panhypopituitarism. Of the 24 cases in the literature, 14 cases
presented with decreased or impaired visual acuity and 12 cases
presented with panhypopituitarism. In addition, intermittent
epistaxis was reported in one patient with a large SCO (4), two cases exhibited weight loss
(2,5) and one case had long-term
musculoskeletal pain (6). A total
of three cases had decreased libido or sexual dysfunction (7–9) and
two cases presented with oligomenorrhea or amenorrhea (10,11).
Consistent with previous studies, one patient (case 1) in the
present study presented with decreased visual acuity, amen-orrhea
and lactation. However, the other patient (case 2) did not exhibit
any clear symptoms or signs. Furthermore, postoperative
panhypopituitarism and diabetes insipidus occurred in four cases
(12–15). One case required hormone
replacement therapy for 15 years (12). In the present two cases,
postoperative diabetes insipidus occurred and was treated following
oral administration of desmopressin acetate for two weeks and two
months, respectively. Therefore, similar to other sellar tumors,
SCO often leads to pituitary hormone disorders and is associated
with postoperative complications, such as diabetes insipidus.
SCO is often misdiagnosed preoperatively. Of the 24
cases of SCO in the literature, 14 cases were misdiagnosed as being
nonfunctional adenomas. Due to its spindle-like shape, four cases
were misdiagnosed as being schwannomas. In addition, one case was
misdiagnosed as being a craniopharyngioma due to recurrent
intratumoral bleeding (13).
Furthermore, two cases were misdiagnosed as being null cell
pituitary adenomas (4). In the
present two cases, one was misdiagnosed as being a nonfunctional
adenoma and the other was misdiagnosed as being a pituitary
prolactin adenoma.
SCO shares similar imaging features with
nonfunctional adenoma, exhibiting no dural attachment or invasion
(19). Of the 24 cases in the
literature, the size and site of the tumor on MRI images were
described in 23 cases. Suprasellar or intrasellar tumors were
reported in 20 cases. Only two cases reported that the tumor was
located within the sella turcica (10,11).
Tumor invasion to the cavernous sinus and compression of the
temporal lobe was reported in one case (9). In addition, one case reported that
the tumor grew forward and invaded into the sphenoid, ethmoid,
nasopharynx and posterior nasal cavity, leading to intermittent
epistaxis (4). Borota et al
(6) reported one case of SCO,
which had invaded into the sphenoid sinuses and disrupted the body
of the sphenoid bone, including the sella turcica. In the present
two cases, the two tumors were large and suprasellar, without
invasion into the cavernous sinuses. These findings are consistent
with a meta-analysis of the world literature since 1893 by
Covington et al (20),
revealing that SCO is either suprasellar or intra- and
supra-sellar. Computed tomography (CT) images of SCO were only
reported in three cases (5,13,16).
Borges et al (13) reported
that ~50% of SCO tissues exhibited calcification on CT images,
consistent with the local hyperintense signals on T1-weighted MRI.
In addition, Singh et al (16) demonstrated that SCO exhibited
isointensity to the cerebral parenchyma on CT images without
intratumoral calcification or bleeding. In addition, as SCO
commonly has a rich blood supply, the tumor may exhibit enhancement
on MRI. In the present cases, the two SCO tumors exhibited marked
homogeneous enhancement. Similarly, Fujisawa et al (15) reported that SCO exhibited numerous
and faint intratumoral vessels on a magnetic resonance angiogram
(MRA); angiography revealed that the SCO was extensively fed by the
bilateral internal carotid arteries and draining veins were
observed in the arterial phase (15). Of five cases of SCO with a rich
blood supply as determined by a preoperative MRA, severe
intraoperative bleeding occurred in four cases (6,8,9,15).
However, as SCO was misdiagnosed as nonfunctional adenoma
preoperatively, angiography was not performed in the majority of
cases of SCO. Therefore, preoperative angiography should be
performed to evaluate the blood supply of SCO if a rich blood
supply is suspected on the MRA, thereby reducing the risk of
intraoperative bleeding.
Of the 24 cases of SCO in the literature, 13 cases
described the intraoperative findings. The SCO was described to be
pale gray (10), grayish
gelatinous (7) and yellow
(11). Similarly, the SCO was gray
or yellow in the present two cases. The texture was similar to that
of normal brain tissue with a rich blood supply. In addition, 11
cases described the SCO as a firm and vascular tumor. Kloub et
al (4) reported that two
recurrent SCOs were invasive with an unclear boundary with the
surrounding tissues and necrosis was identified in one tumor. Tumor
invasion into the base of the sella turcica occurred in two cases
(4,9). No tumor invasion was reported via
intraop-erative inspection in any of the other cases. Dahiya et
al (9) reported a case of SCO
that was firm and difficult to dissect and residual tumors were
found around the internal carotid artery following the second
surgical procedure. Intratumoral bleeding was reported in two cases
and tumors with a rich blood supply were reported in seven cases.
Severe intraopera-tive blood loss (600–900 ml) was also reported
(13,15).
SCO tumors are immunopositive for vimentin, S-100
and EMA (1,2,4–6,10–12,14,15,16).
Electron microscopy revealed that SCO cells contain abundant
swollen mitochondria and that bundles of intermediate filaments are
entrapped in the lysosomes and profiles of the rough endoplasmic
reticulum (1). Roncaroli et
al (1) and Borges et al
(13) reported that well-formed
desmosomes and intermediate junctions, but not secretory granules,
were observed in SCO cells. However, several other studies revealed
that occasional electron dense secretory granules, but not
desmosomes or intercellular junctions, were observed in SCO cells
(4,9,16).
Based on the immunohistochemical and ultrastructural similarities
shared by SCO and folliculostellate cells (FSCs), SCO is theorized
to originate from FSCs (1,21,22).
FSCs are star-like nonhor-mone-secreting cells in the anterior
pituitary, which provide structural support for hormone-secreting
cells, accounting for 5–6% of the pituitary cell population
(23,24). FSCs are hypothesized to be adult
stem cell-like pituitary cells, which have a capacity for divergent
differentiation (4).
Lee et al (25) described the expression of TTF-1 in
eight cases of SCO and demonstrated that TTF-1 was generally
expressed in the fetal neurohypophysis. Similarly, Mlika et
al (14) reported one case of
SCO with positive TTF-1 expression. In the present study, positive
TTF-1 expression was identified in the two cases of SCO. These
findings suggested that this marker may be specific to human
pituicytes. The positive expression of TTF-1 in these 11 cases of
SCO may facilitate further studies on the classification of these
rare sellar tumors and may suggest that SCO and pituicytoma have a
similar origin (25). In addition,
Mete et al (26) reported
positive TTF-1 expression in seven cases of SCO, four cases of
pituicytomas and three cases of granular cell tumors of the
pituitary; while all cases were negative for FSCs. The authors
hypothesized that SCO and granular cell tumors are variants of
pituicytoma and proposed the terms ‘oncocytic pituicytoma’ and
‘granular cell pituicytoma’ to refine the classification of these
lesions (26). Alexandrescu et
al (11) observed that SCO was
positive for CD44, nestin and SMI-131, suggesting that SCO has
features that are similar to those of neuronal precursors, which
may explain the recurrence of SCO in certain cases.
Of the 24 cases of SCO in the literature, recurrence
occurred in eight cases with an average recurrence time of 3.3
years (range, 5–13 years). The mean MIB-1 labeling index was 3%. A
total of two cases with a high MIB-1 labeling index (10–20%)
exhibited recurrence. The other six cases with a low MIB-1 labeling
index also exhibited recurrence. Of the six cases treated with
radiotherapy (doses of 50–55 Gy), recurrence occurred in four
cases. No intra- or extracranial metastases were identified.
However, the longest follow-up period was 16 years (12). These findings suggested that SCO
patients should be followed up for five years or more and it may
not be appropriate to define SCO as a WHO grade I tumor with a
short follow-up period. In addition, Ogiwara et al (7) found that an incomplete resection of
the tumor was a significant risk factor for the recurrence of SCO.
Therefore, a complete resection of the tumor is necessary to
prevent the recurrence of SCO.
In conclusion, SCO was identified as a novel type of
tumor in the WHO classification of tumors of the CNS in 2007
(3). However, to date, only 24
cases of SCO have been reported in the literature and little
information regarding SCO is available. Similar to nonfunctional
adenoma, the most common clinical manifestation of SCO is
panhypopituitarism. Tumors with an enhancement on an MRI should be
considered as SCO and MRA and/or angiography should be performed to
assess the blood supply of the tumor, thus preventing the risk of
severe intraoperative bleeding. Complete removal of the tumor is
important to prevent tumor recurrence. The intraoperative findings,
including the texture and the blood supply of the tumor, may
provide valuable clinical information to guide the surgical
procedures.
References
|
1
|
Roncaroli F, Scheithauer BW, Cenacchi G,
et al: ‘Spindle cell oncocytoma’ of the adenohypophysis: A tumor of
folliculostellate cells? Am J Surg Pathol. 26:1048–1055. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matyja E, Maksymowicz M, Grajkowska W,
Olszewski W, Zieliński G and Bonicki W: Spindle cell oncocytoma of
the adenohypophysis - a clinicopathological and ultrastructural
study of two cases. Folia Neuropathol. 48:175–184. 2010.PubMed/NCBI
|
|
3
|
Fuller GN: The WHO classification of
tumours of the central nervous system, 4th edition. Arch Pathol Lab
Med. 132:9062008.PubMed/NCBI
|
|
4
|
Kloub O, Perry A, Tu PH, Lipper M and
Lopes MBS: Spindle cell oncocytoma of the adenohypophysis: report
of two recurrent cases. Am J Surg Pathol. 29:247–253. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coire CI, Horvath E, Smyth HS and Kovacs
K: Rapidly recurring folliculostellate cell tumor of the
adenohypophysis with the morphology of a spindle cell oncocytoma:
case report with electron microscopic studies. Clin Neuropathol.
28:303–308. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Borota OC, Scheithauer BW, Fougner SL,
Hald JK, Ramm-Pettersen J and Bollerslev J: Spindle cell oncocytoma
of the adenohypophysis: report of a case with marked cellular
atypia and recurrence despite adjuvant treatment. Clin Neuropathol.
28:91–95. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ogiwara H, Dubner S, Shafizadeh S, Raizer
J and Chandler JP: Spindle cell oncocytoma of the pituitary and
pituicytoma: Two tumors mimicking pituitary adenoma. Surg Neurol
Int. 2:1162011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Demssie YN, Joseph J, Dawson T, Roberts G,
de Carpentier J and Howell S: Recurrent spindle cell oncocytoma of
the pituitary, a case report and review of literature. Pituitary.
14:367–370. 2011. View Article : Google Scholar
|
|
9
|
Dahiya S, Sarkar C, Hedley-Whyte ET, et
al: Spindle cell oncocytoma of the adenohypophysis: report of two
cases. Acta Neuropathol. 110:97–99. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Romero-Rojas AE, Melo-Uribe MA,
Barajas-Solano PA, Chinchilla-Olaya SI, Escobar LI and
Hernandez-Walteros DM: Spindle cell oncocytoma of the
adenohypophysis. Brain Tumor Pathol. 28:359–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alexandrescu S, Brown RE, Tandon N and
Bhattacharjee MB: Neuron precursor features of spindle cell
oncocytoma of adeno-hypophysis. Ann Clin Lab Sci. 42:123–129.
2012.PubMed/NCBI
|
|
12
|
Vajtai I, Sahli R and Kappeler A: Spindle
cell oncocytoma of the adenohypophysis: Report of a case with a
16-year follow-up. Pathol Res Pract. 202:745–750. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Borges MT, Lillehei KO and
Kleinschmidt-DeMasters BK: Spindle cell oncocytoma with late
recurrence and unique neuro-imaging characteristics due to
recurrent subclinical intratumoral bleeding. J Neurooncol.
101:145–154. 2011. View Article : Google Scholar
|
|
14
|
Mlika M, Azouz H, Chelly I, et al: Spindle
cell oncocytoma of the adenohypophysis in a woman: a case report
and review of the literature. J Med Case Rep. 5:642011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujisawa H, Tohma Y, Muramatsu N, Kida S,
Kaizaki Y and Tamamura H: Spindle cell oncocytoma of the
adenohypophysis with marked hypervascularity. Case report. Neurol
Med Chir (Tokyo). 52:594–598. 2012. View Article : Google Scholar
|
|
16
|
Singh G, Agarwal S, Sharma MC, et al:
Spindle cell onco-cytoma of the adenohypophysis: Report of a rare
case and review of literature. Clin Neurol Neurosurg. 114:267–271.
2012. View Article : Google Scholar
|
|
17
|
Farooq MU, Bhatt A and Chang HT: Teaching
neuroimage: spindle cell oncocytoma of the pituitary gland.
Neurology. 71:e32008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vajtai I, Beck J, Kappeler A and Hewer E:
Spindle cell oncocytoma of the pituitary gland with follicle-like
component: organotypic differentiation to support its origin from
folliculo-stellate cells. Acta Neuropathol. 122:253–258. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roncaroli F and Scheithauer BW: Papillary
tumor of the pineal region and spindle cell oncocytoma of the
pituitary: New tumor entities in the 2007 WHO classification. Brain
Pathol. 17:314–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Covington MF, Chin SS and Osborn AG:
Pituicytoma, spindle cell oncocytoma, and granular cell tumor:
clarification and meta-analysis of the world literature since 1893.
AJNR Am J Neuroradiol. 32:2067–2072. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Devnath S and Inoue K: An insight to
pituitary folliculo-stellate cells. J Neuroendocrinol. 20:687–691.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hori S, Hayashi N, Fukuoka J, et al:
Folliculostellate cell tumor in pituitary gland. Neuropathology.
29:78–80. 2009. View Article : Google Scholar
|
|
23
|
Horvath E and Kovacs K: Folliculo-stellate
cells of the human pituitary: a type of adult stem cell?
Ultrastruct Pathol. 26:219–228. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Inoue K, Mogi C, Ogawa S, Tomida M and
Miyai S: Are folliculo-stellate cells in the anterior pituitary
gland supportive cells or organ-specific stem cells? Arch Physiol
Biochem. 110:50–53. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee EB, Tihan T, Scheithauer BW, Zhang PJ
and Gonatas NK: Thyroid transcription factor 1 expression in sellar
tumors: a histogenetic marker? J Neuropathol Exp Neurol.
68:482–488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mete O, Lopes MB and Asa SL: Spindle cell
oncocytomas and granular cell tumors of the pituitary are variants
of pituicytoma. Am J Surg Pathol. 37:1694–1699. 2013. View Article : Google Scholar : PubMed/NCBI
|