Introduction
Malignant neoplastic disease is one of the most
common causes of mortality worldwide. ~90% of all cancer
mortalities are the result of metastases, rather than of the
primary tumors (1). The major risk
factors for malignant melanoma are personal or family history of
melanoma, exposure to intense and intermittent ultraviolet
irradiation, phenotypic characteristics, and multiple nevi
(2). The process of tumor
metastasis involves tumor cell proliferation, expansion,
reorganization, degradation and migration through the surrounding
stroma’s microenvironment and the extracellular matrix (ECM), into
the circulation to invade other tissues (3).
The process of tumor metastasis is accompanied with
changes in gene expression, including mutation, overexpression,
loss, activation or inactivation of numerous genes (4). The matrix metalloproteinase (MMP)
family is a large group of secreted proteinases which require zinc
for their catalytic activity. MMPs, which are proteolytic enzymes
in the extracellular matrix (ECM), mainly contribute to cell
motility, tumor invasion, angiogenesis and metastasis (5–7).
Among these MMPs, MMP-2 (gelatinase-A) and MMP-9 (gelatinase-B) are
mainly involved in cancer metastasis and invasion. They
predominantly degrade gelatin and type IV, V, XI and XVI collagen,
the major structural component of basement membrane, which appears
to be crucial in tumor cell invasion and metastasis (8–10). A
previous study reported that oroxylin A could inhibit migration and
invasion of human breast MDA-MB-435 cancer cells via the inhibition
of MMP-2 and MMP-9 (11). Thus,
MMP-2 and MMP-9 have been considered as targets in the development
of drugs against tumor invasion and metastasis (12,13).
The mitogen-activated protein kinase (MAPK) pathway
is associated with tumor proliferation and survival, motility and
invasion (14). In melanoma, MAPK
signalling cascades are critical and constitutively activated by a
variety of mechanisms, making it a target for pathway targeting
therapies. The MAPK signalling pathway is one of the most important
cellular mechanisms responsible for melanoma metastasis by
promoting cell proliferation, survival, invasion and tumor
angiogenesis. Extracellular signal-regulated kinase 1 and 2
(ERK1/2) and p38/MAPK are major MAPKs, which are associated with
tumor metastasis (15). They have
a central role in regulating the expression of MMPs. The up- or
downregulation of MAP kinases and their phosphorylation are
involved in the regulation of MMP-9 expression in cancer cells
(16,17).
Medicinal plants have been used as traditional
remedies for hundreds of years. Radix Ophiopogon japonicus
is an important medicinal herb widely used for the treatment of
various tumors, inflammatory diseases, hepatitis and diarrhea in
East Asian countries, including China, Korea, Taiwan, and Japan
(18–21). The plant has been reported to
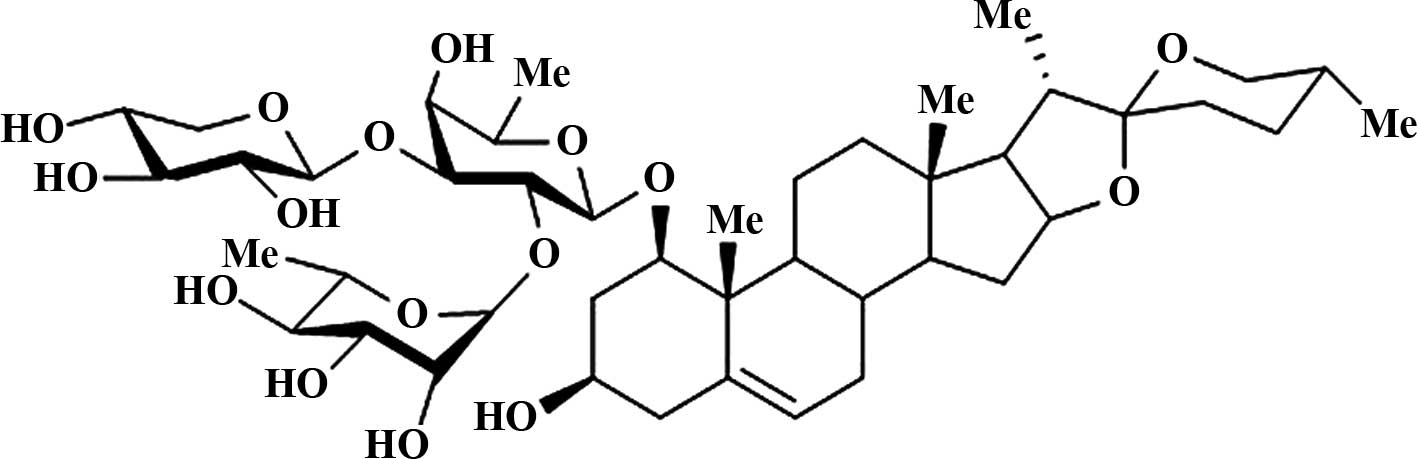
contain a large number of steroidal saponins. Ophiopogonin-D
(ruscogenin 1-O-α-L-rhamnopyranosyl-(1→2)-β-D-xylopyran
osyl- (1→3)-β-D-fucopyranoside) (Fig.
1) is one of the steroidal saponins isolated from the root of
Ophiopogon japonicus (22).
According to previous studies, the ophiopogonin-D processes
anti-inflammatory and anti-oxidant properties, including prevention
of H2O2-induced injury in primary human
umbilical vascular endothelial cells (HUVECs) (23), anti-thrombotic activity (24), anti-inflammation (25) and suppressed leukocyte migration
in vivo (26). However, to
the best of our knowledge, the anti-tumor activity of
ophiopogonin-D has not been evaluated in vitro.
The present study investigated the molecular
mechanisms by which ophiopogonin-D suppresses the migration and
invasion of MDA-MB-435 cells in vitro. Furthermore, the
underlying mechanism of the effect of ophiopogonin-D on MDA-MB-435
cells was explored by assessing its effect on MMP activity and the
MAPK pathway. The present study provided evidence that
ophiopogonin-D is suitable for use in the treatment of metastasis
of melanoma.
Materials and methods
Chemicals and reagents
Ophiopogonin-D was isolated from the root of
Ophiopogon japonicus according to the procedure of a
previous study (27). Compounds
were dissolved in dimethyl sulfoxide (DMSO; Nanjing Sunshine
Biotechnology Ltd., Nanjing, China) and diluted with Dulbecco’s
medium Essential medium (DMEM; Invitrogen Life Technologies,
Carlsbad, CA, USA) without serum prior to each experiment. The
final concentration of DMSO in the culture medium never exceeded
0.1% (v/v), a concentration known not to affect cell proliferation,
and control groups were always treated with 0.1% DMSO in the
corresponding experiments. MTT, Rose Bengal and Triton X-100 were
purchased from Sigma-Aldrich (St. Louis, MO, USA). High-glucose
DMEM was purchased from Gibco-BRL (Invitrogen Life Technologies).
Antibodies specific for phosphorylated (p-)ERK and p-p38/MAPK or
total ERK, p38/MAPK, β-actin, MMP-2 and MMP-9 were obtained from
Cell Signaling Technology (Cell Signaling Technology, Beverly, MA,
USA). The cell culture plate and Transwell plate (8- μm pore
size, 6.5-mm diameter) were obtained from Corning Costar
Corporation (Corning, NY, USA). Matrigel was purchased from BD
Biosciences (La Jolla, CA, USA).
Cells lines and culture
The human breast carcinoma cell line MDA-MB-435 was
obtained from Keygene Corporation (Nanjing, China), grown in
high-glucose DMEM supplemented with 10% heat-inactivated fetal
bovine serum, 100 U/ml penicillin, 100 U/ml streptomycin and 3.7
g/l sodium bicarbonate (all purchased from Nanjing Sunshine
Biotechnology Ltd.). HUVECs were obtained from Shanghai FuMeng Gene
Biotechnology Co., Ltd. (Shanghai, China). The cells were
maintained under a humidified atmosphere of 95% air and 5%
CO2 at 37°C and used for experiments whilst in
logarithmic phase.
Cell adhesion of HUVECs and
fibronectin
The tumor cell adhesion with HUVECs was detected
according to the method by Jones et al (28) with certain modifications. HUVECs
were seeded in 96-well plates in complete HUVEC medium at a density
of 104 cells per well for 24 h. The HUVEC medium was
then removed and cells were washed with phosphate-buffered saline
(PBS). MDA-MB-435 cells were trypsinized and suspended at a final
concentration of 5×105 cells/ml with various
concentrations of ophiopogonin-D (5, 10, 20, 40 and 80 μM)
dissolved in serum-free DMEM. After 3 h, the medium was removed and
the undetached cells were washed with PBS twice and incubated with
Rose Bengal for 5 min. The cells were washed with PBS twice and
fixed with 100 μl mixed solution (95% ethanol/PBS 1:1) per
well for 30 min at room temperature. The ratio of adhesion was
detected by measuring the absorbance of Rose Bengal at a wavelength
of 600 nm using an ELISA reader (Bio-Tek Instruments, Winooski, VT,
USA).
Cell adhesion to fibronectin was assayed as
described previously with a few alterations (29). Briefly, 96-well plates were coated
with fibronectin (Merck, Whitehouse Station, NJ, USA) at 4°C
overnight. MDA-MB-435 cells, which were trypsinized and suspended
at a final concentration of 5×105 cells/ml in serum-free
DMEM with various concentrations of ophiopogonin-D and cultured for
3 h. The unattached cells were removed by discarding the media and
washing with PBS twice. A total of 100 μl MTT (0.5 mg/ml in
PBS) was added to the wells and the cells were cultured for another
3 h. Following discarding the MMT solution, DMSO was then added to
the wells. After agitation for 10 min at room temperature, the
cytotoxicity was determined by measuring the absorbance of the
converted dye at a wavelength of 570 nm and a reference wavelength
of 650 nm in an ELISA reader.
Cell invasion assay
The cell invasion assay was determined as described
previously with a few modifications (30). Briefly, the Transwell membrane (8
μm pore size, 6.5 mm diameter; Corning Costar Corporation)
was pre-coated with Matrigel. The MDA-MB-435 cells, which were
trypsinized and suspended at a final concentration of
5×105 cells/ml in serum-free DMEM with various
concentrations of ophiopogonin-D, were added to the upper well,
while 500 μl DMEM containing 10% FBS was added to the lower
well. After 20 h, assays were stopped by removal of the medium from
the upper wells. The cells on the membrane were fixed with 90%
ethanol and cells on the upper side of the membrane, which had not
transgressed through the membrane, were subsequently wiped off
using cotton buds. The cells on the lower side of the membrane were
stained with 0.1% methyl violet. After 10 min, the chamber was
washed with PBS and images of the membrane were captured using a
microscope (Axiovert40CFL; Carl Zeiss, Oberkochen, Germany).
Finally, the chamber was extracted with 10% acetic acid, and the
absorbance of the extract was measured at 570 nm in an ELISA
reader.
Gelatin zymography
The activities of MMP-2 and MMP-9 were assayed by
gelatin zymography (31,32). Gelatin zymography was performed in
10% SDS-polyacrylamide gel containing 0.1% gelatin. After treatment
with various concentrations of ophiopogonin-D (5, 10, 20, 40 and 80
μM) for 24 h, the supernatants were collected. Samples were
mixed with loading buffer and electrophoresed. Gels were then
washed twice in zymography washing buffer (2.5% Triton X-100, 50
mmol/l Tris-HCl, 5 mmol/l CaCl2; pH 7.6) for 45 min at
room temperature to remove SDS, followed by rinsing twice in
washing buffer for 20 min. The gels were then incubated at 37°C for
40 h in zymography reaction buffer (50 mmol/l Tris, 150 mmol/l
NaCl,10 mmol/l CaCl2, 0.02% NaN3, pH 7.5) and stained
with 0.05% Coomassie blue R-250 (diluted in 30% methanol and 10%
acetic acid) for 3 h and destained with destaining solution (10%
methanol and 5% acetic acid in H2O2). The
field of the destained band was quantified using the Quantity One
System (Bio-Rad, Hercules, CA, USA).
Western blot analysis
Activation of MMP-2/9 and MAPK were assessed by
western blot analysis. MDA-MB-435 cells were pre-incubated with
various concentrations of ophiopogonin-D for 20 h, and cells were
collected. Cells were then lysed in lysis buffer, and the equal
amount of proteins was separated by SDS-PAGE and transferred onto
the polyvinylidene difluoride membranes. Membranes were blocked
with blocking buffer for 1.5 h at room temperature, followed by
overnight incubation at 4°C in the relevant primary antibody
(1:1,000), and finally blocked for 1 h with a secondary antibody
HRP-conjugate (1:2,000). The band detection was revealed by
enhanced chemiluminescence (ECL) using ECL western blotting
detection reagents (Vazyme Biotech Co., Ltd., Nanjing, China) and
exposure to ECL hyperfilm on Kodak film (Eastman Kodak, Rochester,
NY, USA) and analyzed using the Quantity One System.
Statistical analysis
Values are expressed as the mean ± standard
deviation. The experimental data were analyzed using GraphPad Prism
5 software (GraphPad Inc., La Jolla, CA, USA). All comparisons were
made relative to the control groups and significance of differences
was indicated as *P<0.05 and
**P<0.01.
Results
Ophiopogonin-D inhibits MDA-MB-435 cell
adhesion and invasion
In a preliminary experiment, the cytotoxic effects
of ophiopogonin-D were determined using an MTT assay (data not
shown). Ophiopogonin-D demonstrated no obvious cytotoxicity on
MDA-MB-435 cells under 80 μM. Concentrations of
ophiopogonin-D (5, 10, 20, 40 and 80 μM) with no apparent
cytotoxicity on MDA-MB-435 cells were selected for all subsequent
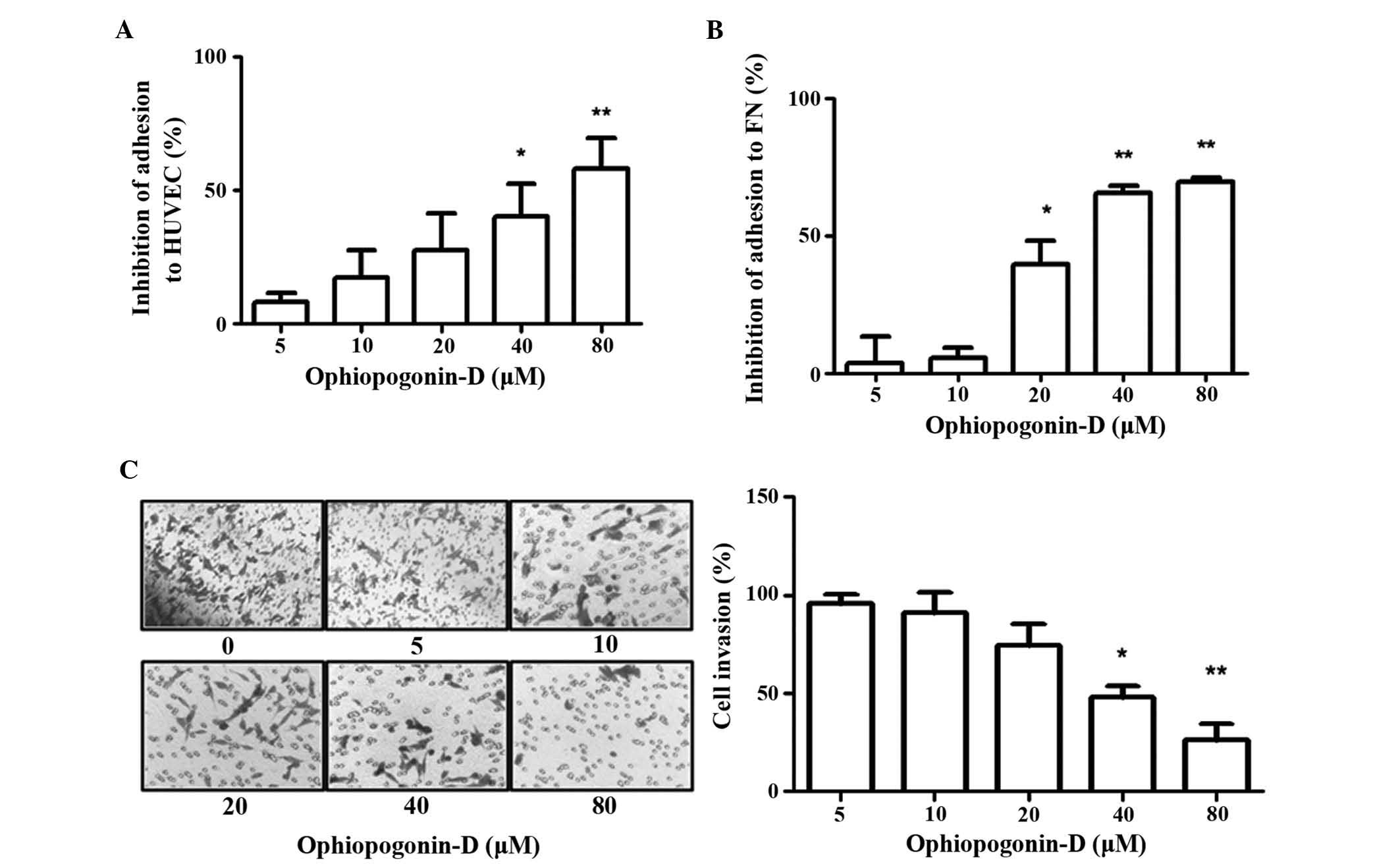
experiments. As shown in Fig. 2A,
ophiopogonin-D was able to inhibit the adhesion of MDA-MB-435 cells
to HUVECs. Ophiopogonin-D at concentrations of 40 and 80 μM
significantly reduced the number of MDA-MB-435 cells adhered to
HUVECs, and the inhibition rates were ~50 and 65%, respectively. In
addition, MDA-MB-435-cell adhesion to fibronectin was detected for
more accurate determination of the effect of ophiopogonin-D on the
adhesion of melanoma cells. Fig.
2B shows the inhibitory effect of ophiopogonin-D on the
adhesion of MDA-MB-435 cells to fibronectin. At concentrations of
40 and 80 μM, ophiopogonin-D significantly inhibited the
adhesion of MDA-MB-435 cells to fibronectin at rates of ~64 and
80%, respectively. Furthermore, the effect of ophiopogonin-D on the
invasive ability of MDA-MB-435 cells was evaluated using a
Transwell assay. Fig. 2C indicates
that after treatment with ophiopogonin-D the invasive capacity of
MDA-MB-435 cells was markedly inhibited. The inhibition rate of 80
μM of ophiopogonin-D was ~78%.
Ophiopogonin-D decreases MMP-9, but not
MMP-2 enzyme activity
The expression of MMP-2 and MMP-9 has been reported
to have a critical role in degrading the basement membrane in tumor
invasion and migration (33). A
gelatin zymography assay showed that the activity of MMP-9 was
reduced with increasing concentration of ophiopogonin-D (Fig. 3). MMP-9 was obviously decreased
following incubation with 40 and 80 μM ophiopogonin-D, while
there was no obvious decrease in MMP-2. This result indicated that
ophiopogonin-D mostly inhibits tumor metastasis through MMP-9, not
MMP-2.
Effect of ophiopogonin-D on the
expression of MMP-2/9 and MAPKs
MMPs have significant roles in cancer metastasis.
Furthermore, the activity of MMP-2/9 is associated with the
adhesion, invasion and angiogenesis of cancer metastasis (34,35).
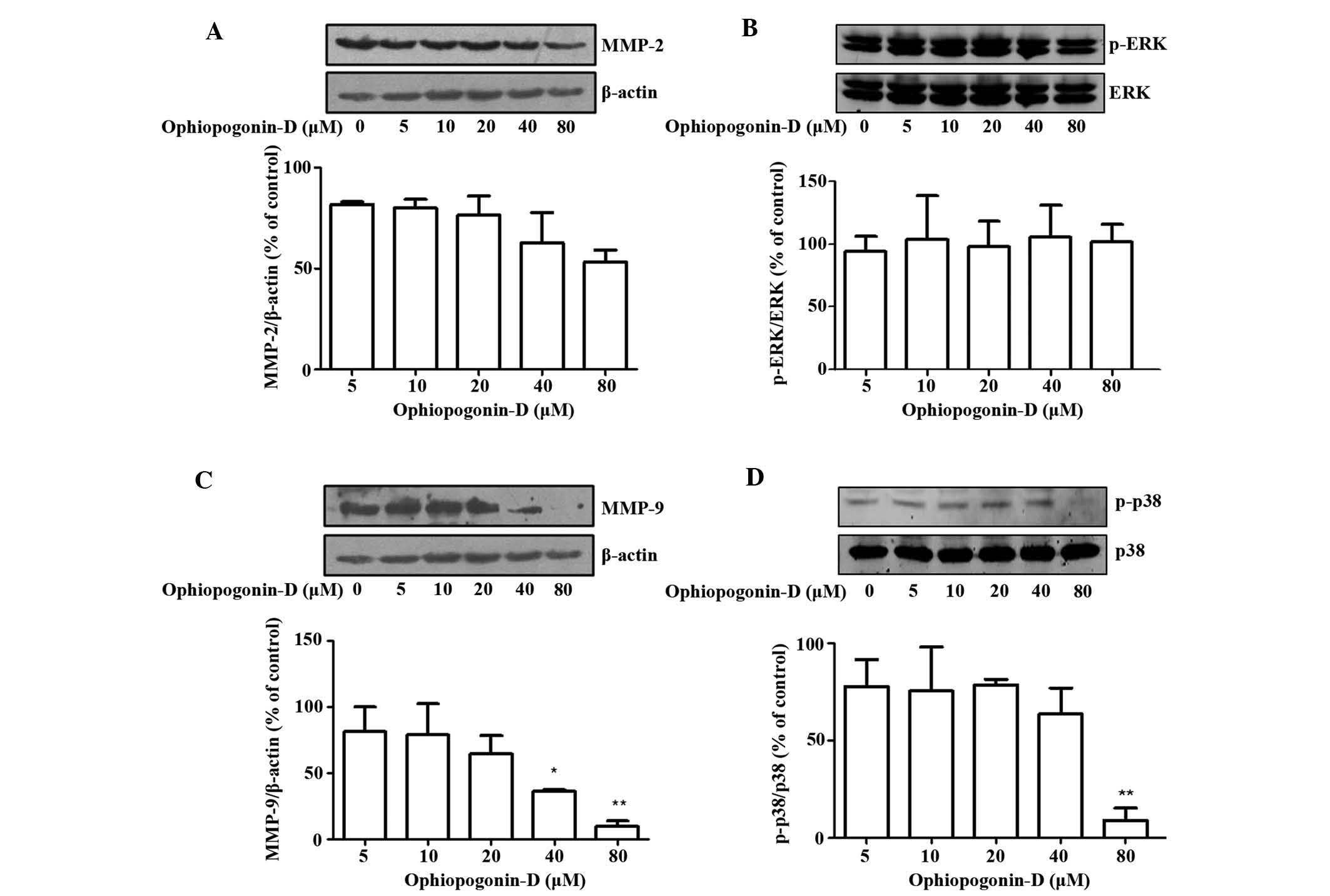
Therefore, the effect of ophiopogonin-D on the expression of MMP-2
and MMP-9 was deteced by western blot analysis. The results
indicated that ophiopogonin-D inhibited the expression of MMP-9 at
concentrations of 40 and 80 μM (Fig. 4). With increasing ophiopogonin-D
concentration, the expression of MMP-9 declined, while there was no
obvious decrease in MMP-2 expression. The result of the western
blot analysis corresponded with the results of the adhesion and
invasion experiments. Furthermore, the expression and
phosphorylation of ERK1/2 and p38/MAPK were examined to assess
whether the MAPK signaling pathway was involved in the
anti-metastatic effect of ophiopogonin-D in melanoma cells
(Fig. 4). The phosphorylation of
p38 was inhibited by ophiopogonin-D at the concentration of 80
μM, while the phosphorylation of ERK was not inhibited by
ophiopogonin-D. Further investigation is required to fully
elucidate the underlying mechanism of the anti-metastatic effect of
ophiopogonin-D on melanoma cells.
Discussion
Tumor growth, invasion and metastasis are multistep
and complex processes that include cell division and proliferation,
proteolytic digestion of the extracellular matrix, cell migration
through basement membranes to reach the circulatory system, as well
as re-migration and growth of tumors at the metastatic sites
(36). The present study
demonstrated that ophiopogonin-D, derived from the root of
Ophiopogon japonicus, was able to inhibit the metastatic
process in MDA-MB-435 cancer cells. It was found that
ophiopogonin-D inhibited the proliferation, adhesion and invasion
of MDA-MB-435 cells. Furthermore, the expression of MMP-9 and the
activation of p38 were inhibited by ophiopogonin-D.
MMPs have a significant role in cancer metastasis
(37). The activities of MMP-2 and
MMP-9 are associated with the adhesion, invasion and angiogenesis
of cancer metastasis (38,39). Invasiveness inhibitors may act by
downregulating extracellular barrier-degrading proteinases,
including MMP-2 and/or MMP-9 (40). To further explore the mechanism of
inhibition of invasion induced by ophiopogonin-D, the present study
examined the effects of ophiopogonin-D on the expression and
activity of MMP-2 and -9 in MDA-MB-435 human breast cancer cells.
The results showed that ophiopogonin-D was able to inhibit the
expression of MMP-9 and zymographic assays showed full development
of the gelatinolytic potential of MMP-9 in the presence of
invasion-restraining concentrations of ophiopogonin-D. It was
demonstrated that ophiopogonin-D was able to
concentration-dependently inhibit the secretion of MMP-9 in
MDA-MB-435 cells. However, ophiopogonin-D had no obvious effects on
the expression and secretion of MMP-2 in MDA-MB-435 cells. MMP-9,
but not MMP-2, has an important role in the mechanism of the
anti-tumor metastatic action of ophiopogonin-D. The results of the
western blot analysis corresponded with the results of the adhesion
and invasion assays.
To assess whether the MAPK signaling pathway is
involved in the mechanism of action of ophiopogonin-D, the present
study examined the expression and phosphorylation of ERK1/2 and p38
MAPK in ophiopogonin-D-treated cells. The expression of p-p38 was
inhibited by ophiopogonin-D, but not the expression of p-ERK. The
results indicated that ophiopogonin-D inhibited cell metastasis,
likely through inactivation of p38, but not ERK1/2. It has been
reported that a p38 pathway inhibitor was able to reduce MMP-9
expression and secretion, as well as in vitro invasion of
cancer cells (41,42). According to the results of the
present study, ophiopogonin-D was able to reduce the expression and
secretion of MMP-9 and the expression of p38/MAPK, and inhibit the
invasion of MDA-MB-435 cells. Therefore, it is concluded that
ophiopogonin-D has the ability to act as a p38 inhibitor to impede
tumor metastasis. Further investigation is required to fully
elucidate the detailed mechanism of ophiopogonin-D on cancer
metastasis, particularly with regard to the MAPK pathway and
additional kinases.
In conclusion, the present study demonstrated that
ophiopogonin-D, derived from the root of Ophiopogon
japonicus, was able to inhibit the metastatic capacity of
MDA-MB-435 cancer cells. Ophiopogonin-D effectively inhibited the
proliferation, adhesion and invasion of MDA-MB-435 cells.
Furthermore, the expression of MMP-9 was inhibited by
ophiopogonin-D. The present study suggested that ophiopogonin-D
inhibits tumor metastasis through downregulation of MMP-9 and
suppression of the p38/MAPK pathway. Hence, ophiopogonin-D is
suggested to be a therapeutic agent for inhibiting the metastasis
and invasion of cancer.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81274131) and a General
Financial Grant from the China Postdoctoral Science Foundation (no.
2012M521150).
References
|
1
|
Böhle AS and Kalthoff H: Molecular
mechanisms of tumor metastasis and angiogenesis. Langenbecks Arch
Surg. 384:133–140. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller AJ and Mihm MC Jr: Melanoma. N Engl
J Med. 355:51–65. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torzilli PA, Bourne JW, Cigler T and
Vincent CT: A new paradigm for mechanobiological mechanisms in
tumor metastasis. Semin Cancer Biol. 22:385–395. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perlikos F, Harrington KJ and Syrigos KN:
Key molecular mechanisms in lung cancer invasion and metastasis: a
comprehensive review. Crit Rev Oncol Hematol. 87:1–11. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao XH, Yang XQ, Wang BC, Liu SP and Wang
FB: Overexpression of twist and matrix metalloproteinase-9 with
metastasis and prognosis in gastric cancer. Asian Pac J Cancer
Prev. 14:5055–5060. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shuman Moss LA, Jensen-Taubman S and
Stetler-Stevenson WG: Matrix metalloproteinases: changing roles in
tumor progression and metastasis. Am J Pathol. 181:1895–1899. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bolkun L, Lemancewicz D, Sobolewski K,
Mantur M, Semeniuk J, Kulczynska A, Kloczko J and Dzieciol J: The
evaluation of angiogenesis and matrix metalloproteinase-2 secretion
in bone marrow of multiple myeloma patients before and after the
treatment. Adv Med Sci. 58:118–125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vandooren J, Van den Steen PE and
Opdenakker G: Biochemistry and molecular biology of gelatinase B or
matrix metallopro-teinase-9 (MMP-9): the next decade. Crit Rev
Biochem Mol Biol. 48:222–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Monsonego-Ornan E, Kosonovsky J, Bar A,
Roth L, Fraggi-Rankis V, Simsa S, Kohl A and Sela-Donenfeld D:
Matrix metalloproteinase 9/gelatinase B is required for neural
crest cell migration. Dev Biol. 364:162–177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Frankowski H, Gu YH, Heo JH, Milner R and
Del Zoppo GJ: Use of gel zymography to examine matrix
metalloproteinase (gelatinase) expression in brain tissue or in
primary glial cultures. Methods Mol Biol. 814:221–233. 2012.
|
|
11
|
Sun Y, Lu N, Ling Y, Gao Y, Chen Y, Wang
L, Hu R, Qi Q, Liu W, Yang Y, You Q and Guo Q: Oroxylin A
suppresses invasion through down-regulating the expression of
matrix metallopro-teinase-2/9 in MDA-MB-435 human breast cancer
cells. Eur J Pharmacol. 603:22–28. 2009. View Article : Google Scholar
|
|
12
|
Jin ML, Park SY, Kim YH, Park G and Lee
SJ: Halofuginone induces the apoptosis of breast cancer cells and
inhibits migration via downregulation of matrix
metalloproteinase-9. Int J Oncol. 44:309–318. 2014.
|
|
13
|
Liao CL, Lin JH, Lien JC, Hsu SC, Chueh
FS, Yu CC, Wu PP, Huang YP, Lin JG and Chung JG: The crude extract
of Corni Fructus inhibits the migration and invasion of U-2 OS
human osteosarcoma cells through the inhibition of matrix
metallopro-teinase-2/-9 by MAPK signaling. Environ Toxicol.
30:53–63. 2015. View Article : Google Scholar
|
|
14
|
Cheng Y, Zhang G and Li G: Targeting MAPK
pathway in melanoma therapy. Cancer Metastasis Rev. 32:567–584.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen T, Heo SI and Wang MH: Involvement of
the p38 MAPK and ERK signaling pathway in the anti-melanogenic
effect of methyl 3,5-dicaffeoyl quinate in B16F10 mouse melanoma
cells. Chem Biol Interact. 199:106–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Davidson B, Givant-Horwitz V, Lazarovici
P, Risberg B, Nesland JM, Trope CG, Schaefer E and Reich R: Matrix
metalloproteinases (MMP), EMMPRIN (extracellular matrix
metalloproteinase inducer) and mitogen-activated protein kinases
(MAPK): co-expression in metastatic serous ovarian carcinoma. Clin
Exp Metastasis. 20:621–631. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu KC, Yang ST, Hsia TC, Yang JS, Chiou
SM, Lu CC, Wu RS and Chung JG: Suppression of cell invasion and
migration by propofol are involved in down-regulating matrix
metalloproteinase-2 and p38 MAPK signaling in A549 human lung
adenocarcinoma epithelial cells. Anticancer Res. 32:4833–4842.
2012.PubMed/NCBI
|
|
18
|
Chen M, Du Y, Qui M, Wang M, Chen K, Huang
Z, Jiang M, Xiong F, Chen J, Zhou J, Jiang F, et al: Ophiopogonin
B-induced autophagy in non-small cell lung cancer cells via
inhibition of the PI3K/Akt signaling pathway. Oncol Rep.
29:430–436. 2013.
|
|
19
|
Li N, Zhang L, Zeng KW, Zhou Y, Zhang JY,
Che YY and Tu PF: Cytotoxic steroidal saponins from Ophiopogon
japonicus. Steroids. 78:1–7. 2013. View Article : Google Scholar
|
|
20
|
Lan S, Yi F, Shuang L, Chenjie W and Zheng
XW: Chemical constituents from the fibrous root of Ophiopogon
japonicus and their effect on tube formation in human myocardial
micro-vascular endothelial cells. Fitoterapia. 85:57–63. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Liu J, Kou J, Yu J and Yu B:
DT-13 suppresses MDA-MB-435 cell adhesion and invasion by
inhibiting MMP-2/9 via the p38 MAPK pathway. Mol Med Rep.
6:1121–1125. 2012.PubMed/NCBI
|
|
22
|
Yu BY, Qiu SX, Zaw K, Xu GJ, Hirai Y,
Shoji J, Fong HH and Kinghorn AD: Steroidal glycosides from the
subterranean parts of Liriope spicata var. prolifera.
Phytochemistry. 43:201–206. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qian J, Jiang F, Wang B, Yu Y, Zhang X,
Yin Z and Liu C: Ophiopogonin D prevents H2O2-induced injury in
primary human umbilical vein endothelial cells. J Ethnopharmacol.
128:438–445. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kou J, Tian Y, Tang Y, Yan J and Yu B:
Antithrombotic activities of aqueous extract from Radix Ophiopogon
japonicus and its two constituents. Biol Pharm Bull. 29:1267–1270.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kou J, Sun Y, Lin Y, Cheng Z, Zheng W, Yu
B and Xu Q: Anti-inflammatory activities of aqueous extract from
Radix Ophiopogon japonicus and its two constituents. Biol Pharm
Bull. 28:1234–1238. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang YL, Kou JP, Ma L, Song JX and Yu BY:
Possible mechanism of the anti-inflammatory activity of ruscogenin:
role of intercellular adhesion molecule-1 and nuclear
factor-kappaB. J Pharmacol Sci. 108:198–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Asano T, Murayama T, Hirai Y and Shoji J:
Comparative studies on the constituents of ophiopogonis tuber and
its congeners. VIII Studies on the glycosides of the subterranean
part of Ophiopogon japonicus Ker-Gawler cv. Nanus. Chem Pharm Bull
(Tokyo). 41:566–570. 1993. View Article : Google Scholar
|
|
28
|
Jones J, Marian D, Weich E, Engl T, Wedel
S, Relja B, Jonas D and Blaheta RA: CXCR4 chemokine receptor
engagement modifies integrin dependent adhesion of renal carcinoma
cells. Exp Cell Res. 313:4051–4065. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu ZJ, Ren YQ, Wang GP, Song Q, Li M,
Jiang SS, Ning T, Guan YS, Yang JL and Luo F: Biological behaviors
and proteomics analysis of hybrid cell line EAhy926 and its parent
cell line A549. J Exp Clin Cancer Res. 28:162009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen HW, Lee JY, Huang JY, Wang CC, Chen
WJ, Su SF, Huang CW, Ho CC, Chen JJ, Tsai MF, Yu SL and Yang PC:
Curcumin inhibits lung cancer cell invasion and metastasis through
the tumor suppressor HLJ1. Cancer Res. 68:7428–7438. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hung SH, Shen KH, Wu CH, Liu CL and Shih
YW: Alpha-mangostin suppresses PC-3 human prostate carcinoma cell
metastasis by inhibiting matrix metalloproteinase-2/9 and
urokinase-plasminogen expression through the JNK signaling pathway.
J Agric Food Chem. 57:1291–1298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kupai K, Szucs G, Cseh S, Hajdu I, Csonka
C, Csont T and Ferdinandy P: Matrix metalloproteinase activity
assays: Importance of zymography. J Pharmacol Toxicol Methods.
61:205–209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hwang ES and Park KK: Magnolol suppresses
metastasis via inhibition of invasion, migration and matrix
metallopro-teinase-2/-9 activities in PC-3 human prostate carcinoma
cells. Biosci Biotechnol Biochem. 74:961–967. 2010. View Article : Google Scholar
|
|
34
|
Song C, Zhu S, Wu C and Kang J: Histone
deacetylase (HDAC) 10 suppresses cervical cancer metastasis through
inhibition of matrix metalloproteinase (MMP) 2 and 9 expression. J
Biol Chem. 288:28021–28033. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ho YL, Li KC, Chao W, Chang YS and Huang
GJ: Korean red ginseng suppresses metastasis of human hepatoma
SK-Hep1 cells by inhibiting matrix metalloproteinase-2/-9 and
urokinase plasminogen activator. Evid Based Complement Alternat
Med. 2012:9658462012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Geho DH, Bandle RW, Clair T and Liotta LA:
Physiological mechanisms of tumor-cell invasion and migration.
Physiology (Bethesda). 20:194–200. 2005. View Article : Google Scholar
|
|
37
|
Wu D, Huang P, Wang L, Zhou Y, Pan H and
Qu P: MicroRNA-143 inhibits cell migration and invasion by
targeting matrix metalloproteinase 13 in prostate cancer. Mol Med
Rep. 8:626–630. 2013.PubMed/NCBI
|
|
38
|
Khasigov PZ, Podobed OV, Gracheva TS,
Salbiev KD, Grachev SV and Berezov TT: Role of matrix
metalloproteinases and their inhibitors in tumor invasion and
metastasis. Biochemistry (Mosc). 68:711–717. 2003. View Article : Google Scholar
|
|
39
|
Hamsa TP and Kuttan G: Inhibition of
invasion and experimental metastasis of murine melanoma cells by
Ipomoea obscura (L) is mediated through the down-regulation of
inflammatory mediators and matrix-metalloproteinases. J Exp Ther
Oncol. 9:139–151. 2011.
|
|
40
|
Ordoñez R, Carbajo-Pescador S,
Prieto-Dominguez N, García-Palomo A, González-Gallego J and Mauriz
JL: Inhibition of matrix metalloproteinase-9 and nuclear factor
kappaB contribute to melatonin prevention of motility and
invasiveness in HepG2 liver cancer cells. J Pineal Res. 56:20–30.
2013. View Article : Google Scholar
|
|
41
|
Yang JS, Lin CW, Hsieh YS, Cheng HL, Lue
KH, Yang SF and Lu KH: Selaginella tamariscina (Beauv) possesses
antimetastatic effects on human osteosarcoma cells by decreasing
MMP-2 and MMP-9 secretions via p38 and Akt signaling pathways. Food
Chem Toxicol. 59:801–807. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Khadjavi A, Valente E, Giribaldi G and
Prato M: Involvement of p38 MAPK in haemozoin-dependent MMP-9
enhancement in human monocytes. Cell Biochem Funct. 32:5–15. 2014.
View Article : Google Scholar
|