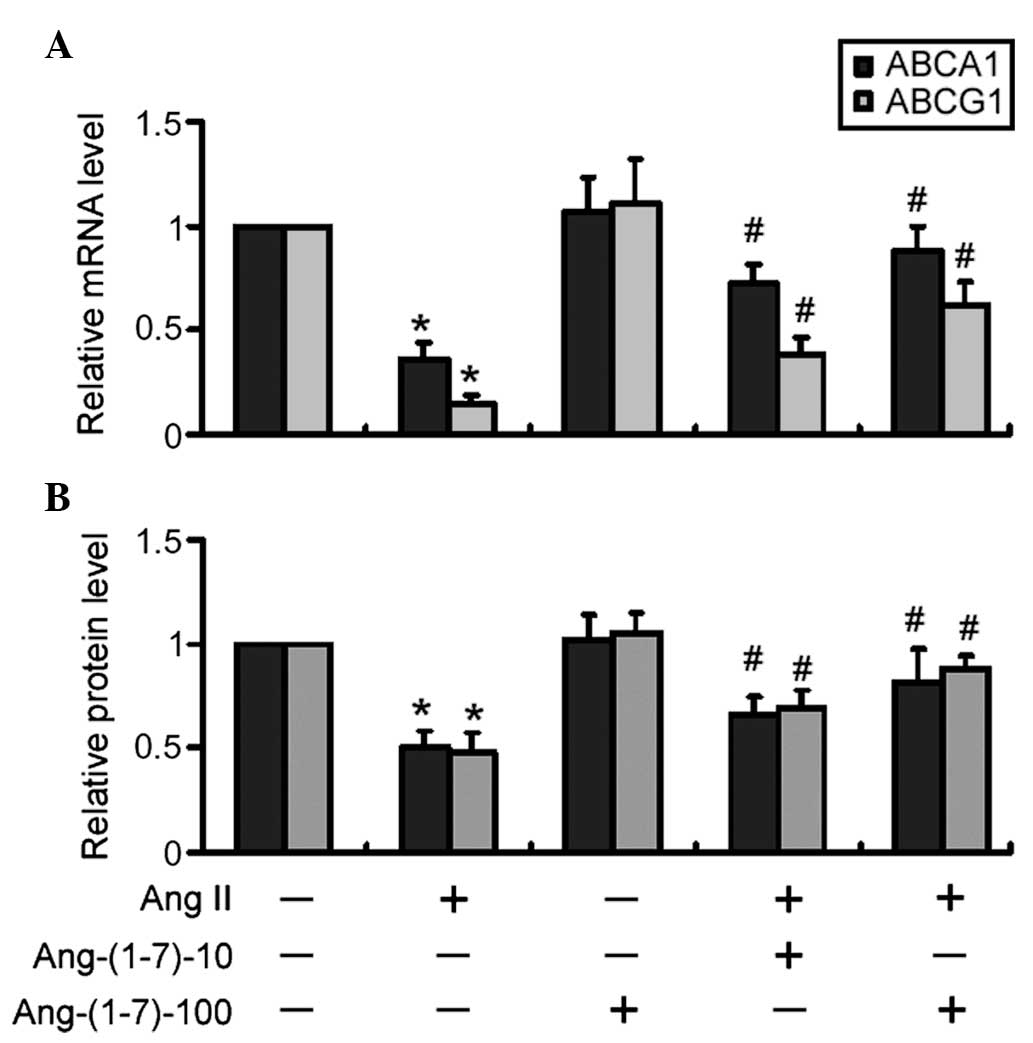
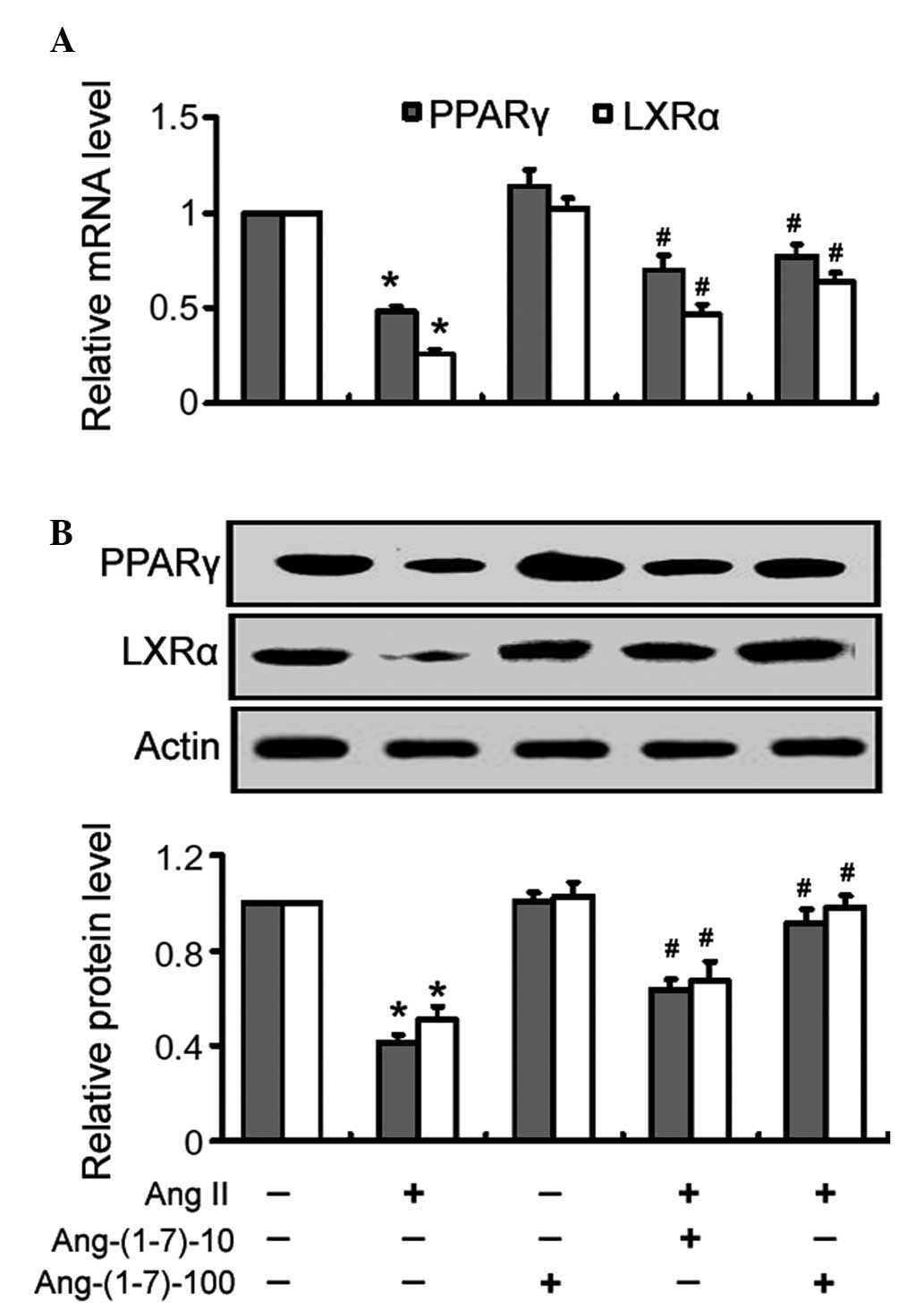
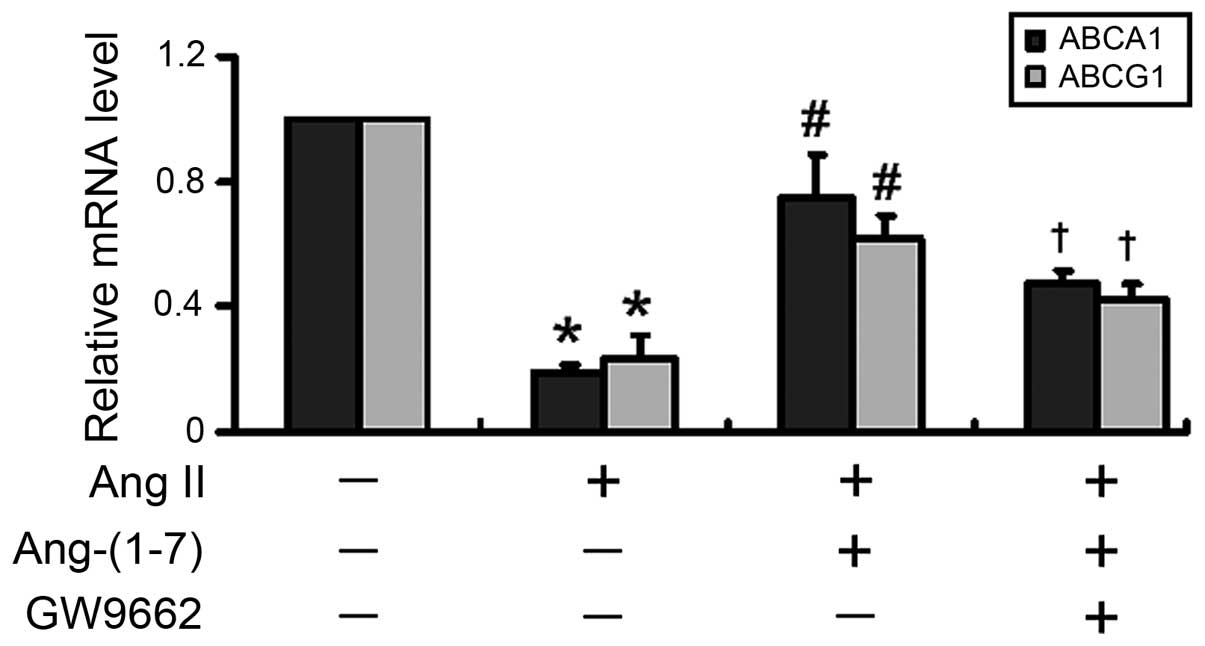
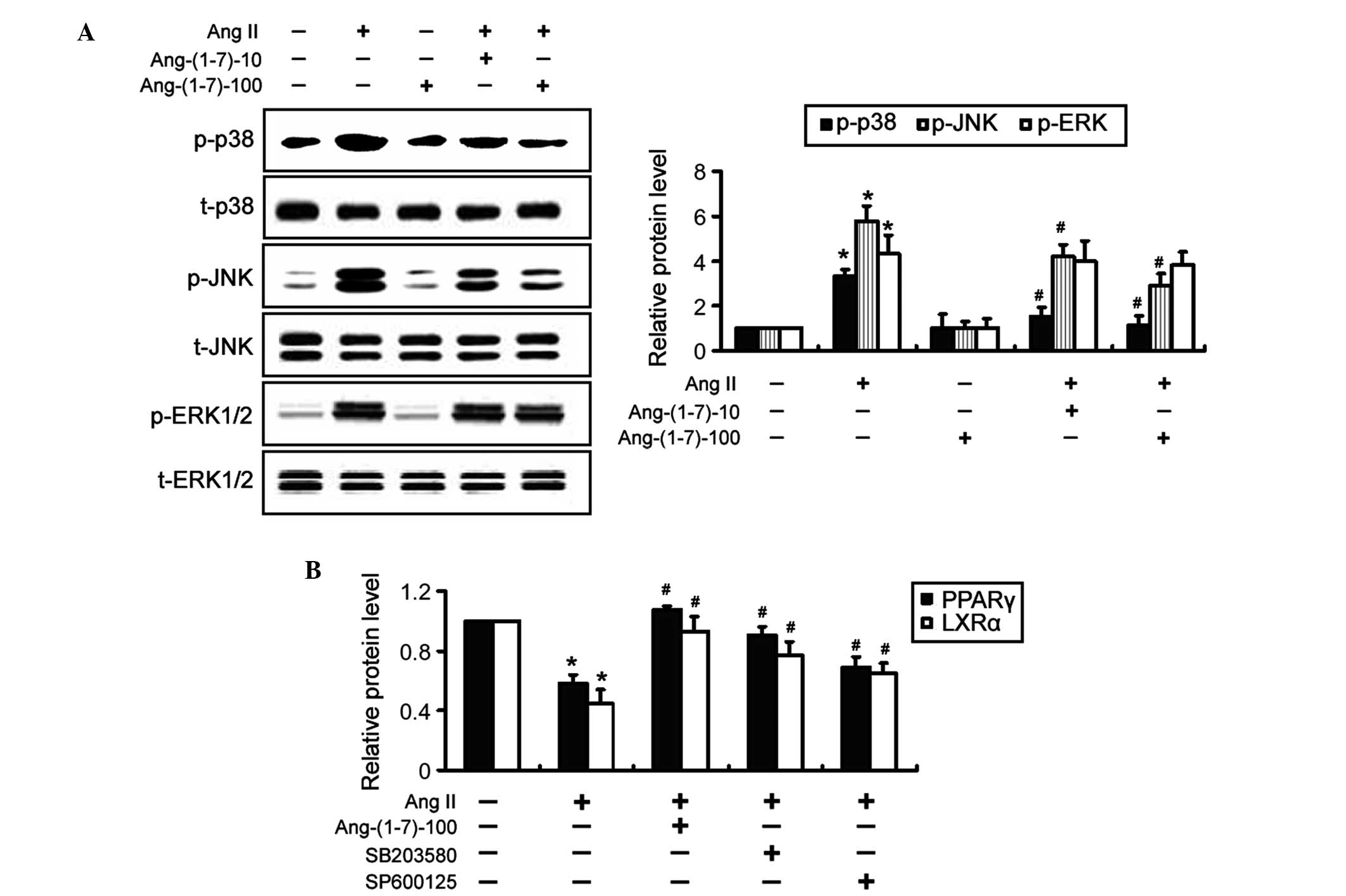
|
1
|
Libby P, Ridker PM and Maseri A:
Inflammation and atherosclerosis. Circulation. 105:1135–1143. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fenyo IM and Gafencu AV: The involvement
of the monocytes/macrophages in chronic inflammation associated
with atherosclerosis. Immunobiology. 218:1376–1384. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu XH, Fu YC, Zhang DW, Yin K and Tang CK:
Foam cells in atherosclerosis. Clin Chim Acta. 424:245–252. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yvan-Charvet L, Wang N and Tall AR: Role
of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and
immune responses. Arterioscler Thromb Vasc Biol. 30:139–143. 2010.
View Article : Google Scholar :
|
|
5
|
Smith JD, Le Goff W, Settle M, Brubaker G,
Waelde C, Horwitz A and Oda MN: ABCA1 mediates concurrent
cholesterol and phospholipid efflux to apolipoprotein A-I. J Lipid
Res. 45:635–644. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsuura F, Wang N, Chen W, Jiang XC and
Tall AR: HDL from CETP-deficient subjects shows enhanced ability to
promote cholesterol efflux from macrophages in an apoE- and
ABCG1-dependent pathway. J Clin Invest. 116:1435–1442. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Costet P, Luo Y, Wang N and Tall AR:
Sterol-dependent transactivation of the ABC1 promoter by the liver
X receptor/retinoid X receptor. J Biol Chem. 275:28240–28245.
2000.PubMed/NCBI
|
|
8
|
Kennedy MA, Venkateswaran A, Tarr PT,
Xenarios I, Kudoh J, Shimizu N and Edwards PA: Characterization of
the human ABCG1 gene: liver X receptor activates an internal
promoter that produces a novel transcript encoding an alternative
form of the protein. J Biol Chem. 276:39438–39447. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chawla A, Boisvert WA, Lee CH, et al: A
PPARγ-LXR-ABCA1 pathway in macrophages is involved in cholesterol
efflux and atherogenesis. Mol Cell. 7:161–171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagy ZS, Czimmerer Z and Nagy L: Nuclear
receptor mediated mechanisms of macrophage cholesterol metabolism.
Mol Cell Endocrinol. 368:85–98. 2013. View Article : Google Scholar
|
|
11
|
Wang Y, Tikellis C, Thomas MC and Golledge
J: Angiotensin converting enzyme 2 and atherosclerosis.
Atherosclerosis. 226:3–8. 2013. View Article : Google Scholar
|
|
12
|
Kawahito H, Yamada H, Irie D, et al:
Periaortic adipose tissue-specific activation of the renin
angiotensin system contributes to atherosclerosis development in
uninephrectomized apoE−/− mice. Am J Physiol Heart Circ Physiol.
305:H667–H675. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fujisaka T, Hoshiga M, Hotchi J, et al:
Angiotensin II promotes aortic valve thickening independent of
elevated blood pressure in apolipoprotein-E deficient mice.
Atherosclerosis. 226:82–87. 2013. View Article : Google Scholar
|
|
14
|
Santos RA, Ferreira AJ, Verano-Braga T and
Bader M: Angiotensin-converting enzyme 2, angiotensin-(1-7) and
Mas: new players of the renin-angiotensin system. J Endocrinol.
216:R1–R17. 2013. View Article : Google Scholar
|
|
15
|
Yang HY, Bian YF, Zhang HP, et al:
Angiotensin-(1-7) treatment ameliorates angiotensin II-induced
HUVEC apoptosis. Clin Exp Pharmacol Physiol. 39:1004–1010. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang JM, Dong M, Meng X, et al:
Angiotensin-(1-7) dose-dependently inhibits atherosclerotic lesion
formation and enhances plaque stability by targeting vascular
cells. Arterioscler Thromb Vasc Biol. 33:1978–1985. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takata Y, Chu V, Collins AR, et al:
Transcriptional repression of ATP-binding cassette transporter A1
gene in macrophages: a novel atherosclerotic effect of angiotensin
II. Circ Res. 97:e88–e96. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sampaio WO, Souza dos Santos RA,
Faria-Silva R, da Mata Machado LT, Schiffrin EL and Touyz RM:
Angiotensin-(1-7) through receptor Mas mediates endothelial nitric
oxide synthase activation via Akt-dependent pathways. Hypertension.
49:185–192. 2007. View Article : Google Scholar
|
|
19
|
Hwang JS, Kang ES, Ham SA, et al:
Activation of peroxisome proliferator-activated receptor γ by
rosiglitazone inhibits lipopolysaccharide-induced release of high
mobility group box 1. Mediators Inflamm. 2012:3528072012.
View Article : Google Scholar
|
|
20
|
Trasino SE, Kim YS and Wang TT: Ligand,
receptor, and cell type-dependent regulation of ABCA1 and ABCG1
mRNA in prostate cancer epithelial cells. Mol Cancer Ther.
8:1934–1945. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
22
|
Rosenbaugh EG, Savalia KK, Manickam DS and
Zimmerman MC: Antioxidant-based therapies for angiotensin
II-associated cardiovascular diseases. Am J Physiol Regul Integr
Comp Physiol. 304:R917–R928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kanome T, Watanabe T, Nishio K, Takahashi
K, Hongo S and Miyazaki A: Angiotensin II upregulates
acyl-CoA:cholesterol acyltransferase-1 via the angiotensin II Type
1 receptor in human monocyte-macrophages. Hypertens Res.
31:1801–1810. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Chen Z, Liao Y, et al: Angiotensin
II increases the cholesterol content of foam cells via
down-regulating the expression of ATP-binding cassette transporter
A1. Biochem Biophys Res Commun. 353:650–654. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mario EG, Santos SH, Ferreira AV, Bader M,
Santos RA and Botion LM: Angiotensin-(1-7) Mas-receptor deficiency
decreases peroxisome proliferator-activated receptor γ expression
in adipocytes. Peptides. 33:174–177. 2012. View Article : Google Scholar
|
|
26
|
Dhaunsi GS, Yousif MH, Akhtar S, Chappell
MC, Diz DI and Benter IF: Angiotensin-(1-7) prevents
diabetes-induced attenuation in PPAR-gamma and catalase activities.
Eur J Pharmacol. 638:108–114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ren J, Jin W and Chen H: oxHDL decreases
the expression of CD36 on human macrophages through PPARgamma and
p38 MAP kinase dependent mechanisms. Mol Cell Biochem. 342:171–181.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rohde E, Schallmoser K, Reinisch A, et al:
Pro-angiogenic induction of myeloid cells for therapeutic
angiogenesis can induce mitogen-activated protein kinase
p38-dependent foam cell formation. Cytotherapy. 13:503–512. 2011.
View Article : Google Scholar :
|
|
29
|
Mei S, Gu H, Ward A, et al: p38
mitogen-activated protein kinase (MAPK) promotes cholesterol ester
accumulation in macrophages through inhibition of macroautophagy. J
Biol Chem. 287:11761–11768. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Min KJ, Um HJ, Cho KH and Kwon TK:
Curcumin inhibits oxLDL-induced CD36 expression and foam cell
formation through the inhibition of p38 MAPK phosphorylation. Food
Chem Toxicol. 58:77–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yin R, Dong YG and Li HL: PPARγ
phosphorylation mediated by JNK MAPK: a potential role in
macrophage-derived foam cell formation. Acta Pharmacol Sin.
27:1146–1152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Santos SH, Andrade JM, Fernandes LR, et
al: Oral Angiotensin-(1-7) prevented obesity and hepatic
inflammation by inhibition of resistin/TLR4/MAPK/NF-κB in rats fed
with high-fat diet. Peptides. 46:47–52. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moon JY, Tanimoto M, Gohda T, et al:
Attenuating effect of angiotensin-(1-7) on angiotensin II-mediated
NAD(P)H oxidase activation in type 2 diabetic nephropathy of
KK-A(y)/Ta mice. Am J Physiol Renal Physiol. 300:F1271–F1282. 2011.
View Article : Google Scholar : PubMed/NCBI
|