Introduction
Autophagy is a key intracellular pathway, which
involves the degradation of damaged organelles and misfolded
proteins through the actions of lysosomes (1). Autophagy is involved in cell survival
and cell death, depending on the stimuli (2,3).
Previous studies have demonstrated that certain plant- and
animal-derived compounds are able to induce autophagy, indicating
that they may possess potential anticancer properties (4,5). For
example, resveratrol, a naturally occurring polyphenol in a number
of plants, has been observed to induce autophagy in ovarian cancer
cells and in human U373 glioma cells (6); curcumin induces autophagy by
activating the Akt/mammalian target of rapamycin (mTOR)/p70S6
kinase and extracellular signal-regulated kinase (ERK)1/2 signaling
pathways (7); and arenobufagin has
been reported to induce apoptosis and autophagy in human
hepatocellular carcinoma cells by inhibiting the phosphoinositide-3
kinase (PI3K)/Akt/mTOR pathway (8).
PI3K and Akt have been implicated in the activation
of mTOR protein kinase. The PI3K/Akt/mTOR signaling pathway is a
key regulator of a wide range of physiological cell functions,
including proliferation, motility, differentiation, growth,
survival, metabolism, autophagy and apoptosis (9).
The mitogen-activated protein kinase (MAPK) pathways
have been observed to serve key functions in the development and
progression of cancer (10). The
three major MAPK pathways include the p38 MAPK pathway, the ERK1/2
(p44/p42) pathway and the c-Jun N-terminal kinase (JNK) pathway
(11). Activation of the ERK1/2
pathway has been associated with cell survival, proliferation and
differentiation, and the JNK pathway has been observed to regulate
diverse biological functions, including cytoprotection, apoptosis
and metabolism (10). Previous
studies have indicated that JNK and p38 are activated by
chemotherapeutic drugs, inflammatory cytokines and reactive oxygen
species (ROS) (12,13).
Sann-Joong-Kuey-Jian-Tang (SJKJT), a traditional
Chinese medicine, has been observed to inhibit the proliferation of
MCF-7 and MDA-MB-231 human breast cancer cells by inhibiting the
progression of the cell cycle and inducing apoptosis (14). SJKJT has also been found to induce
apoptosis via upregulating the protein expression of
microtubule-associated protein light chain 3 (15), Fas and tumor necrosis factor-α
(TNF-α) (16) and upregulating the
antitumor activity of 5-fluorouracil in colo 205 cells (17). It also reduces the protein
expression levels of myeloid cell leukemia 1 and translationally
controlled tumor protein, and upregulates the protein expression
levels of TNF-α and B-cell-associated X protein (Bax) in pancreatic
carcinoma cells (18). SJKJT
contains several active ingredients, including baicalin, berberine,
gentiopicroside, glycyrrhizin, palmatine, mangiferin and wogonin
(19). Our previous study reported
that SJKJT induces apoptosis in HepG2 cells by increasing the
expression levels of TNF-α, caspase-8, caspase-3 and Bax (20). Although SJKJT has been demonstrated
to induce autophagy in HepG2 cells, the underlying mechanism of
action remains to be elucidated. In the present study, the
molecular pathways through which SJKJT induces autophagy in the
human HepG2 hepatocellular carcinoma cell line were
investigated.
Materials and methods
Chemical reagents
The MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide],
dimethyl sulfoxide (DMSO) and acridine orange were obtained from
Merck Millipore (Darmstadt, Germany). Paraformaldehyde, Triton
X-100 and propidium iodide (PI) were obtained from Sigma-Aldrich
(St. Louis, MO, USA). Minimum essential medium (MEM-α), fetal
bovine serum (FBS), 10X phosphate-buffered saline (PBS) and
penicillin-streptomycin were obtained from Gibco Life Technologies
(Grand Island, NY, USA). Lipofectamine 2000 transfection reagent
and 4′6-diamidino-2-phenylindole (DAPI) were obtained from
Invitrogen Life Technologies (Carlsbad, CA, USA). The 10X
radioimmunoprecipitation (RIPA) lysis buffer was obtained from EMD
Millipore (Billerica, MA, USA). Tween 20 was obtained from AMRESCO,
Inc. (Solon, OH, USA). WesternBright Quantum enhanced
chemiluminescence (ECL) horseradish peroxidase (HRP) was obtained
from Advansta (Menlo Park, CA, USA).
Cell culture
The human HepG2 liver carcinoma cell line was
obtained from the Bioresource Collection and Research Center
(Hsinchu, Taiwan). The cells were maintained in MEM-α medium with
10% FBS, 100 U/ml penicillin and 0.1 mg/ml streptomycin at 37°C in
a humidified atmosphere of 95% air and 5% CO2.
Preparation of SJKJT
SJKJT consists of 17 species of medicinal herbs,
including Glycyrrhiza uralensis Fisch, Coptis
chinensis Franch, Cimicifuga heracleifolia Komar,
Phellodendron amurense Rupr, Anemarrhena
asphodeloides Bunge, Scutellaria baicalensis Georgi,
Gentiana scabra Bunge, Trichosanthes cucumer oides
Maxim, Platycodon grandiflour, Laminaria japonica
Aresch, Bupleurum chinese DC, Curcuma aeruginosa
Roxb, Sparganium stoloniferum Bucch, Forsythia
suspense Vahl, Pueraria lobata Ohwi, Paeonia
lactiflora Pall and Angelica sinensis Diels (12). The crude extract of SJKJT used in
the present study was obtained from Chuang Song Zong Pharmaceutical
Co., Ltd. (Ligang Plant, Taiwan). The SJKJT was diluted in
distilled sterilized PBS to create a stock solution (100 mg/ml),
which was then stored at −20°C, according to the manufacture’s
instructions. The final concentrations of SJKJT were 0.8, 1.6 and 2
mg/ml.
Measurement of cell viability in the
HepG2 cells
The cell viability was assessed using an MTT assay.
The HepG2 cells were plated in a 96-well plate at a density of
2×104 cells/well and were incubated overnight at 37°C.
Subsequent to the removal of the MEM-α medium, the cells were
treated with various concentrations (0.5, 1, 1.5, 2, 2.5 or 5
mg/ml) of SJKJT for 24, 48 or 72 h. Following treatment with SJKJT,
the cells were treated with MTT (1 mg/ml) and were incubated for 2
h at 37°C. The medium was removed and the purple-blue MTT formazan
precipitate was dissolved in 100 µl DMSO. The absorbance was
measured at a wavelength of 590 nm, with the results expressed as a
percentage of the untreated controls. The percentage of
proliferation was calculated using the following formula:
Proliferation (%) = (ODtest − ODblank × 100, where ODtest and
ODblank represent the optical density of the test substances and
the blank controls, respectively.
Acridine orange staining for the analysis
of autophagy
Autophagy is characterized by the formation of
acidic vesicular organelles (AVOs). To detect AVOs, cells can be
stained with acridine orange, a nucleic acid-specific fluorescent
cationic dye (21). The cells were
seeded at a density of 2×105 cells in six-well plates
and allowed to attach. Subsequent to treatment with 0.8 mg/ml SJKJT
for 6 h at 37°C, the cells were stained with 1 µg/ml
acridine orange for 10 min at 37°C, collected by trypsinization
(Gibco Life Technologies) and resuspended in PBS. The green
(510–530 nm) and red (650 nm) fluorescence, which was emitted from
1×104 cells illuminated with blue (488 nm) excitation
light, were measured using a BD accuri C5 flow cytometer and BD
Accuri™ C6 version 1.0.264.21 software (BD Biosciences, Franklin
Lakes, NJ, USA).
Green fluorescent protein (GFP-LC3)
plasmid transfection
HepG2 cells (1×105) were seeded onto
six-well plates and transfected with a GFP-LC3 expression plasmid
(kind gift from Dr Lin, Institute of Biomedical Science, National
Chung-Hsing University, Taichung, Taiwan) using Lipofectamine 2000
transfection reagent. Following transfection for 24 h at 37°C, the
cells were treated with 0.8 mg/ml SJKJT or 2 µg/ml rapamycin
(EMD Millipore) for 12 h at 37°C. The cells were then fixed with 4%
paraformaldehyde for 30 min at 37°C and washed twice in PBS. The
cell nuclei were then counterstained with 1 mg/ml DAPI and images
of the cells were captured from four non-overlapping fields using a
Leica SP5 confocal laser-scanning microscope (Leica Microsystems
GmbH, Wetzlar, Germany).
Nuclei PI staining analysis
The HepG2 Cells (1×105) were plated onto
12-well plates and treated with SJKJT (0.8 mg/ml) for four 0, 3, 6
or 12 h. The cells were then fixed with 4% formaldehyde for 30 min
at room temperature, and were washed twice with PBS. The cells were
then permeabilized in 0.25% Triton-X 100 for 10 min at room
temperature and then washed three times in PBS. The nuclei were
stained using PI (5 µg/ml) for 10 min and were then examined
under an Olympus IX81 microscope (Olympus, Tokyo, Japan).
Cell lysis and western blot analysis
Following SJKJT treatment, the HepG2 cells were
washed with ice-cold PBS. The cells were lysed in 1X RIPA lysis
buffer, containing protease inhibitors. The cells were then removed
and collected into eppendorf tubes (Quality Scientific Plastics,
Inc., San Diego, CA, USA), which were agitated for 30 min at 4°C,
followed by centrifugation at 13,000 × g for 10 min at 4°C (5415D;
Eppendorf, Hamburg, Germany). The protein concentrations were
determined using a Bicinchoninic Acid Protein Assay kit (Thermo
Fisher Scientific, Waltham, MA, USA). Equal quantities of sample
(10 µg/lane) were loaded into wells containing 6–10%
SDS-polyacrylamide gel (Bio-Rad Laboratories, Inc., Hercules, CA,
USA), and were separated by SDS-PAGE. The separated proteins were
then electrophoretically transferred onto polyvinylidene difluoride
membranes (EMD Millipore) at 400 mA for 2 h. The membranes were
then incubated in blocking buffer (PBS with 0.05% Tween 20 and 5%
non fat dry-milk) for 1 h at room temperature, followed by
incubation with the following primary antibodies overnight at 4°C:
Rabbit polyclonal Beclin-1 (cat. no. 3738); rabbit polyclonal LC3B
(cat. no. 2775); rabbit monoclonal p62 (cat. no. 8025); rabbit
polyclonal phosphorylated (p)-PI3K (cat. no. 4228); rabbit
polyclonal PI3K (cat. no. 4292); rabbit polyclonal p-mTOR (cat. no.
2971); rabbit monoclonal mTOR (cat. no. 2983); rabbit polyclonal
p-Akt (cat. no. 9275); rabbit polyclonal Akt (cay. no. 9272);
rabbit monoclonal p-ERK1/2 (cat. no. 4370); rabbit monclonal ERK1/2
(cat. no. 4695); rabbit monoclonal p-SAPK/JNK (cat. no.4668);
rabbit polyclonal SAPK/JNK (cat. no. 9252); rabbit monoclonal p-p38
(cat. no. 4511); rabbit polyclonal p38 (cat. no. 9212) (all Cell
Signaling Technology, Inc., Danvers, MA, USA); rabbit monoclonal
Atg-3 (cat. no. GTX63041); and rabbit monoclonal Atg-5 (cat. no.
GTX62601; both GeneTex, Inc., Irvine, CA, USA) and mouse monoclonal
β-actin (cat. no. A5441; Sigma-Aldrich. All primary antibodies were
used at 1:1,000 dilutions. The membranes were then incubated with
HRP-conjugated goat anti-rabbit (1:10,000; cat. no. AP132P) and
goat anti-mouse (1:10,000; cat. no. AP124P) secondary antibodies
(Merck Millipore) for 1 h at room temperature. The blots were
washed three times in 1X PBS-Tween 20 solution and incubated for 1
min with WesternBright Quantum enhanced chemiluminescence reagents.
The results were visualized by exposing the blots to Super RX-N
film (Fujifilm Corporation, Tokyo, Japan). The protein expression
levels were quantified using Image J software (1.42q; National
Institutes of Health, Bethesda, MD, USA, 2009).
Statistical analyses
The data are expressed as the mean ± standard
deviation, and were compared using Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using GraphPad Prism software,
version 4.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Treatment with SJKJT inhibits the
proliferation of HepG2 cells
The HepG2 cells were treated with various
concentrations of SJKJT (0, 0.5, 1, 1.5, 2, 2.5 or 5 mg/ml) for 24,
48 and 72 h and cell viability was measured using an MTT assay. The
half-maximal inhibitory concentration (IC50) was 2.91
mg/ml at 24 h, 1.64 mg/ml at 48 h and 1.26 mg/ml at 72 h, thus a
dose-dependent reduction in proliferation was observed with the
administration of SJKJT (Fig.
1).
 | Figure 1HepG2 cells (2×104
cells/well) treated with various concentrations of SJKJT (0, 0.5,
1, 1.5, 2, 2.5 or 5 mg/ml) for 24, 48 or 72 h. Cell viability was
measured using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.
The cytotoxicity of SJKJT in the HepG2 cells was dose-dependent.
The data are expressed as the mean ± standard deviation of three
experiments. *P<0.001, vs. control (0 mg/ml). SJKJT,
Sann-Joong-Kuey-Jian-Tang. |
SJKJT induces autophagy in HepG2
cells
The HepG2 cells were treated with 0.8 mg/ml SJKJT
for 6 h, stained with 1 µg/ml acridine orange, and examined
by flow cytometry. The results demonstrated that exposure to 0.8
mg/ml SJKJT for 6 h was effective at inducing autophagy in the
HepG2 cells (Fig. 2).
Subsequently, GFP-LC3 plasmids and DAPI staining were used to
observe the efficiency of autophagosome/lysosome fusion in cells
treated with or without 0.8 mg/ml SJKJT for 12 h. The numbers of
GFP-LC3B-labled puncta in the cytosol were markedly higher in the
SJKJT group compared with the control group (Fig. 3).
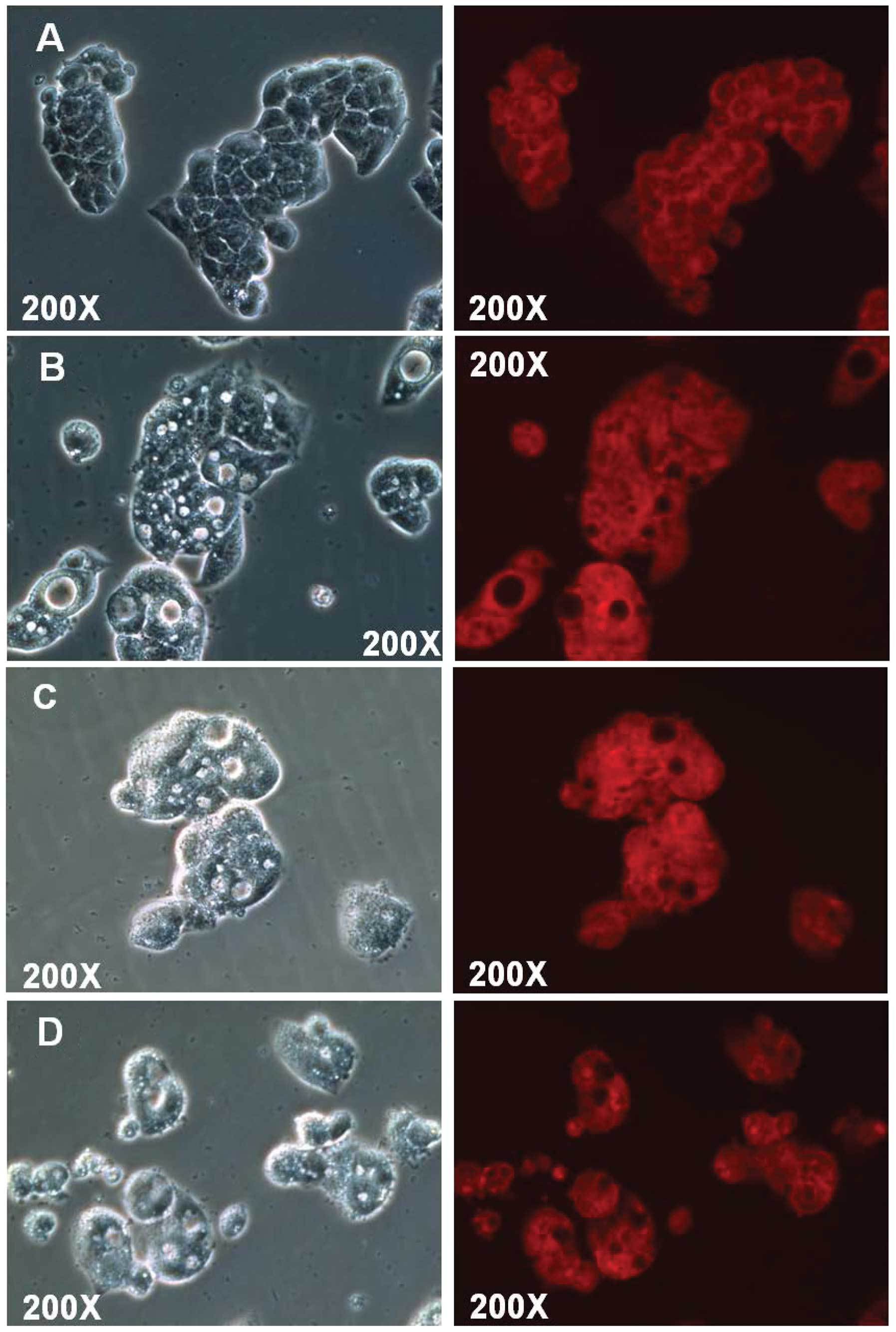
PI nuclear staining detects morphological
alterations in HepG2 cells
The HepG2 cells were treated with 0.8 mg/ml SJKJT
for different durations (0, 3, 6 and 12 h) and were then fixed with
4% paraformaldehyde. Following permeabilization of the cell
membranes, the nuclei were stained with PI (5 µg/ml) in
order to detect morphological alterations in the HepG2 cells. The
results indicated that the number of cells with a vacuolated
cytoplasm was markedly higher in the SJKJT-treated group compared
with the control group (Fig.
4).
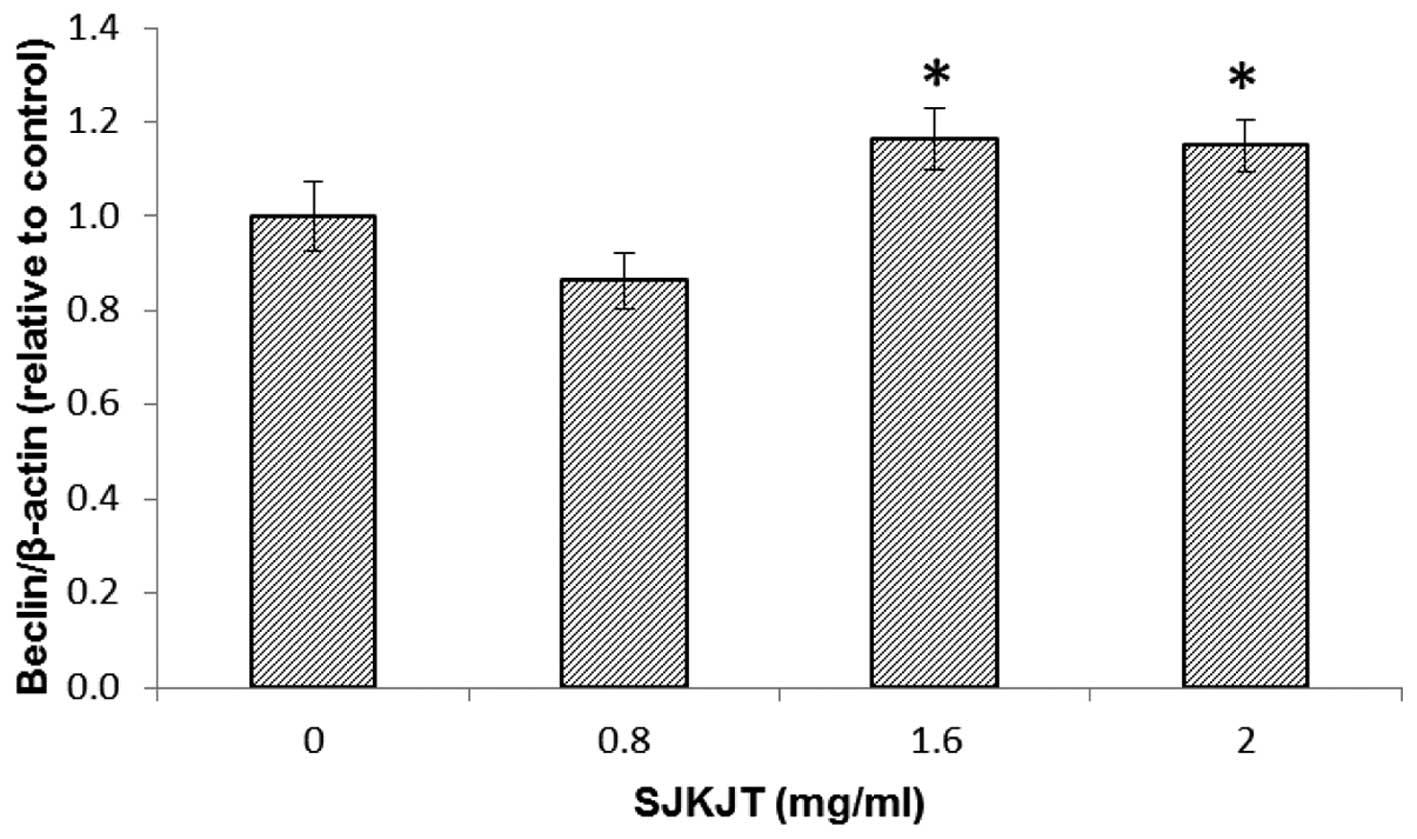
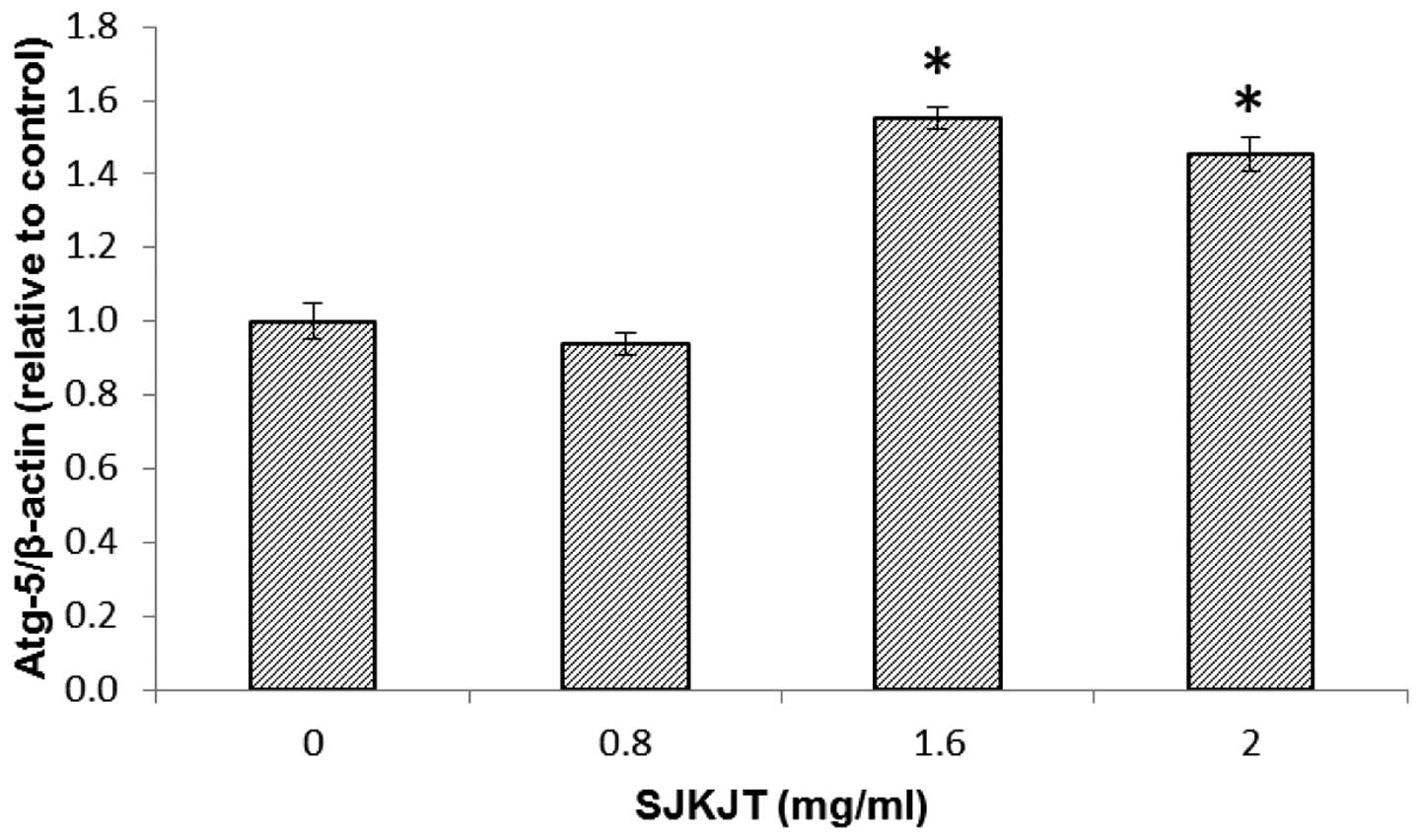
SJKJT alters the levels of
autophagy-associated proteins in HepG2 cells
Western blot analysis was performed to detect
changes in the expression levels of the Beclin, Atg-3, Atg-5,
LC3B-II and p62 autophagy-associated proteins in the HepG2 cells
following exposure to various concentrations of SJKJT (0, 0.8, 1.6
and 2 mg/ml) for 24 h. The results revealed that the expression
levels of Beclin, Atg-3, Atg-5 and LC3B-II were significantly
increased (P<0.001) and expression levels of p62 were
significantly reduced (P<0.001) following treatment with SJKJT
at 0.8 mg/ml (Fig. 5). Figs. 6 and 7 indicate the protein expression of
Beclin and Atg-5 in the HepG2 cells, respectively following
treatment with SJKJT (0, 0.8, 1.6 or 2 mg/ml). The results revealed
that the expression levels of Beclin and Atg-5 were significantly
increased (P<0.001) following treatment with SJKJT at 1.6 and 2
mg/ml.
SJKJT induces autophagy in HepG2 cells by
upregulating the PI3K/Akt/mTOR pathway
The PI3K/Akt/mTOR signaling pathway is a well-known
survival pathway, involved in the regulation of cell growth,
tumorigenesis and the cell cycle (22). Western blot analysis was performed
to measure changes in protein expression levels of p-PI3K, PI3k,
p-mTOR, mTOR, p-Akt and Akt in the HepG2 cells following treatment
with various concentrations of SJKJT (0, 0.8, 1.6 and 2 mg/ml) and
in vehicle-treated control cells after 24 h. The results
demonstrated that the expression levels of p-PI3K, p-mTOR and p-Akt
were significantly lower in the HepG2 cells than in the untreated
control cells (Fig. 8).
 | Figure 8Effects on SJKJT on the PI3K/Akt/mTOR
pathway. The cells were treated with various concentrations of
SJKJT (0, 0.8, 1.6 or 2 mg/ml) for 24 h and the expressions of
p-PI3K, PI3K, mTOR, p-mTOR, p-Akt and Akt in HepG2 cells were
analyzed by western blot analysis. β-actin served as a loading
control. SJKJT, Sann-Joong-Kuey-Jian-Tang; PI3K, phosphoinositide-3
kinase; mTOR, mammalian target of rapamysin; p-,
phosphorylated. |
SJKJT induces autophagy in HepG2 cells by
upregulating the ERK1/2 and JNK1/2MAPK pathways
MAPK signaling is important in the outcome of, and
sensitivity to, anticancer therapies (23). These activated kinases transmit
extracellular signals, which regulate cell proliferation, growth,
differentiation, migration and apoptosis (24). To examine whether SJKJT activates
the ERK1/2 and JNK1/2 MAPK pathways in HepG2 cells, western blot
analysis was performed to detect the expression levels of p-ERK1/2,
ERK1/2, p-JNK, JNK, p-p38 and p38. It was observed that, following
treatment of the HepG2 cells with various concentrations of SJKJT
(0, 0.8, 1.6 and 2 mg/ml) for 24 h, the expression levels of
p-ERK1/2 increased and that of p-p38 was reduced (Fig. 9).
Discussion
SJKJT, a traditional Chinese medicine consisting of
17 species of medicinal herbs, has been demonstrated to exhibit
antitumor and antiproliferative effects (15,17,25).
In our previous study, SJKJT was observed to induce apoptosis in
HepG2 cells by increasing the expression levels of TNF-α,
caspase-8, caspase-3 and Bax (20). In the present study, SJKJT was
observed to induce autophagy, via a mechanism involving the
PI3K/Akt/mTOR and p38 MAPK pathways, and inhibit the proliferation
of HepG2 cells in a time- and dose-dependent manner (Fig. 1).
Several chemotherapeutic agents have been
demonstrated to induce autophagy in human hepatocellular carcinoma
cells (26), including matrine and
bufalin (27). Certain anticancer
therapeutic agents have been identified to target pathways involved
in autophagy, including dihydroartemisinin, which is reported to
inhibit the nuclear translocation of nuclear factor-κB (28); thiazolidinedione, which induces
autophagy in breast cancer cells by activating peroxisome
proliferator-activated receptor-γ (29); curcumin, which suppresses the
growth of malignant gliomas by inducing autophagy through a
mechanism mediated by the Akt and ERK signaling pathways (30); and E platinum, which induces
autophagy by inhibiting the phosphorylation of mTOR in BGC-823
gastric carcinoma cells (31).
These results suggested that basal autophagy is crucial in the
suppression of spontaneous tumorigenesis.
Autophagy has been observed to serve a key function
in tumor suppression (32) and
previous studies have indicated the inhibition of autophagy as a
promising target for cancer therapy (33,34).
A number of signaling pathways are involved in autophagy, including
the class I PI3K/Akt/mTOR pathway (35). The results of the present study
indicated that SJKJT inducedcell death by inhibiting the activation
of PI3K in the HepG2 cells (Fig.
8). The inhibition of PI3K also resulted in the downregulation
of p-mTOR, an essential protein for the induction of autophagy
(Fig. 8). In addition, JNK
activation has been found to be involved in the regulation of
autophagy and apoptosis (36). The
results of the present study demonstrated that SJKJT induced
autophagy in the HepG2 cells via activation of the MAPK signaling
pathways, including the ERK1/2 pathways (Fig. 9).
The association between autophagy and apoptosis has
been widely investigated. Several pathways have been demonstrated
to be involved in the regulation of autophagy and apoptosis, and
the induction of autophagy-associated genes, including LC3-II,
which is localized to preautophagosomes and autophagosomes
(37), B-cell lymphoma (Bcl-2) and
Bcl-extra large oncogenic proteins (38) and the induction of ROS (12). In the present study, treatment of
HepG2 cells with SJKJT resulted in the formation of autophagosomes,
accumulation of AVOs (Fig. 2),
increase in cytoplasmic puncta (Fig.
3), increased protein expression of LC3-II and reduced
expression of p62 (Fig. 5),
indicating that SJKJT induced autophagy in the HepG2 cells.
In conclusion, the present study is the first, to
the best of our knowledge, to demonstrate that SJKJT may induce
autophagy and inhibit cell growth, by regulation of the
PI3K/Akt/mTORand p38 MAPK pathways in HepG2 cells.
Acknowledgments
The present study was supported by a grant from the
Changhua Christian Hospital, Changhua, Taiwan (grant no.
100-CCH-ICO-06-3).
References
|
1
|
Levine B and Klionsky DJ: Development by
self-digestion: molecular mechanisms and biological functions of
autophagy. Dev Cell. 6:463–477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kondo Y, Kanzawa T, Sawaya R and Kondo S:
The role of autophagy in cancer development and response to
therapy. Nat Rev Cancer. 5:726–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yorimitsu T and Klionsky DJ: Autophagy:
molecular machinery for self-eating. Cell Death Differ. 12(Suppl
2): 1542–1552. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kung CP, Budina A, Balaburski G,
Bergenstock MK and Murphy M: Autophagy in tumor suppression and
cancer therapy. Crit Rev Eukaryot Gene Expr. 21:71–100. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thorburn A: Apoptosis and autophagy:
Regulatory connections between two supposedly different processes.
Apoptosis. 13:1–9. 2008. View Article : Google Scholar :
|
|
6
|
Yamamoto M, Suzuki SO and Himeno M:
Resveratrol-induced autophagy in human U373 glioma cells. Oncol
Lett. 1:489–493. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shinojima N, Yokoyama T, Kondo Y and Kondo
S: Roles of the Akt/mTOR/p70S6 K and ERK1/2 signaling pathways in
curcumin-induced autophagy. Autophagy. 3:635–637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang DM, Liu JS, Deng LJ, et al:
Arenobufagin, a natural bufadienolide from toad venom, induces
apoptosis and autophagy in human hepatocellular carcinoma cells
through inhibition of PI3K/Akt/mTOR pathway. Carcinogenesis.
34:1331–1342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li H, Jin X, Zhang Z, Xing Y and Kong X:
Inhibition of autophagy enhances apoptosis induced by the
PI3K/AKT/mTor inhibitor NVP-BEZ235 in renal cell carcinoma cells.
Cell Biochem Funct. 31:427–433. 2013. View
Article : Google Scholar
|
|
10
|
Fan M and Chambers TC: Role of
mitogen-activated protein kinases in the response of tumor cells to
chemotherapy. Drug Resist Updat. 4:253–267. 2001. View Article : Google Scholar
|
|
11
|
Raman M, Chen W and Cobb MH: Differential
regulation and properties of MAPKs. Oncogene. 26:3100–3112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mei S, Gu H, Ward A, et al: p38
mitogen-activated protein kinase (MAPK) promotes cholesterol ester
accumulation in macrophages through inhibition of macroautophagy. J
Biol Chem. 287:11761–11768. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cui Q, Tashiro S, Onodera S, Minami M and
Ikejima T: Oridonin induced autophagy in human cervical carcinoma
HeLa cells through Ras, JNK, and P38 regulation. J Pharmacol Sci.
105:317–325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsu YL, Yen MH, Kuo PL, et al:
San-Zhong-Kui-Jian-Tang, a traditional Chinese medicine
prescription, inhibits the proliferation of human breast cancer
cell by blocking cell cycle progression and inducing apoptosis.
Biol Pharm Bull. 29:2388–2394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng CY, Lin YH and Su CC:
Sann-Joong-Kuey-Jian-Tang increases the protein expression of
microtubule-associated protein II light chain 3 in human colon
cancer colo 205 cells. Mol Med Rep. 2:707–711. 2009.PubMed/NCBI
|
|
16
|
Cheng CY, Lin YH and Su CC:
Sann-Joong-Kuey-Jian-Tang up-regulates the protein expression of
Fas and TNF-α in colo 205 cells in vivo and in vitro. Mol Med Rep.
3:63–67. 2010.PubMed/NCBI
|
|
17
|
Cheng CY, Lin YH and Su CC: Anti-tumor
activity of Sann-Joong-Kuey-Jian-Tang alone and in combination with
5-fluorouracil in a human colon cancer colo 205 cell xenograft
model. Mol Med Rep. 3:227–231. 2010.
|
|
18
|
Chien SY, Kuo SJ, Chen DR and Su CC:
Sann-Joong-Kuey-Jian-Tang decreases the protein expression of Mcl 1
and TCTP and increases that of TNF-α and Bax in BxPC-3 pancreatic
carcinoma cells. Int J Mol Med. 32:85–92. 2013.PubMed/NCBI
|
|
19
|
Lin SJ, Tseng HH, Wen KC and Suen TT:
Determination of gentiopicroside, mangiferin, palmatine, berberine,
baicalin, wogonin and glycyrrhizin in the traditional Chinese
medicinal preparation Sann-Joong-Kuey-Jian-Tang by high-performance
liquid chromatography. J Chromatogr A. 730:17–23. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen YL, Yan MY, Chien SY, et al:
Sann-Joong-Kuey-Jian-Tang inhibits hepatocellular carcinoma Hep-G2
cell proliferation by increasing TNF-α, Caspase-8, Caspase- 3 and
Bax but by decreasing TCTP and Mcl-1 expression in vitro. Mol Med
Rep. 7:1487–1493. 2013.PubMed/NCBI
|
|
21
|
Paglin S, Hollister T, Delohery T, et al:
A novel response of cancer cells to radiation involves autophagy
and formation of acidic vesicles. Cancer Res. 61:439–444.
2001.PubMed/NCBI
|
|
22
|
Martelli AM, Chiarini F, Evangelisti C, et
al: Two hits are better than one: targeting both
phosphatidylinositol 3-kinase and mammalian target of rapamycin as
a therapeutic strategy for acute leukemia treatment. Oncotarget.
3:371–394. 2012.PubMed/NCBI
|
|
23
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Plotnikov A, Zehorai E, Procaccia S and
Seger R: The MAPK cascades: signaling components, nuclear roles and
mechanisms of nuclear translocation. Biochim Biophys Acta.
1813:1619–1633. 2011. View Article : Google Scholar
|
|
25
|
Hsu YL, Yen MH, Kuo PL, et al:
San-Zhong-Kui-Jian-Tang, a traditional Chinese medicine
prescription, inhibits the proliferation of human breast cancer
cell by blocking cell cycle progression and inducing apoptosis.
Biol Pharm Bull. 29:2388–2394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo XL, Li D, Hu F, et al: Targeting
autophagy potentiates chemotherapy-induced apoptosis and
proliferation inhibition in hepatocarcinoma cells. Cancer Lett.
320:171–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miao Q, Bi LL, Li X, et al: Anticancer
effects of bufalin on human hepatocellular carcinoma HepG2 cells:
roles of apoptosis and autophagy. Int J Mol Sci. 14:1370–1382.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu W, Chen SS, Zhang JL, Lou XE and Zhou
HJ: Dihydroartemisinin induces autophagy by suppressing NF-κB
activation. Cancer Lett. 343:239–248. 2014. View Article : Google Scholar
|
|
29
|
Zhou J, Zhang W, Liang B, et al: PPARgamma
activation induces autophagy in breast cancer cells. Int J Biochem
Cell Biol. 41:2334–2342. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aoki H, Takada Y, Kondo S, Sawaya R,
Aggarwal BB and Kondo Y: Evidence that curcumin suppresses the
growth of malignant gliomas in vitro and in vivo through induction
of autophagy: role of Akt and extracellular signal-regulated kinase
signaling pathways. Mol Pharmacol. 72:29–39. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu C, Zou MJ, Zhao L, et al: E Platinum, a
newly synthesized platinum compound, induces autophagy via
inhibiting phosphorylation of mTOR in gastric carcinoma BGC-823
cells. Toxicol Lett. 210:78–86. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Janku F, McConkey DJ, Hong DS and Kurzrock
R: Autophagy as a target for anticancer therapy. Nat Rev Clin
Oncol. 8:528–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bincoletto C, Bechara A, Pereira GJ, et
al: Interplay between apoptosis and autophagy, a challenging
puzzle: New perspectives on antitumor chemotherapies. Chem Biol
Interact. 206:279–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meijer AJ and Codogno P: Regulation and
role of autophagy in mammalian cells. Int J Biochem Cell Biol.
36:2445–2462. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu YT, Tan HL, Huang Q, Ong CN and Shen
HM: Activation of the PI3K-Akt-mTOR signaling pathway promotes
necrotic cell death via suppression of autophagy. Autophagy.
5:824–834. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wei Y, Sinha S and Levine B: Dual role of
JNK1-mediated phosphorylation of Bcl-2 in autophagy and apoptosis
regulation. Autophagy. 4:949–951. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kabeya Y, Mizushima N, Ueno T, et al: LC3,
a mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shimizu S, Yoshida T, Tsujioka M and
Arakawa S: Autophagic cell death and cancer. Int J Mol Sci.
15:3145–3153. 2014. View Article : Google Scholar : PubMed/NCBI
|