Introduction
Cervical cancer is one of the most commonly
diagnosed types of cancer, and the rates of mortality associated
with this disease are ranked fourth highest of all malignant
neoplasms amongst women worldwide (1). It has been estimated that there are
~500,000 novel cases of cervical cancer diagnosed annually. Of
those diagnosed in the USA, approximately one-third will succumb to
the disease, and this number is significantly higher amongst women
in developing countries, where 70% of cases are diagnosed at an
advanced stage (2). Current
treatment strategies include surgery, radiotherapy and
chemotherapy. However, the majority of patients exhibit metastatic
disease at the time of diagnosis or tumor recurrence following
treatment. The survival of women with locally-advanced or
metastatic disease has remained poor throughout the last two
decades, and the long-term outlook has not significantly improved.
Therefore, the identification and development of novel agents with
high efficacy and low toxicity, that are able to overcome drug
resistance and radioresistance, and have improved pharmacologic
profiles is of significance worldwide.
Bioactive natural or synthetic chemical agents are
frequently utilized in cancer therapeutics to reverse, suppress or
prevent cancer progression. It has previously been reported that
pseudolaric acid B (PAB) (3), a
diterpene acid isolated from the bark of the root and trunk of
Pseudolarix kaempferi Gordon (Pinaceae), possesses multiple
biological activities, including anti-fungal (4), anti-fertility, cytotoxic,
antimicrobial (5), anti-tubulin
(6), anti-tumor (7) and anti-angiogenic activities
(8). Previous studies have also
revealed that PAB is able to induce growth inhibition, cell cycle
arrest and apoptosis in various types of cancer, including ovarian
cancer, lung cancer, prostate cancer and leukemia (9–11).
However, to date, the mechanisms underlying the anticancer effects
of PAB have remained to be elucidated.
The Akt signaling pathway has a critical role in the
regulation of cell metabolism, growth, apoptosis, survival and
tumorigenesis (12,13), and activation of the Akt signaling
pathway enhances the resistance of cancer cells to apoptosis and
cell cycle progression, thus contributing to the survival and
proliferation of cancer cells.
In the present study, the effects of PAB on cell
viability, cell apoptosis and the Akt signaling pathway were
examined in HeLa cervical cancer cells, and the antitumor
mechanisms of PAB were analyzed.
Materials and methods
Materials
Cervical cancer cells (HeLa) were obtained from the
Cell Bank of the Shanghai Institute of Biochemistry and Cell
Biology, Chinese Academy of Sciences (Shanghai, China). PAB was
purchased from the China Institute of Biological Products (purity
>99.0%; Beijing, China). RPMI-1640 medium, fetal bovine serum
(FBS), penicillin-streptomycin, pancreatin, glutamine and the
bicinchoninic acid (BCA) protein assay kit were purchased from
Beyotime Institute of Biotechnology (Suzhou, China). An Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) Apoptosis
kit was purchased from Roche Diagnostics (Shanghai, China). MTT and
Rhodamine 123 were purchased from Sigma-Aldrich (St. Louis, MO,
USA). A Caspase-3 Colorimetric Assay kit was obtained from Nanjing
Keygen Biotech Co. Ltd (Nanjing, China). The membranes were
incubated overnight at 4°C with the following primary antibodies
from Cell Signaling Technology, Inc. (Danvers, MA, USA): Rabbit
polyclonal human Akt (#9272S; 1:1,000), rabbit monoclonal
phosphorylated-Akt (Ser473; p-Akt; #4060S; 1:2,000), rabbit
monoclonal p-glycogen synthase kinase (GSK)-3β (Ser9; #5558S;
1:1,000), rabbit polyclonal B cell lymphoma-2 (Bcl-2; #2876S;
1:1,000), rabbit polyclonal Bcl-2-associated X protein (Bax;
#2772S; 1:1,000) and rabbit polyclonal β-actin (#4967S; 1:1,000).
The anti-rabbit horseradish peroxidase-conjugated secondary
antibodies (1:1,000) were purchased from Wuhan Boster Biotechnology
Co., Ltd. (Wuhan, China). All other chemicals were of reagent grade
and obtained from commercial sources.
Cell culture
HeLa cells were cultured in RPMI-1640 medium
supplemented with heat-inactivated 10% FBS and 1% antibiotics (100
IU penicillin and 100 µg/ml streptomycin) in a humidified
incubator at 37°C and 5% CO2. Logarithmically growing
cells were used in all the subsequent experiments.
Cell viability
An MTT assay was used to analyze the viability of
HeLa cells following PAB treatment. Briefly, HeLa cells were seeded
into 96-well plates (6.0×103 cells/well). Following
cellular adhesion, the medium was replaced with fresh medium
supplemented with various concentrations of PAB (0, 2, 4, 8 and 16
µmol/l) and further cultured for the indicated time-periods
(24, 48 and 72 h). The control culture received only the culture
medium. Following incubation with PAB or culture medium, MTT was
added at a concentration of 5 mg/ml, and the cells were incubated
for 4 h at 37°C. The medium was subsequently discarded and dimethyl
sulfoxide (Sigma-Aldrich) was added to dissolve the MTT formazan
crystals. The absorbance of each well was determined using a
microplate reader (Bio-Rad 680; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) at a wavelength of 570 nm. The wells without PAB
and the free cells (culture medium alone) were used as background.
The cell growth inhibition rate was defined as the relative
absorbance of treated versus untreated cells.
Cell apoptosis assay
In order to quantify apoptosis, cells were stained
with Annexin V and PI using an Annexin V-FITC/PI Apoptosis kit
according to the manufacturer’s instructions. Briefly, HeLa cells
were cultured in six-well plates (2×105 cells/well) and
allowed to adhere overnight. Following cellular adhesion, the cells
were treated for a further 48 h with PAB (0, 2, 4 and 8
µmol/l). Subsequently, the cells were washed twice with cold
phosphate-buffered saline (PBS; Wuhan Boster Biotechnology Co.,
Ltd.) and resuspended in Annexin V-FITC binding buffer. Annexin
V-FITC was then added and mixed gently, prior to incubation of the
cells for 15 min at room temperature in the dark. The mixture was
then centrifuged at 1,000 × g for 5 min at room temperature and
resuspended in Annexin V-FITC binding buffer. PI staining solution
was then added and combined. The cells were kept on ice in the dark
and immediately analyzed by flow cytometry (FACScan; BD
Biosciences, San Diego, CA, USA).
Caspase-3 activity determination
A Caspase-3 colorimetric assay kit was used to
evaluate Caspase-3 activity. The assay is based on the cleavage of
DEVD-pNA, the chromogenic substrate, by Caspase-3. HeLa cells were
seeded into 96-well white opaque plates (6×103
cells/well) and a corresponding optically transparent 96-well
plate, and allowed to adhere overnight, according to the
manufacturer’s instructions. Following cellular adhesion, cells
were treated with PAB (0, 2, 4 and 8 µmol/l) for 48 h. At
the end of the incubation period, cells were harvested, lysed in
chilled lysis buffer (Beyotime Institute of Biotechnology, Suzhou,
China) on ice for 10 min and centrifuged for 5 min at 1,500 × g.
Subsequently, Caspase substrate solution, containing the specific
peptide substrate, was added to the supernatant and incubated for 2
h at 37°C. Finally, Caspase-3 activity was spectrophotometrically
quantified at a wavelength of 405 nm using the UV-2250
Spectrophotometer (Shimadzu Corporation, Kyoto, Japan).
Measurement of mitochondrial membrane
potential
Rhodamine 123 staining was used to measure the
mitochondrial membrane potential. HeLa cells were cultured in
six-well plates (2×105 cells/well) and allowed to attach
overnight. The cells were then treated for a further 48 h with PAB
(0, 2, 4 and 8 µmol/l) as mentioned above. Cells were
harvested, washed twice with PBS, incubated with 1 ml Rhodamine 123
staining solution (1 µg/ml) at 37°C in the dark for 30 min,
washed twice with PBS and centrifuged at 500 × g for 10 min.
Finally, absorbance was determined using a spectrofluorometer
(F-2500; Hitachi, Ltd., Tokyo, Japan), at an excitation wavelength
of 505 nm and an emission wavelength of 534 nm.
Western blot assay
Protein expression levels were analyzed by western
blotting. Briefly, HeLa cells were seeded in six-well plates at a
density of 2.5×105 cells/well and were incubated
overnight at 37°C prior to treatment. Following treatment of the
cells with PAB (0, 2, 4 and 8 µmol/l, respectively) for 48
h, the cells were washed with PBS, lysed with lysis buffer and
incubated at 4°C for 1 h. The extracts were cleared by
centrifugation at 1,900 × g for 20 min at 4°C. The protein
concentration was determined using a BCA protein assay kit
according to the manufacturer’s instructions. Protein samples were
loaded at a concentration of 40 µg/lane, separated by 12.5%
SDS-PAGE and transferred onto a nitrocellulose membrane (EMD
Millipore, Bedford, MA, USA) using a wet transfer system. The
membrane was blocked with 10% non-fat dried milk in Tris-buffered
saline with 0.1% Tween-20 (pH 8.0; Beyotime Institute of
Biotechnology, Shanghai, China) and then incubated with primary
antibodies against Akt, p-Akt, p-GSK-3β, Bax, Bcl-2 and actin
overnight at 4°C. The membrane was subsequently incubated with the
appropriate horseradish peroxidase-conjugated secondary antibodies
at a dilution of 1:3,000. The results were detected with an
enhanced chemiluminescence system (EMD Millipore) and Hyperfilm
X-ray film (Kodak, Rochester, NY, USA).
Statistical analysis
SPSS software, version 13.0 (SPSS, Inc., Chicago,
IL, USA) was used for the statistical analysis. All continuous
values are expressed as the mean ± standard deviation. Student’s
t-test was used to compare the differences between two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
PAB decreases the viability of HeLa
cells
In order to evaluate the effects of PAB on cell
growth, HeLa cells were treated with increasing concentrations of
PAB for various time-periods, and the viability of the cells was
assessed by MTT assay. As revealed in Fig. 1, following PAB treatment, the
viability of HeLa cells was significantly decreased in a dose- and
time-dependent manner.
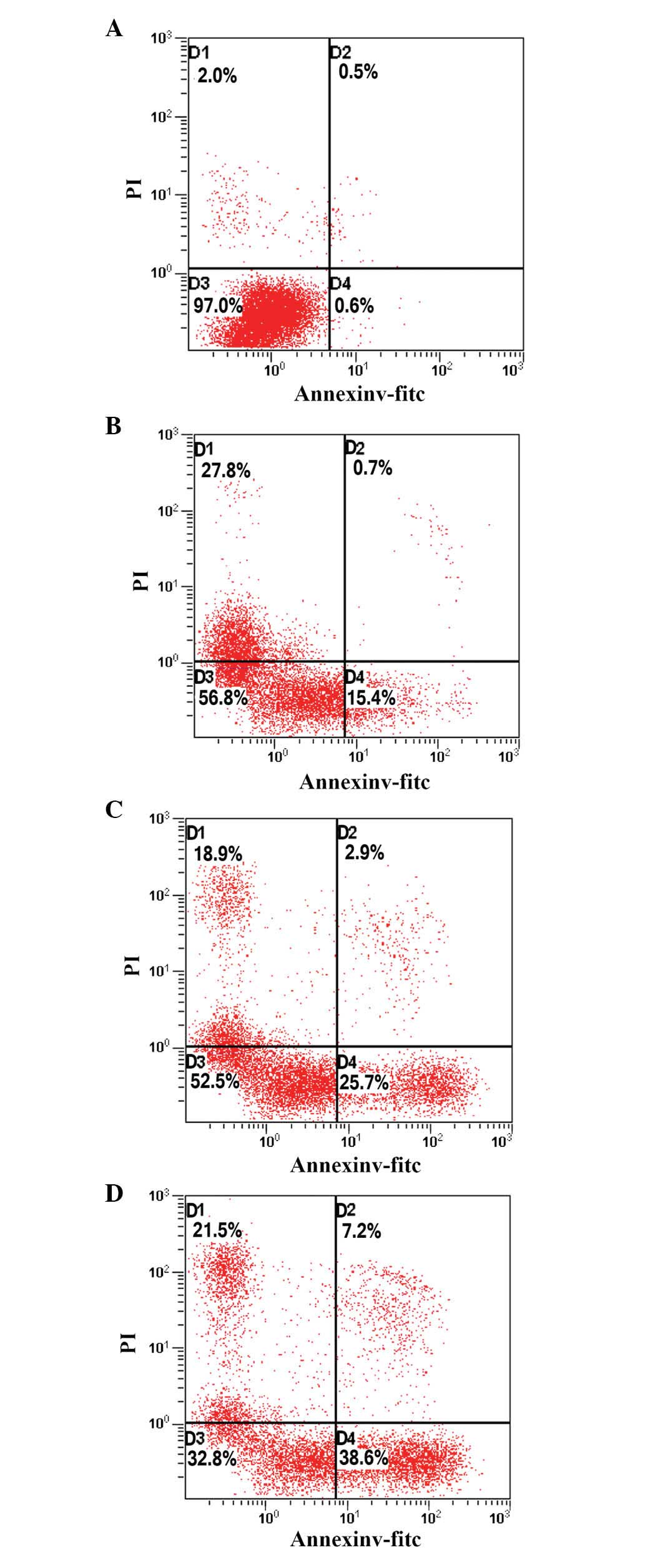
PAB induces apoptosis in HeLa cells
To determine whether the growth-inhibitory effect of
PAB was associated with the induction of apoptosis, HeLa cells
treated with PAB for 48 h were analyzed using flow cytometry. As
indicated in Fig. 2, the
proportion of apoptotic cells increased from 15.4 to 38.6%
following PAB treatment, in a dose-dependent manner, indicating
that PAB may inhibit the growth of HeLa cells via the induction of
apoptosis.
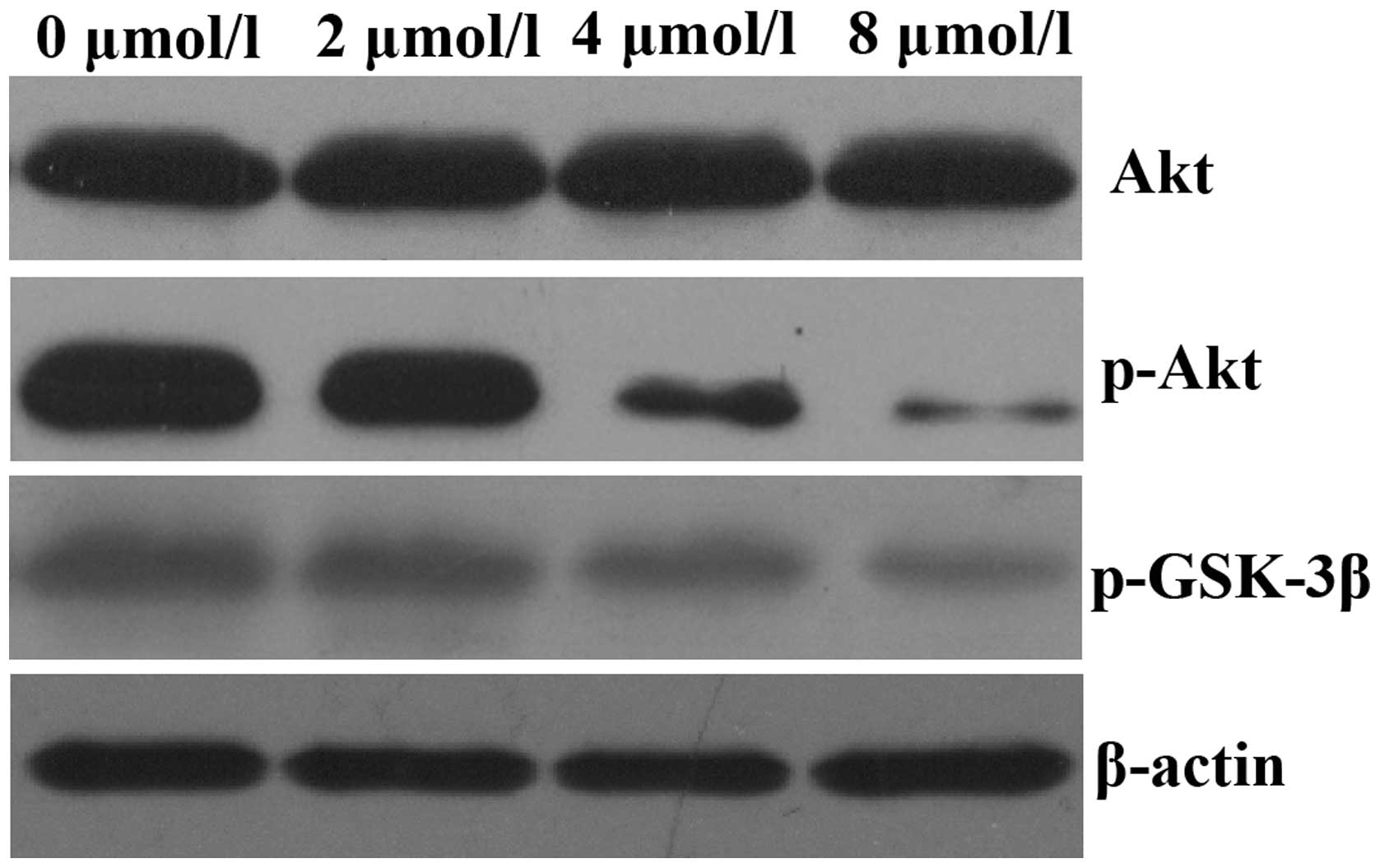
PAB inhibits Akt kinase activation in
HeLa cells
It has previously been reported that aberrant
activation of the Akt signaling pathway occurs in a number of human
malignancies (14). Therefore, the
present study aimed to assess the effects of PAB on the Akt
signaling pathway. HeLa cells were exposed to 0, 2, 4 and 8
µmol/l PAB for 48 h and the expression of p-AKT was assessed
by western blot analysis. As demonstrated in Fig. 3, treatment of HeLa cells with PAB
for 48 h decreased the expression of p-AKT in a dose-dependent
manner; however, total Akt expression levels remained unchanged.
The expression of p-GSK-3β, a downstream effector of Akt, was also
decreased.
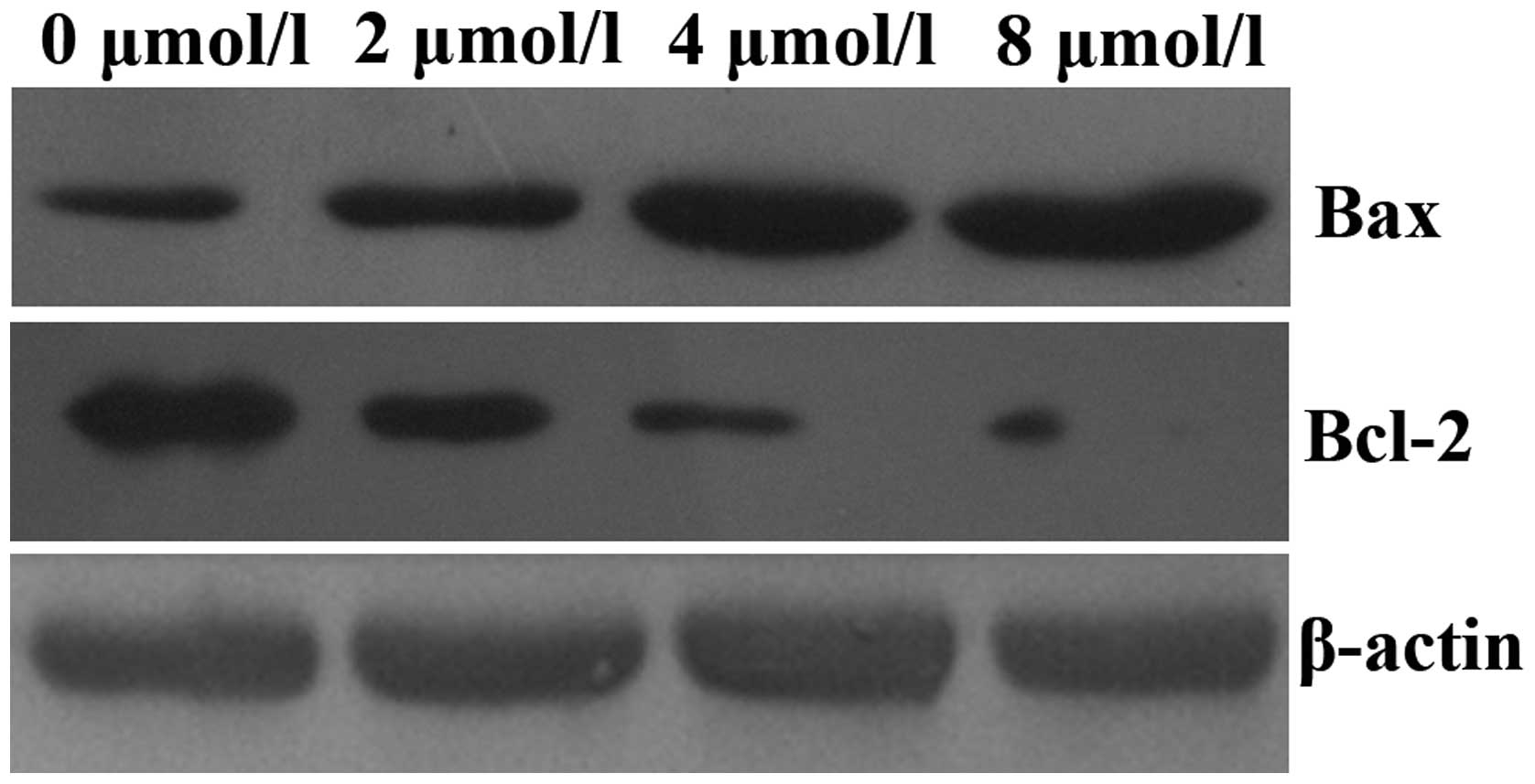
PAB upregulates caspase-3 activity and
alters the protein expression levels of Bax and Bcl-2 in HeLa
cells
In order to elucidate the mechanisms underlying the
induction of apoptosis by PAB in HeLa cells, mitochondrial features
of the intrinsic apoptotic pathway were analyzed. Proapoptotic
members of the Bcl-2 family, including Bax, are required for the
induction of mitochondrial dysfunction during apoptosis. The
protein expression levels of Bax and Bcl-2 were assessed by western
blotting. The results indicated that treatment of HeLa cells with
increasing doses of PAB for 48 h enhanced the expression of Bax and
downregulated the expression of anti-apoptotic Bcl-2 (Fig. 4). In addition, the activity of
caspase-3 was upregulated (Fig.
5).
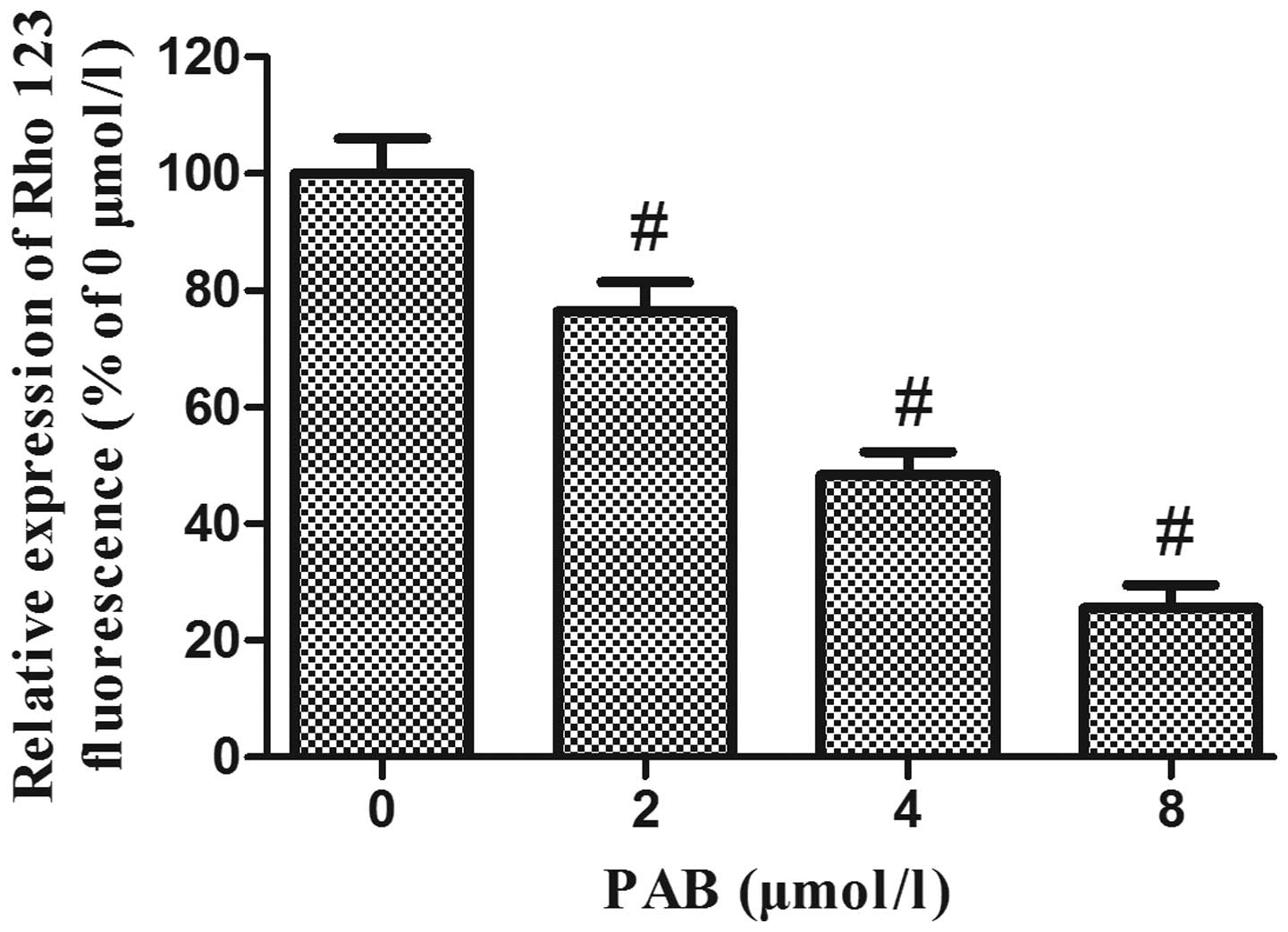
PAB decreases the mitochondrial membrane
potential in HeLa cells
A variety of studies have demonstrated that the
disruption of mitochondrial integrity is a critical stage, which
occurs in cells undergoing apoptosis, and a decrease in
mitochondrial membrane potential is associated with such
mitochondrial dysfunction (15–17).
A loss of mitochondrial membrane potential therefore has a
significant role in facilitating mitochondrial-mediated apoptosis.
As shown in Fig. 6, Rhodamine 123
fluorescence intensity was significantly decreased following
treatment of HeLa cells with increasing concentrations of PAB for
48 h, suggesting that PAB treatment of HeLa cells may induce
apoptosis via the mitochondrial apoptosis pathway.
Discussion
Cervical cancer, a common gynecological malignancy,
is one of the leading causes of cancer-associated mortality amongst
females worldwide (18). There are
>500,000 novel cases of cervical cancer diagnosed annually, as
well as >270,000 mortalities, worldwide. Greater than 85% of
these cases and mortalities occur in developing countries (1). The treatment strategy for cervical
cancer depends on the disease status at the time of diagnosis. For
patients with earlier-stage disease, standard treatments focus on
radical surgery, whereas the treatment for patients with bulky,
locally-advanced or metastatic stage disease is more often focused
on radical radiotherapy or primary concurrent chemoradiation
(19,20). Although there have been marked
improvements in the treatment of various types of cancer over the
past 30 years, the prognosis of patients with locally-advanced or
metastatic cervical cancer has remained virtually unchanged.
Therefore, the development of innovative treatment strategies is
required, in order to improve the prognosis of the disease.
PAB has been reported to induce growth inhibition,
cell cycle arrest and apoptosis in various types of cancer in
vitro and in vivo (9–11).
However, the underlying mechanisms of these effects have remained
to be elucidated. In the present study, it was demonstrated that
PAB effectively inhibited HeLa cell proliferation in a dose- and
time-dependent manner. Whether the growth-inhibitory effect of PAB
was associated with the induction of apoptosis was also examined,
and the results of Annexin V/PI staining indicated that treatment
of HeLa cells with PAB induced cell apoptosis in a
concentration-dependent manner.
It has been universally acknowledged that
alterations in the balance between cell proliferation and apoptosis
represent a significant factor in the process of carcinogenesis,
and that the induction of apoptosis is one of the most effective
approaches for tumor therapies. Apoptosis is a highly regulated
process, by which cells undergo inducible non-necrotic cellular
suicide, and involves the coordination of anti- and pro-apoptotic
proteins (21). Pro-apoptotic
members of the Bcl-2 family, including Bax, are necessary for the
induction of mitochondrial dysfunction during apoptosis. The
results of western blot analysis indicated that the expression of
pro-apoptotic factor Bax was markedly upregulated, whereas
expression of the anti-apoptotic factor Bcl-2 was significantly
reduced following treatment with PAB. Furthermore, caspase-3
activation was significantly enhanced. In addition, treatment of
HeLa cells with PAB also induced a decrease in the mitochondrial
membrane potential. These results suggested that Bcl-2 inhibited
Bax activity, which reduced the mitochondrial membrane potential,
resulting in caspase-3 upregulation and inducing cell apoptosis
(22). Together, these results
indicated that PAB was able to induce apoptosis in HeLa cells via
activation of the mitochondrial apoptosis pathway. Additionally,
the mechanisms underlying the decrease in cell proliferation and
the enhancement of apoptosis in HeLa cells induced by PAB were
investigated.
Akt (also known as protein kinase B), a subfamily of
the serine/threonine kinase family, has a vital role in cell
metabolism, growth, apoptosis and tumorigenesis (12,13).
Once activated, p-Akt, a significant regulatory factor involved in
multiple apoptotic processes, resists apoptosis through
antagonization and inactivation of various components of the
apoptotic cascade, including pro-apoptotic factor Bax (23). It has also been reported that
activated Akt may induce or suppress downstream target proteins,
including Bcl-2-associated death promoter, caspase-9, nuclear
factor-κB, GSK-3β and forkhead transcription factor Foxo1, thereby
regulating proliferation, differentiation, apoptosis and metastasis
(24–26). It has been confirmed that Akt and
p-Akt are overexpressed in a variety of human malignancies
(14,27), and aberrant activation of the Akt
pathway may also directly or indirectly interact with other
pathways (28,29). Therefore, inhibition of the Akt
pathway may be an effective approach for the prevention and
treatment of certain malignancies. In the present study, it was
demonstrated that Akt phosphorylation was decreased following PAB
treatment. Furthermore, the PAB-induced inhibition of Akt
phosphorylation was coupled with the suppression of p-GSK-3β. These
results demonstrated that inhibition of Akt phosphorylation may be
one of the underlying mechanisms of PAB-induced apoptosis.
In conclusion, the results of the present study
demonstrated that PAB inhibited cell proliferation and induced
apoptosis in HeLa cells, and that the anti-tumor effects of PAB
were associated with the inhibition of Akt phosphorylation, thus
suppressing the Akt signaling pathway. These results broaden the
potential medicinal applications of PAB, and additional studies are
required to further investigate the underlying apoptotic
mechanisms. It was hypothesized that PAB may function as a novel
therapeutic agent for the treatment of human cervical cancer, alone
or in combination with conventional therapeutics.
Acknowledgments
The present study was supported by a grant from the
Natural Science Foundation of Hubei Province (no.
2010CDB06903).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tewari KS, Sill MW, Long HJ III, et al:
Improved survival with bevacizumab in advanced cervical cancer. N
Engl J Med. 370:734–743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yan Z, Hua H, Xu Y and Samaranayake LP:
Potent antifungal activity of pure compounds from Traditional
Chinese Medicine extracts against six oral Candida species and the
synergy with fluconazole against azole-resistant Candida albicans.
Evid Based Complement Alternat Med. 2012:1065832012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang J, Yan LT, Yuan EL, et al:
Antifungal activity of compounds extracted from cortex
Pseudolaricis against Colletotrichum gloeosporioides. J Agric Food
Chem. 62:4905–4910. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chiu P, Leung LT and Ko BC: Pseudolaric
acids: isolation, bioactivity and synthetic studies. Nat Prod Rep.
27:1066–1083. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sarkar T, Nguyen TL, Su ZW, et al:
Interaction of pseudolaric acid B with the colchicine site of
tubulin. Biochem Pharmacol. 84:444–450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu B, Yue DM, Shu LH, Li NJ and Wang JH:
Pseudolaric acid B induces caspase-dependent cell death in human
ovarian cancer cells. Oncol Rep. 31:849–857. 2014.
|
|
8
|
Miao ZH, Feng JM and Ding J: Newly
discovered angiogenesis inhibitors and their mechanisms of action.
Acta Pharmacol Sin. 33:1103–1111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guan T and Yang Y: Role of pseudolaric
acid B in A549 lung cancer cell proliferation and apoptosis. Mol
Med Rep. 9:144–148. 2014.
|
|
10
|
Ma G, Chong L, Li XC, Khan IA, Walker LA
and Khan SI: Selective inhibition of human leukemia cell growth and
induction of cell cycle arrest and apoptosis by pseudolaric acid B.
J Cancer Res Clin Oncol. 136:1333–1340. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tong J, Yin S, Dong Y, Guo X, Fan L, Ye M
and Hu H: Pseudolaric acid B induces caspase-dependent apoptosis
and autophagic cell death in prostate cancer cells. Phytother Res.
27:885–891. 2013. View
Article : Google Scholar
|
|
12
|
Brazil DP, Yang ZZ and Hemmings BA:
Advances in protein kinase B signalling: AKTion on multiple fronts.
Trends Biochem Sci. 29:233–242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Manning BD and Cantley LC: AKT/PKB
signaling: navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang B, Meng N, Zhao B, Zhao J, Zhang Y,
Zhang S and Miao J: Protective effects of a synthesized
butyrolactone derivative against chloroquine-induced autophagic
vesicle accumulation and the disturbance of mitochondrial membrane
potential and Na+, K+-ATPase activity in vascular endothelial
cells. Chem Res Toxicol. 22:471–475. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Galluzzi L, Vitale I, Abrams JM, et al:
Molecular definitions of cell death subroutines: Recommendations of
the Nomenclature Committee on Cell Death 2012. Cell Death Differ.
19:107–120. 2012. View Article : Google Scholar :
|
|
17
|
Vandenabeele P, Galluzzi L, Vanden Berghe
T and Kroemer G: Molecular mechanisms of necroptosis: An ordered
cellular explosion. Nat Rev Mol Cell Biol. 11:700–714. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
19
|
Green JA, Kirwan JM, Tierney JF, Symonds
P, Fresco L, Collingwood M and Williams CJ: Survival and recurrence
after concomitant chemotherapy and radiotherapy for cancer of the
uterine cervix: a systematic review and meta-analysis. Lancet.
358:781–786. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rogers L, Siu SS, Luesley D, Bryant A and
Dickinson HO: Radiotherapy and chemoradiation after surgery for
early cervical cancer. Cochrane Database Syst Rev.
5:CD0075832012.PubMed/NCBI
|
|
21
|
Tang D, Lotze MT, Kang R and Zeh HJ:
Apoptosis promotes early tumorigenesis. Oncogene. 30:1851–1854.
2011. View Article : Google Scholar
|
|
22
|
Youle RJ and Strasser A: The BCL-2 protein
family: opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar
|
|
23
|
Coffer PJ, Jin J and Woodgett JR: Protein
kinase B (c-Akt): a multifunctional mediator of
phosphatidylinositol 3-kinase activation. Biochem J. 335:1–13.
1998.PubMed/NCBI
|
|
24
|
Wang Z, Yang J, Fisher T, Xiao H, Jiang Y
and Yang C: Akt activation is responsible for enhanced migratory
and invasive behavior of arsenic-transformed human bronchial
epithelial cells. Environ Health Perspect. 120:92–97. 2012.
View Article : Google Scholar :
|
|
25
|
Mosca E, Barcella M, Alfieri R, Bevilacqua
A, Canti G and Milanesi L: Systems biology of the metabolic network
regulated by the Akt pathway. Biotechnol Adv. 30:131–141. 2012.
View Article : Google Scholar
|
|
26
|
Wang R, Cong WH, Guo G, Li XX, Chen XL, Yu
XN and Li H: Synergism between carnosic acid and arsenic trioxide
on induction of acute myeloid leukemia cell apoptosis is associated
with modulation of PTEN/Akt signaling pathway. Chin J Integr Med.
18:934–941. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lindsley CW: The Akt/PKB family of protein
kinases: a review of small molecule inhibitors and progress towards
target validation: a 2009 update. Curr Top Med Chem. 10:458–477.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oki E, Baba H, Tokunaga E, et al: Akt
phosphorylation associates with LOH of PTEN and leads to
chemoresistance for gastric cancer. Int J Cancer. 117:376–380.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song L, Xiong H, Li J, et al: Sphingosine
kinase-1 enhances resistance to apoptosis through activation of
PI3K/Akt/NF-κB pathway in human non-small cell lung cancer. Clin
Cancer Res. 17:1839–1849. 2011. View Article : Google Scholar : PubMed/NCBI
|