Introduction
Acute lung injury (ALI) and its more severe form,
acute respiratory distress syndrome (ARDS), are critical illnesses
with high rates of mortality (1).
The pathological hallmarks of these conditions are diffuse
alveolo-capillary injury and pulmonary hyperpermeability,
associated with a dysregulated inflammatory response (2,3).
These abnormalities are associated with the clinical manifestations
of acute onset and progressive deterioration of respiratory
function (4).
Over the past decades, modulation of the
inflammatory response and visceral organ function by the autonomic
nervous system have been extensively investigated. In the lungs,
rich supplies of C-type sensory nerves, which are vagal afferent
nerves, innervate the alveolar walls, ducts and pulmonary
capillaries (5). Exposure to
exogenous irritants or endogenous inflammatory stimuli triggers
C-type nerve endings to release neuropeptides, including
α-calcitonin gene-related peptide (α-CGRP). In addition to being
released from nerve endings, α-CGRP is synthesized and secreted by
epithelial cells and macrophages under inflammatory stress
conditions in vitro (6,7).
α-CGRP is a 37 amino-acid peptide that functions via
the α-CGRP receptor, which comprises calcitonin receptor-like
receptor (CRLR) and receptor activity-modifying protein 1 (RAMP1)
(8). Initially, α-CGRP was
demonstrated to potentiate inflammation via its vasodilatory
properties (9). α-CGRP later
emerged as an anti-inflammatory agent, which was found to act via
its ability to upregulate interleukin (IL)-10 and IL-10-independent
inducible cyclic adenosine monophosphate early repressor (ICER), as
well as inhibit nuclear factor kappa B activity in vitro
(10–12). Recent studies have indicated that
α-CGRP mediates protective effects in animal models of allergic
airway inflammation, hyperoxic lung injury, lung
ischemia/reperfusion injury and lung fibrosis (13–16).
In addition, studies have demonstrated that α-CGRP prevented
lipopolysaccharide (LPS)-induced lethal endotoxemia and acute liver
injury in mice (17,18). LPS, a Gram-negative bacterial
endotoxin, is a causative agent that initiates ALI/ARDS.
Intratracheal LPS instillation has been widely accepted as an
ALI/ARDS experimental model (19).
Therefore, the present study aimed to investigate whether exogenous
α-CGRP had a protective role in LPS-induced ALI, and examined
α-CGRP and α-CGRP receptor expression during LPS-induced ALI.
Furthermore, the present study aimed to elucidate whether exogenous
α-CGRP effects were associated with ICER.
Materials and methods
Animals and experimental groups
The present study was approved by the ethics
committee of the Harbin Medical University (Harbin, China). All
procedures were performed according to the Harbin Medical
University Institutional Animal Care and Use Committee guidelines
and national guidelines for the treatment of animals. The adult
male Sprague Dawley rats weighing 240–280 g used in the present
study were obtained from Beijing Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China). The rats were maintained
under a controlled temperature (24±2ºC) on a 12 h light/12 h dark
cycle, and were given ad libitum acces to standard
laboratory chow and tap water. Rats were assigned to receive
continuous intraperitoneal infusions of α-CGRP or 0.9% normal
saline. They subsequently underwent either LPS or 0.9% normal
saline intratracheal instillation. Rats were divided into four
groups: i) Saline-saline (S-S) group, n=10; ii)
saline-α-CGRP (S-C) group, n=10; iii) LPS-saline (L-S)
group, n=14 and iv) LPS-α-CGRP (L-C) group, n=14. Four
animals from each group were used for the evaluation of pulmonary
permeability with Evans blue (EB) dye.
Experimental protocol
Rats were anesthetized by intraperitoneal injection
of pentobarbital (60 mg/kg; Shanghai Xitang Biotechnology Co.,
Ltd., Shanghai, China). Subsequently, a tracheostomy was performed,
the rats were mechanically ventilated [fraction of insprired oxygen
(FiO2), 0.6; rate, 65 breaths/min; tidal volume, 8 ml/kg
and positive end-expiratory pressure, 3 cm H2O; Harvard
Inspira ASV, Harvard Apparatus, Holliston, MA, USA] and femoral
arterial and vein cannulas were inserted. Anesthesia and muscle
relaxation were maintained by intravenous pentobarbital (30
mg/kg/h) and pancuronium bromide (0.2 mg/h; Gedeon Richter Ltd.,
Budapest, Hungary) injection. Warming pads were used to maintain
the animals’ body temperature at 36.5–37.5ºC. Electrocardiography
(ECG), arterial pressure and rectal temperatures were continuously
monitored (AcqKnowledge and MP150 version 2.7.2; BIOPAC Systems,
Inc., Goleta, CA, USA), and femoral arterial blood was used for
blood gas analysis. In the S-C and L-C groups, the animals were
intraperitoneally pre-treated with α-CGRP (C0292; Sigma-Aldrich,
St. Louis, MO, USA) at 0.4 μg/kg/min for 30 min. This dose
was determined with pilot experiments and did not result in a
significant decrease in the mean arterial pressure (MAP), while the
oxygenation index was significantly improved. In the S-S and L-S
groups, an equal volume of normal saline was infused over 30 min.
Thereafter, in the L-S and L-C groups, 0.5 mg/kg LPS
(Escherichia coli, O55:B5; Sigma-Aldrich) was administered
intratracheally in 0.3 ml normal saline. In the S-S and S-C groups,
0.3 ml normal saline was administered. MAP, temperature, heart rate
and arterial blood gases (pH, PaO2, PaCO2)
were recorded hourly throughout the experiments. Following 4 h,
animals were sacrificed by exsanguination, and the heart-lung block
was dissected from the thorax. Additionally, bronchoalveolar lavage
fluid (BALF) was obtained from the left lung, the upper right lobe
was harvested for the wet-dry (W/D) weight ratio and the middle
right lobe was harvested for histology. All remaining lung tissue
was frozen in liquid nitrogen and kept at −80ºC for further
analyses.
BALF collection and total cell
counts
The left lung was lavaged three times with 3 ml cold
normal saline through a tracheal cannula. BALF was centrifuged at
1,500 rpm for 10 min at 4ºC. The supernatant was frozen at −20ºC
for later analysis. The cells were resuspended in 1 ml 0.9% normal
saline, subjected to Wright-Giemsa staining (Nanjing Jiancheng
Biotechnology Institute, Nanjing, China) and counted using a
Neubauer chamber (Anxin Optical Instrument Manufacture Co., Ltd.,
Shanghai, China).
The upper right lobe was weighed immediately
following excision, and the dry weight was determined after heating
the lungs at 80ºC for 48 h. The W/D ratio was calculated by
dividing the wet weight by the dry weight.
Pulmonary permeability
To assess pulmonary permeability, EB extravasation
was measured as previously described (20). Animals received EB dye (30 mg/kg;
Sigma-Aldrich) intravenously 2 h prior to sacrification (n=4 per
group). The lungs were perfused free of blood with saline via a
thoracotomy, excised en bloc, blotted dry and weighed. Pulmonary
tissue was homogenized in saline (0.1 ml/100 μg tissue) and
incubated with two volumes of formamide (Merck Millipore,
Billerica, MA, USA) for dye extraction (24 h, 37ºC). The
supernatant was separated from the lung tissue by centrifugation at
5,000 × g for 30 min, and the optical supernatant density was
determined spectrophotometrically at 620 nm. The extravasated EB
concentration was calculated against a standard curve and expressed
as micrograms of EB dye per gram of lung tissue.
Histology
The middle right lobe was fixed in 4%
paraformaldehyde (Sigma-Aldrich) for 48 h, embedded in paraffin and
then cut into 5-μm sections. The sections were stained with
hematoxylin and eosin (H&E; Sigma-Aldrich) and examined by
light microscopy (Eclipse 80i; Nikon Corporation, Tokyo,
Japan).
BALF cytokine/chemokine measurements
BALF tumor necrosis factor (TNF)-α, IL-1β,
macrophage-inflammatory protein-2 (MIP2) and intracellular cell
adhesion molecule 1 (ICAM-1) levels were measured using Rat TNF-α
Boster™ ELISA kit, Rat IL-1β Boster™ ELISA kit, Rat MIP2 Boster™
ELISA kit and Rat ICAM-1 Boster™ ELISA kit, according to the
manufacturer’s instructions (Boster Systems, Inc., Wuhan,
China).
α-CGRP measurement in the lung
parenchyma
α-CGRP concentration in lung tissue from the S-S and
L-S groups was analyzed at each time-point (n=4 per point) with a
commercial ELISA kit (CGRP kit; Uscn Life Science, Inc., Wuhan,
China).
Immunohistochemistry
Immunohistochemistry was performed on
paraffin-embedded sections. Following deparaffinization and
rehydration in two changes of xylene and six different
concentrations of alcohol (Sigma-Aldrich), sections were retrieved
in a retrieval solution (9 mM sodium citrate, 2 mM citric acid) at
120ºC for 3 min and blocked with 2% bovine serum albumin (Boster
Systems, Inc.) in phosphate-buffered saline (PBS) for 30 min once
cool. Sections were immunostained with purified polyclonal goat
anti-rat CRLR (1:20 dilution; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) overnight at 4ºC. Sections were incubated with a
rabbit anti-rat secondary immunoglobulin G antibody (1:400; Boster
Systems, Inc.) for 30 min. Specific labeling was detected with
3,3′-diaminobenzidine substrate (Sigma-Aldrich). All sections were
counterstained with hematoxylin. A sample treated analogously but
with omission of the primary antibody served as a negative
control.
Quantitative reverse-transcription
polymerase chain reaction (RT-qPCR)
Lung tissue total RNA was isolated using an RNA pure
kit (BioTeke Corp., Beijing, China) according to the manufacturer’s
instructions; 1 μg total RNA was used in a 20-μl
reaction to synthesize cDNA with a High-Capacity cDNA Reverse
Transcription kit (Applied Biosystems, Life Technologies, Foster
City, CA, USA). qRT-PCR was performed using an Accupower
2xGreenstar qPCR Master mix (Bioneer, Daejeon, Korea). Rat β-actin
mRNA was used as the internal control. The primer sequences used
were as follows (5′-3′): β-actin forward, GTC AGG TCA TCA CTA TCG
GCA AT and reverse, AGA GGT CTT TAC GGA TGT CAA CGT; CRLR forward,
CAA ACA GAC TTG GGA GTC ACT AG and reverse, CTG CAA CGT CAT TCC AGC
AT; RAMP1 forward, CAC CAA ACT CGT GGC AAA C and reverse, GGG GGA
GCA CAA TGA AAG G; ICER forward, GCT CCT ACT ACT GCT TTG C and
reverse, GCT TTC GAG TTG TTG CTT CTT C; TNF-α forward, CTT CTC ATT
CCT GCT CGT GG and reverse, TCC TCC GCT TGG TGG TTT. All primers
were designed and synthesized by Settlebio, Co. (Harbin, China).
The reaction was performed as follows: Denaturation at 94ºC for 2
min, 30 cycles at 94ºC for 30 sec, 55ºC for 60 sec and 72ºC for 50
sec, followed by 10 min at 75ºC. Each sample was evaluated in
duplicate. The amplification specificity of PCR products was
confirmed by melting curve analysis, agarose gel electrophoresis
and sequencing. Relative messenger RNA (mRNA) levels were
calculated using the 2−Δ(ΔCT) method and normalized to
levels of β-actin as a reference (21).
Western blot analyses
Total protein was obtained from lung tissue
homogenates in a membrane lysis buffer containing a detergent [16
mM 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate, 20
mM Tris-HCl, pH 7.5, 1 mM Na2EDTA and 1 mM
dithiothreitol] (Sigma-Aldrich) with protease inhibitors (1 mM
benzamidine, 1 μg/ml leupeptin, 10 μg/ml soybean
trypsin inhibitor and 0.5 mM PMSF; Sigma-Aldrich), as previously
described (22). Protein
concentrations were determined using a Bradford reagent assay
(23). CRLR and RAMP1 western blot
analysis was performed using 30 μg protein. Proteins were
separated by 10–15% SDS-PAGE and transferred to polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). The
membranes were blocked for 2 h in Tris-buffered saline containing
0.1% Tween and 5% non-fat dried milk (Nanjing Jiancheng
Biotechnology Institute) and incubated with primary antibody at 4ºC
overnight. The primary antibodies used in this experiment were goat
polyclonal anti-CRLR (sc-18007; Santa Cruz Biotechnology, Inc.) at
a 1:200 dilution, rabbit polyclonal anti-RAMP1 (sc-11379; Santa
Cruz Biotechnology, Inc.) at a 1:300 dilution and mouse polyclonal
anti-β-actin (Boster) at a 1:1,000 dilution. Following washing, the
membranes were incubated with horseradish peroxidase-conjugated
enhanced chemiluminescence (ECL) rabbit anti-goat, goat anti-rabbit
or rabbit anti-mouse secondary antibodies. The ECL western blotting
system (Beyotime Institute of Biotechnology, Haimen, China) was
used for detection. Proteins were quantified with a digitized image
and normalized against β-actin.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. MAP, PaO2/FiO2 and α-CGRP analyses
between groups were assessed by repeated measures tests followed by
Tukey’s test. Differences in other data were examined using one-way
analysis of variance followed by least significant difference test
or unpaired Student’s t-test. All statistical assessments were
two-sided and P<0.05 was considered to indicate a statistically
significant difference between values. Statistical analyses were
carried out using either SAS 9.2 (SAS Institute Inc., Cary, NC,
USA) or SPSS 21.0 (IBM, Armonk, NY, USA).
Results
α-CGRP rescues
PaO2/FiO2 decrease following LPS
instillation
The MAP was decreased in the four groups during the
course of the experiment. There were no significant differences in
MAP between the four groups at any time-point (P>0.05; Fig. 1A).
 | Figure 1(A) MAP and (B)
PaO2/FiO2 was determined prior to α-CGRP and
LPS treatment and every hour following LPS treatment in the four
groups. S-S and S-C groups: Intraperitoneal infusion of saline or
α-CGRP, followed by intratracheal instillation of saline. L-S and
L-C groups: Intraperitoneal infusion of saline or α-CGRP, followed
by intratracheal instillation of LPS. Values are expressed as the
mean ± standard error of the mean (n=6 for S-S and S-C groups, n=10
for L-S and L-C groups). #P<0.05 vs. S-S group;
&P<0.05 vs. L-S group. Repeated measures tests followed by
Tukey’s test were performed. MAP, mean arterial blood pressure;
α-CGRP, α-calcitonin gene-related peptide; LPS, lipopolysaccharide;
S-S, saline-saline group; S-C, saline-α-CGRP group; L-S, LPS-saline
group; L-C, LPS-α-CGRP group; PaO2/FiO2,
ratio of oxygen tension to inspired oxygen fraction. |
Instillation of LPS resulted in a significant
decrease in PaO2/FiO2 in the L-S and L-C
groups compared to that of the S-S and S-C groups following 1 h of
injury (P<0.05 at each time-point). Animals in the L-C group had
significantly higher PaO2/FiO2 levels at 3
and 4 h after LPS instillation compared with those in the L-S group
(312.50±17.27 vs. 270.90±27.80 mmHg, P<0.05 at 3 h; 307.4±25.05
vs. 252.6±23.88 mmHg, P<0.05 at 4 h). There were no significant
differences between the two S-S and S-C groups (Fig. 1B).
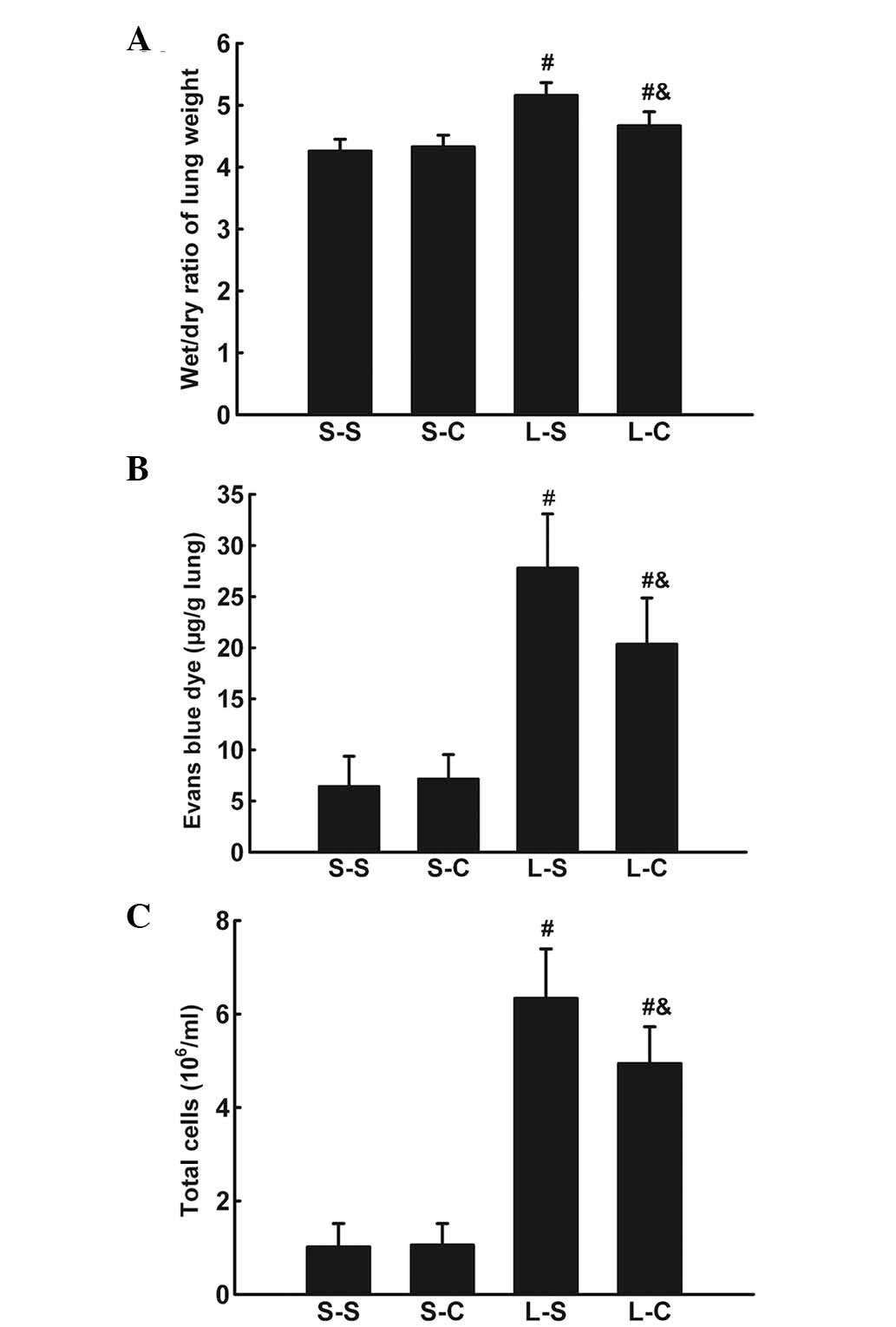
α-CGRP rescues LPS-induced W/D weight
ratio increase and EB dye accumulation
The lung W/D weight ratio was significantly
increased 4 h following LPS instillation (P<0.05 vs. S-S group)
(Fig. 2A). α-CGRP infusion
significantly attenuated the lung W/D weight ratio increase
(4.674±0.22 vs. 5.16±0.21, P<0.05).
LPS induced an increase in EB dye accumulation in
lung parenchyma (P<0.05 vs. S-S group; Fig. 2B). α-CGRP treatments resulted in a
significant decrease in EB dye accumulation (20.35±4.51 vs.
27.8±5.29 μg/g lung, P<0.05).
α-CGRP attenuates the LPS-induced
pulmonary inflammatory response
Total BALF cell numbers were significantly increased
4 h following LPS instillation (P<0.05 vs. S-S group; Fig. 2C) and L-C group cell numbers were
significantly lower than those of the L-S group
(4.94±0.78×106 vs. 6.34±1.06×106,
P<0.05).
The TNF-α, IL-1β, IL-6, MIP-2, and ICAM-1 protein
levels in BALF were significantly increased in the L-S and L-C
groups compared with those of the S-S and S-C groups. The L-C group
had significantly lower TNF-α, IL-1β, MIP-2, and ICAM-1 levels in
BALF compared with those of the L-S group (P<0.05; Fig. 3A–D).
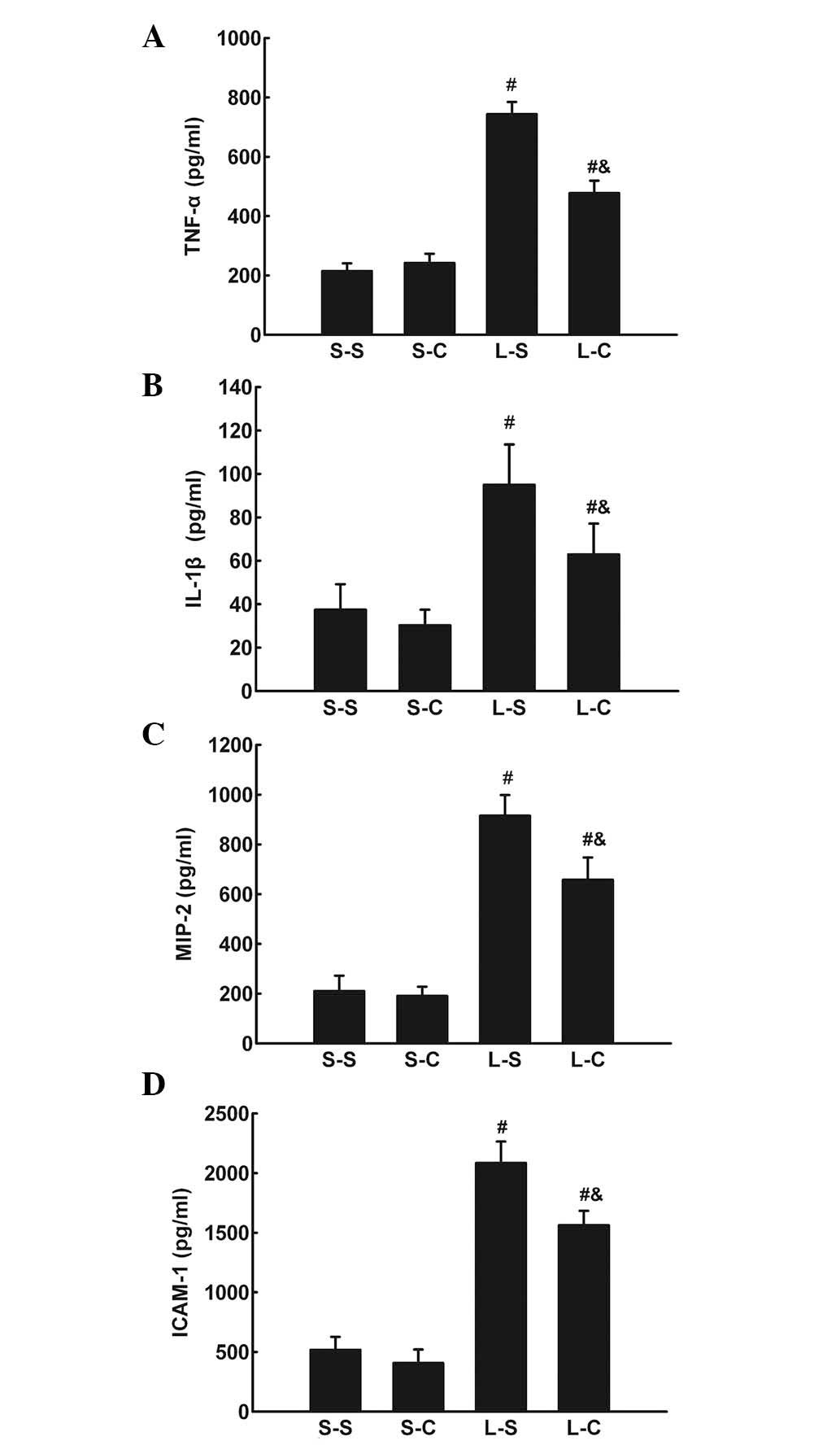 | Figure 3(A) TNF-α, (B) IL-1β, (C) MIP-2 and
(D) ICAM-1 in bronchoalveolar lavage fluid following 4 h in the
S-S, S-C, L-S and L-C groups. Values are expressed as the mean ±
standard error of the mean (n=6 for S-S and S-C groups, n=10 for
L-S and L-C groups). #P<0.05 vs. S-S group;
&P<0.05 vs. L-S group. One-way analysis of
variance followed by least significant difference test were used.
TNF-α, tumor necrosis factor-alpha; IL-1β, interleukin-1 beta;
MIP-2, macrophage inflammatory protein-2; ICAM-1, intercellular
adhesion molecule-1; S-S, saline-saline group; S-C, saline-α-CGRP
group; L-S, LPS-saline group; L-C, LPS-α-CGRP group; α-CGRP,
α-calcitonin gene-related peptide. |
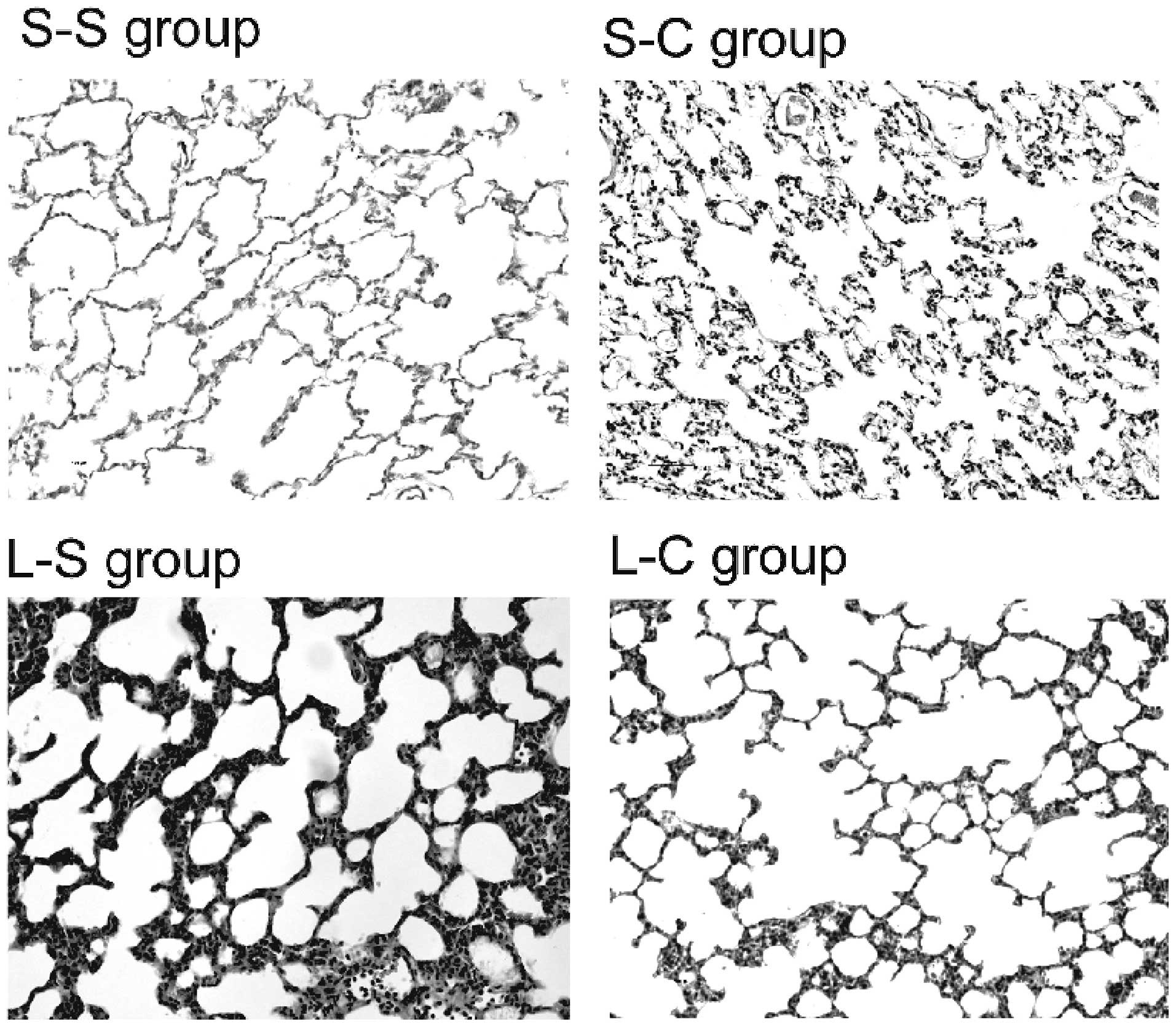
α-CGRP abrogates LPS-induced lung
injury
Histological analysis demonstrated that lungs in the
S-S and S-C groups exhibited no morphological injuries (Fig. 4). A representative lung tissue
section from the L-S group demonstrated increased wall thickness
and marked inflammatory cell infiltration compared with that of the
S-S group. Furthermore, α-CGRP pre-treatment decreased wall
thickness and inflammatory cell infiltration.
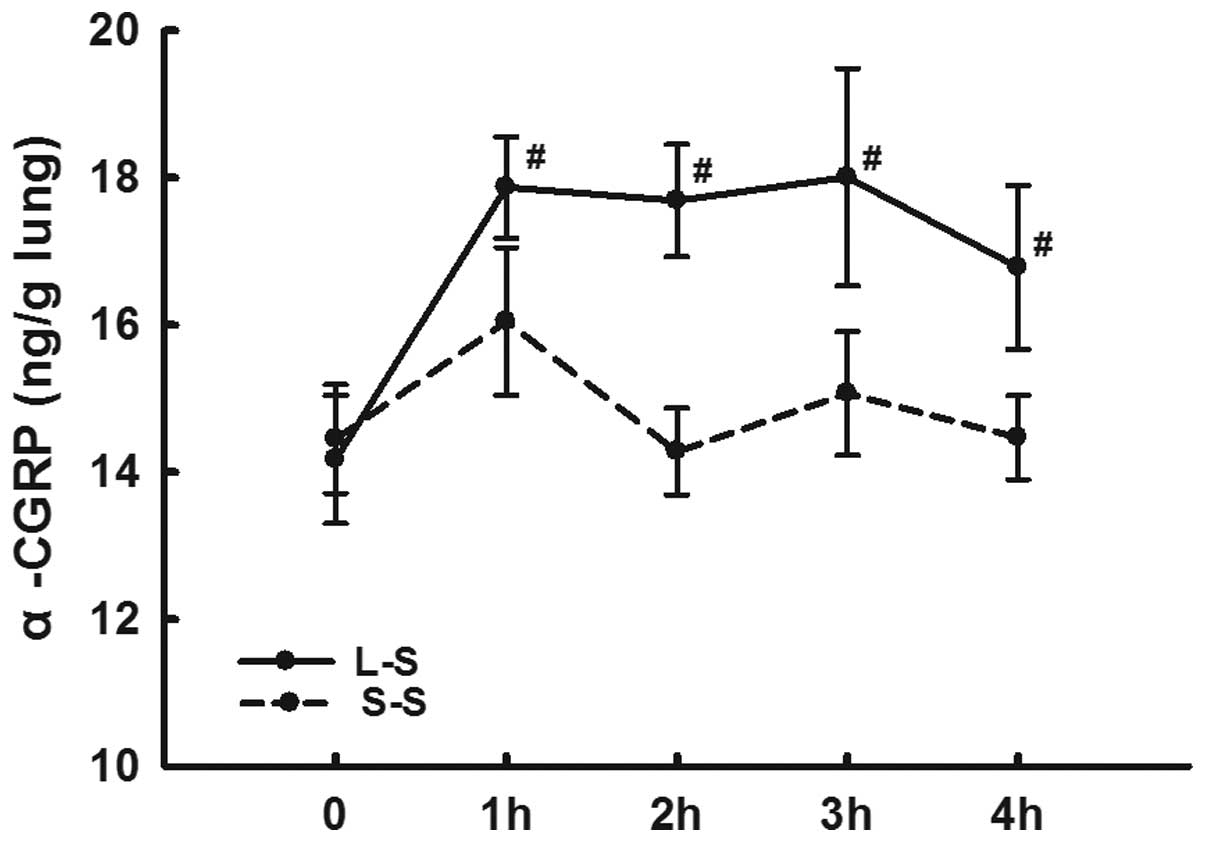
LPS instillation increases α-CGRP
expression levels and decreases α-CGRP receptor expression
levels
The lung homogenate α-CGRP levels in the L-S group
were significantly higher than those in the S-S group from 1 h
following LPS administration at each time-point (P<0.05;
Fig 5).
Immunohistochemistry detected CRLR expression in
pulmonary microvascular endothelial cells and alveolar macrophages
in LPS-induced lung injury (Fig. 6Aa
and Ab). RT-qPCR and western blot analysis revealed lower mRNA
and protein expression levels of CRLR and RAMP1 in the L-S group
compared with those in the S-S group (P<0.05; Fig. 6B and C; Fig. 7).
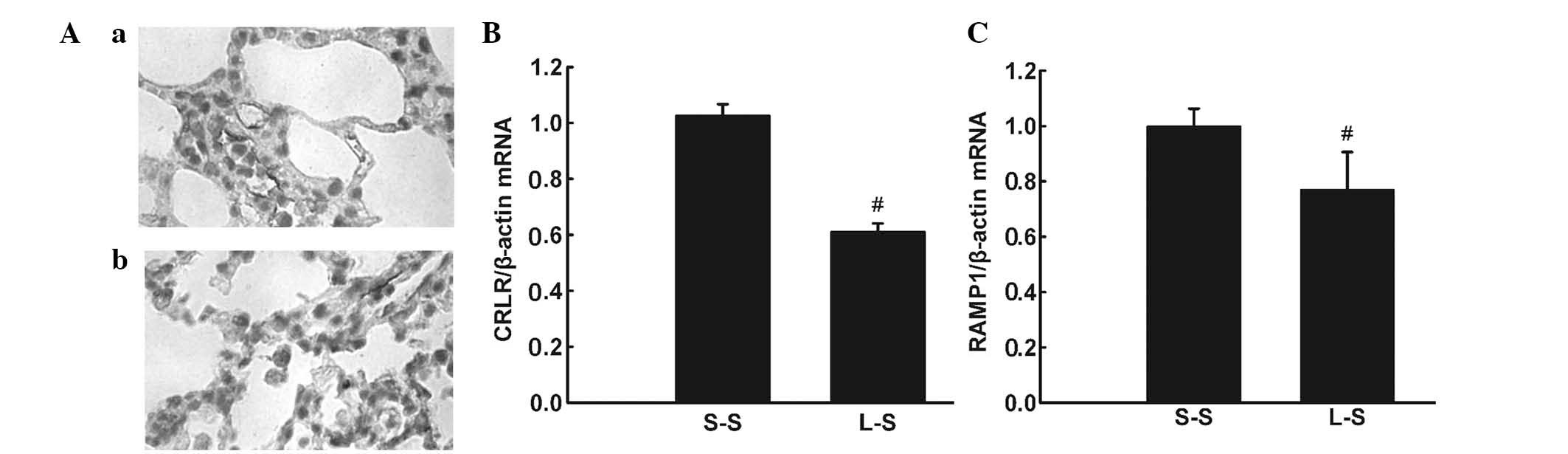 | Figure 6(A) Representative
immunohistochemical images showing CRLR-positive cells in the lung
of the L-S group. (a and b) CRLR-positive endothelial cells and
alveolar macrophage cells (original magnification, ×1,000). (B)
CRLR and (C) RAMP1 were quantified by RT-qPCR in lung tissue 4 h
following treatment in the S-S and L-S groups. For RT-qPCR
analysis, β-actin mRNA was used as an internal control. X-fold
induction was calculated referring to the mRNA levels in rats of
the S-S group. Values are expressed as the mean ± standard error of
the mean (n=6 for S-S group, n=10 for L-S group).
#P<0.05 vs. S-S group. Significant differences
between the two groups were evaluated using unpaired Student’s
t-test. S-S, saline-saline group; L-S, LPS-saline group; CRLR,
calcitonin receptor-like receptor; RAMP1, receptor
activity-modifying protein 1; RT-qPCR, quantitative reverse
transcription polymerase chain reaction; mRNA, messenger RNA. |
α-CGRP increases ICER mRNA expression and
decreases TNF-α mRNA expression following LPS instillation
RT-qPCR assays demonstrated that ICER mRNA
expression levels were significantly higher in the L-C group at two
and 4 h than those in the L-S group (P<0.05; Fig. 8A). TNF-α mRNA expression was
significantly lower in the L-C group compared with those in the L-S
group at two and 4 h (P<0.05; Fig.
8B).
Discussion
In the present study, it was demonstrated that
intratracheal instillation of LPS induced ALI. α-CGRP infusion
attenuated LPS-induced ALI as indicated by improved oxygenation,
ameliorated histological changes, reduced EB leakage as well as
decreased W/D ratio, total cell count and cytokine levels in BALF.
The role of exogenous α-CGRP in LPS-induced ALI was associated with
upregulation of the transcription factor ICER, which inhibits the
transcription of TNF-α.
α-CGRP is a vasodilatory neuropeptide that exerts
broad regulatory effects in physiological and pathophysiological
situations. Studies have demonstrated beneficial effects of α-CGRP
in cardiovascular diseases (22).
In the respiratory system, α-CGRP mediates protective effects in
allergic airway inflammation, hyperoxic lung injury, lung
ischemia/reperfusion injury and lung fibrosis (13–16).
These observations are consistent with the results of the present
study, in which exogenous α-CGRP attenuated LPS-induced ALI.
In the present study, an increasing amount of α-CGRP
was detected in lung homogenates from 1 h following LPS
instillation. α-CGRP receptor expression by endothelial cells and
alveolar macrophages provided the basis for the protective effects
of α-CGRP supplementation. The increasing amount of α-CGRP observed
is additionally supported by a previous study, in which
intravenously administered LPS resulted in increased α-CGRP in
lungs (24).
The effects of α-CGRP on blood pressure were
evaluated. A previous study demonstrated that systemic
administration of α-CGRP (0.38–38 μg/kg) decreased MAP in a
dose-dependent manner for 30 min in conscious rats (25). According to this observation and
the pilot study preceding the present study, it was demonstrated
that infusion of 0.4 μg/kg/min α-CGRP for 30 min did not
significantly decrease MAP during LPS-induced ALI. Furthermore,
oxygenation capability, expressed as
PaO2/FiO2, was significantly improved with
exogenous α-CGRP administration in LPS-induced ALI. It was
hypothesized that this effect was due to an attenuation of lung
damage following LPS endotoxemia. Although there was no significant
MAP decrease following α-CGRP infusion, the possibility of
deteriorated PaO2/FiO2 due to hypoxic
pulmonary vasoconstriction inhibition cannot be excluded. Further
studies are required in order to clarify the hemodynamic effects of
α-CGRP on LPS-induced ALI.
The inflammatory protein influx and capillary
leakage were evaluated using the extravascular EB dye assay and W/D
ratio. Lung hyperpermeability has been suggested to be a major
characteristic of ALI (26).
α-CGRP pretreatment was demonstrated to significantly decrease
protein influx and capillary leakage. Lung hyperpermeability
reduction protects against LPS-induced ALI (27). It was hypothesized that this effect
of α-CGRP may be associated with endothelial barrier function
stabilization, and potentially with adrenomedullin and intermedin,
which are members of the calcitonin/α-CGRP peptide family (28–30).
Various inflammatory mediators, including the
cytokines TNF-α and IL-1β, the chemokine MIP2, and ICAM-1, have
been demonstrated to cause lung damage in LPS-induced ALI (31–33).
α-CGRP administration attenuated the production of these
inflammatory mediators during ALI. These results are supported by
other studies that have demonstrated various anti-inflammatory
effects of α-CGRP in in vivo and in vitro models. In
a murine endotoxemia model, α-CGRP markedly attenuated serum TNF-α
levels (17). MIP-2, which
possesses neutrophil chemotactic activity, is mainly released from
macrophages (34). Ma et al
(7) demonstrated that exogenous
α-CGRP was able to stimulate, inhibit or have no effect on
inflammatory mediator release in RAW macrophages in a
dose-dependent manner. The results of the present study suggested
that 0.4 μg/kg/min α-CGRP may inhibit macrophage
inflammatory activity in LPS-induced ALI. ICAM-1 mediates adhesion
between pulmonary endothelial cells and neutrophils (35). Therefore, a decrease in ICAM-1
expression by exogenous α-CGRP may indicate protective effects on
the pulmonary endothelium. This hypothesis is similar to the
results of a study by Huang et al (36), which demonstrated that α-CGRP
inhibited LPS-induced chemokine production in human dermal
microvascular endothelial cells. Consistent with lower MIP-2 and
ICAM-1 expression levels, a reduction in the number of inflammatory
cells in BALF was also observed.
Immunohistochemical analysis also demonstrated that
CRLR was mainly expressed by pulmonary endothelial cells and
alveolar macrophages, which was consistent with the results of a
previous human study (37).
Expression of CRLR and RAMP1 was significantly reduced in
LPS-induced ALI. The downregulation of CRLR and RAMP1 may indicate
reduced efficacy of endogenous α-CGRP signalling in LPS-induced
lung injury, and administration of exogenous α-CGRP potentially
amplifies endogenous α-CGRP effects in LPS-induced ALI. α-CGRP
receptor activation increases cellular cAMP levels, leading to the
activation of protein kinse A (PKA) (38). PKA subunits are able to activate
transcription factor cyclic adenosine monophosphate response
element-binding protein, which may promote the transcription of
ICER (11,39). It has been shown that α-CGRP
inhibits the functions of various immune cells and dampens
inflammation by ICER, which directly represses TNF-α transcription
through inhibiting the TNF-α promoter (11,40).
Therefore, the expression of ICER and TNF-α was evaluated. ICER
expression was upregulated and TNF-α mRNA expression levels were
downregulated by α-CGRP during ALI. Therefore, it was hypothesized
that α-CGRP attenuated ALI partially via ICER induction.
Aoki-Nagase et al (41) observed that endogenous α-CGRP
expression partially mediated acid-induced ALI in a 2-h mouse model
that differed from the model used in the present study. It was
suggested that this result was observed because HCl instillation
can directly stimulate the C sensory nerve and cause a large,
simultaneous release of α-CGRP and substance P (42). A biphasic injury (with 1- and 4-h
peaks in acid-induced ALI) also indicated that the initial injury
may be caused by neurogenic inflammation (43). In LPS-induced ALI, however, LPS
does not directly stimulate the C sensory nerve. Instead, LPS binds
to Toll-like receptor 4 on parenchyma pulmonary cells to induce
inflammation and lung damage, which stimulates C-type sensory
neurons to release α-CGRP (44).
The enhanced α-CGRP release under inflammatory stress exerts a
negative feedback function by inhibiting local immunoreactions
(45).
There were several limitations of the present study.
α-CGRP effects were only evaluated up to 4 h following LPS
administration; however, injury usually peaks at ~24–48 h post LPS
administration. The pulmonary hemodynamic effects of α-CGRP in
LPS-induced ALI have also remained elusive. Furthermore, only the
involvement of ICER was evaluated; whether other mechanisms are
responsible for the protective effects of α-CGRP remains to be
elucidated.
In conclusion, the present study indicated that
exogenous α-CGRP infusion improved oxygenation and reduced lung
damage during LPS-induced ALI. The protective effects of α-CGRP
observed were associated with ICER upregulation.
Acknowledgments
The present study was supported by the Major Basic
Research Project of the Second Affiliated Hospital of Harbin
Medical University (Harbin, China; no. 100286).
References
|
1
|
Zambon M and Vincent JL: Mortality rates
for patients with acute lung injury/ARDS have decreased over time.
Chest. 133:1120–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bachofen M and Weibel ER: Structural
alterations of lung parenchyma in the adult respiratory distress
syndrome. Clin Chest Med. 3:35–56. 1982.PubMed/NCBI
|
|
3
|
Ware LB and Matthay MA: The acute
respiratory distress syndrome. N Engl J Med. 342:1334–1349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ware LB: Pathophysiology of acute lung
injury and the acute respiratory distress syndrome. Semin Respir
Crit Care Med. 27:337–349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coleridge JC and Coleridge HM: Afferent
vagal C fibre innervation of the lungs and airways and its
functional significance. Rev Physiol Biochem Pharmacol. 99:1–110.
1984.PubMed/NCBI
|
|
6
|
Wang W, Jia L, Wang T, Sun W, Wu S and
Wang X: Endogenous calcitonin gene-related peptide protects human
alveolar epithelial cells through protein kinase Cepsilon and heat
shock protein. J Biol Chem. 280:20325–20330. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma W, Dumont Y, Vercauteren F and Quirion
R: Lipopolysaccharide induces calcitonin gene-related peptide in
the RAW264.7 macrophage cell line. Immunology. 130:399–409. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McLatchie LM, Fraser NJ, Main MJ, et al:
RAMPs regulate the transport and ligand specificity of the
calcitonin-receptor-like receptor. Nature. 393:333–339. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Buckley TL, Brain SD, Rampart M and
Williams TJ: Time-dependent synergistic interactions between the
vasodilator neuropeptide, calcitonin gene-related peptide (CGRP)
and mediators of inflammation. Br J Pharmacol. 103:1515–1519. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fox FE, Kubin M, Cassin M, et al:
Calcitonin gene-related peptide inhibits proliferation and antigen
presentation by human peripheral blood mononuclear cells: effects
on B7, interleukin 10, and interleukin 12. J Invest Dermatol.
108:43–48. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Harzenetter MD, Novotny AR, Gais P, Molina
CA, Altmayr F and Holzmann B: Negative regulation of TLR responses
by the neuropeptide CGRP is mediated by the transcriptional
repressor ICER. J Immunol. 179:607–615. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ding W, Wagner JA and Granstein RD: CGRP,
PACAP, and VIP modulate Langerhans cell function by inhibiting
NF-kappaB activation. J Invest Dermatol. 127:2357–2367. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rochlitzer S, Veres TZ, Kühne K, et al:
The neuropeptide calcitonin gene-related peptide affects allergic
airway inflammation by modulating dendritic cell function. Clin Exp
Allergy. 41:1609–1621. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dang H, Yang L, Wang S, Fang F and Xu F:
Calcitonin gene-related peptide ameliorates hyperoxia-induced lung
injury in neonatal rats. Tohoku J Exp Med. 227:129–138. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ji P, Jiang T, Wang M, Wang R, Zhang L and
Li Y: Denervation of capsaicin-sensitive C fibers increases
pulmonary inflammation induced by ischemia-reperfusion in rabbits.
J Surg Res. 184:782–789. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hartopo AB, Emoto N, Vignon-Zellweger N,
et al: Endothelin-converting enzyme-1 gene ablation attenuates
pulmonary fibrosis via CGRP-cAMP/EPAC1 pathway. Am J Respir Cell
Mol Biol. 48:465–476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gomes RN, Castro-Faria-Neto HC, Bozza PT,
et al: Calcitonin gene-related peptide inhibits local acute
inflammation and protects mice against lethal endotoxemia. Shock.
24:590–594. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kroeger I, Erhardt A, Abt D, et al: The
neuropeptide calcitonin gene-related peptide (CGRP) prevents
inflammatory liver injury in mice. J Hepatol. 51:342–353. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen H, Bai C and Wang X: The value of the
lipopolysaccharide-induced acute lung injury model in respiratory
medicine. Expert Rev Respir Med. 4:773–783. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moitra J, Sammani S and Garcia JG:
Re-evaluation of Evans Blue dye as a marker of albumin clearance in
murine models of acute lung injury. Transl Res. 150:253–265. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Cueille C, Pidoux E, de Vernejoul MC,
Ventura-Clapier R and Garel JM: Increased myocardial expression of
RAMP1 and RAMP3 in rats with chronic heart failure. Biochem Biophys
Res Commun. 294:340–346. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan Y, Wang X, Zeng Q, Wu HM, Qi YF and
Tang CS: Effects of continuous intermedin infusion on blood
pressure and hemodynamic function in spontaneously hypertensive
rats. J Geriatr Cardiol. 9:17–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okajima K, Isobe H, Uchiba M and Harada N:
Role of sensory neuron in reduction of endotoxin-induced
hypotension in rats. Crit Care Med. 33:847–854. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sirén AL and Feuerstein G: Cardiovascular
effects of rat calcitonin gene-related peptide in the conscious
rat. J Pharmacol Exp Ther. 247:69–78. 1988.PubMed/NCBI
|
|
26
|
Matute-Bello G, Downey G, Moore BB, et al
Acute Lung Injury in Animals Study Group: An official American
Thoracic Society workshop report: features and measurements of
experimental acute lung injury in animals. Am J Respir Cell Mol
Biol. 44:725–738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peng X, Hassoun PM, Sammani S, et al:
Protective effects of sphingosine 1-phosphate in murine
endotoxin-induced inflammatory lung injury. Am J Respir Crit Care
Med. 169:1245–1251. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hippenstiel S, Witzenrath M, Schmeck B, et
al: Adrenomedullin reduces endothelial hyperpermeability. Circ Res.
91:618–625. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aslam M, Pfeil U, Gündüz D, et al:
Intermedin (adreno-medullin2) stabilizes the endothelial barrier
and antagonizes thrombin-induced barrier failure in endothelial
cell monolayers. Br J Pharmacol. 165:208–222. 2012. View Article : Google Scholar :
|
|
30
|
Ogoshi M, Inoue K, Naruse K and Takei Y:
Evolutionary history of the calcitonin gene-related peptide family
in vertebrates revealed by comparative genomic analyses. Peptides.
27:3154–3164. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuwano K and Hara N: Signal transduction
pathways of apoptosis and inflammation induced by the tumor
necrosis factor receptor family. Am J Respir Cell Mol Biol.
22:147–149. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ganter MT, Roux J, Miyazawa B, et al:
Interleukin-1beta causes acute lung injury via alphavbeta5 and
alphavbeta6 integrin-dependent mechanisms. Circ Res. 102:804–812.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamasawa H, Ishii Y and Kitamura S:
Cytokine-induced neutrophil chemoattractant in a rat model of
lipopolysaccharide-induced acute lung injury. Inflammation.
23:263–274. 1999.PubMed/NCBI
|
|
34
|
Wolpe SD, Sherry B, Juers D, Davatelis G,
Yurt RW and Cerami A: Identification and characterization of
macrophage inflammatory protein 2. Proc Natl Acad Sci USA.
86:612–616. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Doerschuk CM, Quinlan WM, Doyle NA, et al:
The role of P-selectin and ICAM-1 in acute lung injury as
determined using blocking antibodies and mutant mice. J Immunol.
157:4609–4614. 1996.PubMed/NCBI
|
|
36
|
Huang J, Stohl LL, Zhou X, Ding W and
Granstein RD: Calcitonin gene-related peptide inhibits chemokine
production by human dermal microvascular endothelial cells. Brain
Behav Immun. 25:787–799. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hagner S, Stahl U, Knoblauch B, McGregor
GP and Lang RE: Calcitonin receptor-like receptor: identification
and distribution in human peripheral tissues. Cell Tissue Res.
310:41–50. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Walker CS, Conner AC, Poyner DR and Hay
DL: Regulation of signal transduction by calcitonin gene-related
peptide receptors. Trends Pharmacol Sci. 31:476–483. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mayr B and Montminy M: Transcriptional
regulation by the phosphorylation-dependent factor CREB. Nat Rev
Mol Cell Biol. 2:599–609. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Altmayr F, Jusek G and Holzmann B: The
neuropeptide calcitonin gene-related peptide causes repression of
tumor necrosis factor-alpha transcription and suppression of ATF-2
promoter recruitment in Toll-like receptor-stimulated dendritic
cells. J Biol Chem. 285:3525–3531. 2010. View Article : Google Scholar :
|
|
41
|
Aoki-Nagase T, Nagase T, Oh-Hashi Y, et
al: Calcitonin gene-related peptide mediates acid-induced lung
injury in mice. Respirology. 12:807–813. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ho CY, Gu Q, Lin YS and Lee LY:
Sensitivity of vagal afferent endings to chemical irritants in the
rat lung. Respir Physiol. 127:113–124. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kennedy TP, Johnson KJ, Kunkel RG, Ward
PA, Knight PR and Finch JS: Acute acid aspiration lung injury in
the rat: biphasic pathogenesis. Anesth Analg. 69:87–92. 1989.
View Article : Google Scholar
|
|
44
|
Reiss LK, Uhlig U and Uhlig S: Models and
mechanisms of acute lung injury caused by direct insults. Eur J
Cell Biol. 91:590–601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dakhama A, Larsen GL and Gelfand EW:
Calcitonin gene-related peptide: role in airway homeostasis. Curr
Opin Pharmacol. 4:215–220. 2004. View Article : Google Scholar : PubMed/NCBI
|