Introduction
Lung carcinoma (LC), is the most common type of
cancer and the leading cause of cancer-related mortality worldwide
(1). Currently available
treatments are ineffective in 60–70% of patients with LC (1,2). LC
is remarkably resistant to chemotherapy and radiotherapy (2). Therefore, novel therapeutic
approaches to LC are required.
Adenoviral vectors may be produced in high titers,
generally do not integrate into DNA and exhibit low pathogenicity
in humans. Therefore, they are commonly used in cancer gene therapy
(3,4). However, adenoviral vectors are rarely
capable of successful amplification in tumor cells, which limits
their therapeutic efficacy in cancer (4). Conditionally replicating adenovirus
(CRAd; oncolytic adenovirus) is currently used for the treatment of
solid tumors (5). Oncolytic
therapy uses viruses that are tumor-specific and cause the
proliferation of progeny viruses in neighboring tumor cells,
eventually resulting in lysis of these cells.
CRAds are currently separated into three categories:
A tumor-specific adenoviral vector that uses tumor-specific
promoters (such as telomerase) to express the early region 1A (E1A)
gene, which is amplified in tumor cells (6,7);
adendovirus 5 (Ad5) Δ-24 vector, which exhibits a 24 base pair (bp)
deletion in the Ad5 E1A conserved domain 2 (CR2) region and is
replicated in tumor cells exhibiting retinoblastoma protein
dysfunctions (8,9); and ONYX-015, which lacks the E1B 55
kilodalton (kDa) gene, dl1520, and is replicated in p53-deficient
tumor cells (10,11).
ONYX-015 exhibited positive experimental results,
with the ONYX-015 vector being able to selectively replicate in or
cause lysis of p53-deficient tumor cells in in vitro and
in vivo experiments (12,13).
However, the outcomes of clinical trials that used ONYX-015 as a
monotherapy were found to be less positive (14,15).
ONYX-015 is therefore combined with therapeutic genes, such as
canstatin and mutant K5 genes, in order to overcome this
limitation. Antitumor gene therapy makes use of angiogenesis
inhibitor genes (16,17). Arresten is an angiogenesis
inhibitor, which may help to inhibit tumor cell growth (18).
In the present study, Ad5 E1B 55 kDa-deficient CRAd
was used in order to investigate its potential in the treatment of
LC. Two expression cassettes that express arresten and tumor
necrosis factor-related apoptosis-inducing ligand (TRAIL) were
inserted into the fiber and the putative early region 4 (E4) of
CRAd in order to investigate the synergistic mechanisms of the two
genes and their potential in the treatment of LC.
Materials and methods
Cell cultures
Cell lines were maintained in a humidified 37°C
atmosphere at 5% CO2. The following cell lines were
purchased from the Shanghai Cell Collection Center (Shanghai,
China), A549 (human lung adenocarcinoma), NCI H460 (human lung
large cell carcinoma), and HeLa and MRC-5 (healthy human lung
cells). The HEK293 human embryonic kidney cell line was purchased
from the American Type Culture Collection (ATCC, Manassas, VA,
USA). Cells were maintained in Dulbecco’s modified Eagle’s medium
or RPMI 1640 medium (Gibco Life Technologies, Carlsbad, CA, USA)
supplemented with 10% heat-inactivated fetal bovine serum (Hangzhou
Sijiqing Co. Ltd., Hangzhou, China), 250 U/ml penicillin and 250
µg/ml streptomycin (North China Pharmaceutical Co., Ltd.,
Shijiazhuang, China).
Construction of adenovirus transfer
plasmids
The human arresten and TRAIL genes were amplified
using polymerase chain reaction (PCR) from a human cDNA library
(Agilent Technologies, Inc., Santa Clara, CA, USA). The following
primers were used: Forward: 5′-aatcgatatgtctgttgatcacggcttc-3′ and
reverse: 5′-atctagattatgttcttctcatacagac-3′ for arresten (with the
restriction site Cla I and the initiation codon atg) and
forward: 5′-aatcgatatggctatgatggaggt-3′ and reverse:
5′-atctagattatgttcttctcataca-3′ for TRAIL (with the restriction
site Xba I). Protocols for PCR and cloning into the shuttle
plasmid, pAd5-cytomegalovirus-(CMV) and pAd5-phosphoglycerate
kinase 1-(PGK) were performed according to the methods described
previously (19). Following
digestion and sequencing of the amplified fragments, the shuttle
plasmid was linearized with Pme I, and then transformed into
Escherichia coli BJ5183-Ad Easy-1 (Agilent Technologies,
Inc.) using electroporation (Multiporator; Eppendorf, Hamburg,
Germany).
The plasmids were termed pAd-arresten and pAd-TRAIL,
and their identifications were confirmed using kanamycin (Amresco
LLC, Solon, OH, USA) selection and restriction digestion. The
recombinant pAd-arresten and pAd-TRAIL were linearized with
Pme I and transfected into HEK293 cells in order to form
CRAd-arresten, CRAd-TRAIL and CRAd-arresten-TRAIL (Fig. 1).
 | Figure 1Constrution of oncolytic adenoviruses.
(A) Schematic diagram of Ad5-CMV-eGFP, CRAd, CRAd-arresten,
CRAd-TRAIL and CRAd-arresten-TRAIL. In CRAd, the E1B 55kDa fragment
was deleted. In CRAd-arresten, the region between modified fiber
and E4 was replaced by one or two expression cassettes of genes
derived from CMV or PGK promoters. (B) The arresten gene was cloned
from the human cDNA library using polymerase chain reaction. (C)
shuttle-arresten was cut using Hind III enzyme. (D)
CRAd-arresten released a fragment of 4.5 kb following Pac I
digestion. (E) The cytopathic effects of HEK293 cell lines infected
with CRAd-arresten. CRAd, conditionally replicating adenovirus;
TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; E4,
putative early region 4; E3, early region 3; kDa, kilodalton; Ad5,
adendovirus 5; CMV, cytomegalovirus; eGFP, enhanced green
fluorescent protein; PGK, phosphoglycerate kinase 1; ITR, inverted
terminal repeat; PA, protective antigen. |
Adenoviruses were detected by observation of a
cytopathic effect using a fluorescence microscope (Observer Z1;
Carl Zeiss AG, Jena, Germany), which is a routine method to detect
the efficiency and activity of adenoviruses. Viruses were
propagated in HEK293 cells, purified using ultracentrifugation in a
cesium chloride (Sigma-Aldrich, St. Louis, MO, USA) gradient and
subjected to dialysis in virus dialysis buffer (10 mM tris-HCl, pH
8.0; 2 mM MgCl2; 4% sucrose) four times for 1 h each
time. Adenoviral functional titers were determined using a plaque
assay of the HEK293 cells (19).
Titers of the viruses were measured using a standard end point
dilution assay, as described previously (19). Replication-deficient
adenovirus-expressing reporter with enhanced green fluorescent
protein (eGFP) with an RGD modification in the HI loop
(Ad5-CMV-eGFP) from stock (Agilent Technologies, Inc.) was used as
the negative control.
Reverse transcription-PCR (RT-PCR)
analysis
Total RNA was extracted using TRIzol® (Invitrogen
Life Technologies, Shanghai, China) from A549 human lung
adenocarcinoma cell lines infected with the five adenoviruses and
treated with DNase I (Takara Bio, Inc., Otsu, Japan; 0.2
U/µl working concentration). First strand cDNA was generated
from 1 µg of RNA using an RNA LA PCR™ (AMV) kit (Takara Bio,
Inc.). PCR was conducted using the following primers: Forward:
5′-acgggggaaaacataagacc-3′ and reverse: 5′-tggcgcacttctaaactcct-3′
for arresten; forward: 5′-acgacaaacaaatggtccaa-3′, and reverse:
5′-actaaaaaggccccgaaaaa-3′ for TRAIL gene; and forward:
5′-ggccaaggtcatccatgacaac-3′ and reverse:
5′-tcccgttcagctcagggatgac-3′ for GAPDH, which was used as a
reference gene. PCR conditions consisted of an initial denaturation
step for 5 min at 94°C, followed by 30 cycles of amplification
(denaturation for 30 sec at 94°C, annealing for 30 sec at 55°C and
extension for 30 sec at 72°C) and a final extension for 10 min at
72°C. PCR products (6 µl) were electrophoresed on 15g/l agarose
gels (Invitrogen Life Technologies) in order to visualize cDNA
products.
Western blot analysis
Following 48 h of infection with one of the five
adenoviruses (CRAd, CRAd-arresten, CRAd-TRAIL, CRAd-arresten-TRAIL
or the negative control, Ad5-CMV-eGFP, the infected A549 cell lines
were harvested and total protein was extracted using a lysis
buffer. Protein concentrations were measured using a protein assay
kit (Bio-Rad Laboratories, Hercules, CA, USA). Total protein (20
µg) was separated on a 12% SDS-polyacrylamide gel (Amresco
LLC) and transferred to a polyvinylidene fluoride membrane (GE
Healthcare Bio-Sciences, Pittsburgh, PA, USA). Standard western
blotting was conducted using polyclonal rabbit antibodies against
human arresten (1:1,000; cat. no. PB0126) and TRAIL (1:500; cat.
no. BA1446), and a secondary antibody (horseradish
peroxidase-conjugated goat anti-rabbit IgG; cat. no. BA1055, Wuhan
Boster Biological Technology, Ltd., Wuhan, China). Cells were
washed three times with phosphate-buffered saline and Tween-20
(PBS-T) and bands were visualized using an enhanced
chemiluminescence western blotting kit (EMD Millipore, Billerica,
MA, USA).
Virus cytotoxicity was measured using a
3-(4,5-dimeth-ylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide
(MTT) assay. Cells were seeded in 96-well tissue culture plates
(2×103 cells/well). Following exposure to adenoviruses
for 24, 48 or 72 h (with five multiplicities of infection), 20
µl of MTT solution (Sigma-Aldrich; 2 mg/ml) was added.
Subsequently the plates were incubated at 37°C for 4 h. The
supernatants were replaced with acid isopropyl alcohol (Tianjin
Chem Co., Ltd., Tianjin, China) in order to dissolve the solid
product. The absorbance at 570 nm was measured using a microplate
reader (Bio-Rad Laboratories). Experiments were repeated three
times.
Animal experiments
Mice received care in compliance with the guidelines
for the care and use of laboratory animals in research (20). Experiments were approved by the
Ethics Committee of Shaanxi Normal University (Xi’an, China). A
total of 30 female Balb/c nude mice, aged 4–6 weeks were obtained
from the Animal Research Committee of the Institute of Biochemistry
and Cell Biology (Shanghai, China). A xenograft tumor model was
established using subcutaneous injections of A549 cell lines
(2×106/ml) into the right flanks of the mice. Once the
tumors had attained a size of 80–100 mm3, mice were
randomly assigned one of five groups: Ad-CMV-eGFP (negative
control), CRAd, CRAd-arresten, CRAd-TRAIL or CRAd-arresten-TRAIL.
Mice were intratumorally injected with (5×108
plaque-forming unit) plaque-forming unit in 100 µl PBS and
tumor size was measured, everyday for a period of eight days. Tumor
sizes were measured using a caliper and tumor volume was calculated
as length × width2/2 = tumor volume (mm3). At
the end of the experiment, the mice were sacrificed by etherization
and the tumors were resected in order to conduct
immunohistochemical analysis.
Immunohistochemical analysis
Tumor tissues removed from treated mice were fixed
in 10% buffered formalin (Tianjin Chem Co., Ltd.), dehydrated using
gradient alcohols and embedded in paraffin. Serial sections (4-
µm) were prepared and stained with hematoxylin and eosin
(H&E). An ABC staining system kit (sc-2019; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) was used in order to perform
immunohistochemical analysis. Sections were washed with PBS and
treated with 1% hydrogen peroxide for 20 min in order to inactivate
the endogenous peroxidase. Subsequently, the sections were blocked
using a blocking serum (Zhongshan Golden Bridge Biotechnology Co.,
Ltd., Beijing, China). Intratumoral microvessels were stained using
a monoclonal rat anti-mouse antibody against the cluster of
differentiation 31 (CD31; sc-71871; 1:200; Santa Cruz
Biotechnology, Co., Ltd.) antigen in order to measure intratumoral
microvessel density (MVD). The number of microvessels was counted
from ten randomly selected visual fields (x400) using a light
microscope (Observer Z1) and MVD was calculated using the Weidner
standard of scoring (19). Steps
were performed according to the manufacturer’s instructions.
H&E was used as a counterstain.
A terminal deoxynucleotidyl
transferase-mediated deoxynucleotide triphosphate-biotin nick
end-labeling (TUNEL) assay
A TUNEL assay was performed in order to detect the
presence of apoptotic cells, according to the manufacturer’s
instructions (Nanjing, Keygen Biotech Co. Ltd., Nanjing, China).
Apoptotic cells were counted on ten randomly selected visual fields
(x400) using a light microscope. The apoptotic index was calculated
using the formula: Apoptotic index = (total number of apoptotic
cells/total number of cells) × 100.
Statistical analysis
Data are presented as the mean ± standard deviation.
Unpaired student’s t-test and one-way analysis of variance were
conducted in order to assess significant differences between
groups. In all cases P<0.05 was considered to indicate a
statistically significant difference.
Results
Gene cloning and the construction of four
oncolytic adenoviruses
A schematic diagram of the five adenoviruses,
including Ad5-CMV-eGFP (negative control), CRAd, CRAd-Arresten,
CRAd-TRAIL and CRAd-Arresten-TRAIL is shown in Fig. 1A. The E1B 55 kDa fragment was
deleted from the CRAd adenovirus. For the CRAd-arresten, CRAd-TRAIL
or CRAd-arresten-TRAIL adenoviruses, the regions between fiber and
E4 were replaced by one or two gene expression cassettes derived
from CMV or PGK promoters (Fig.
1A).
Arresten cDNA (712 bp) was obtained using PCR
amplification (Fig. 1B). The
amplified fragment was inserted into a shuttle vector, downstream
of the cytomegalovirus promoter using ligation and then confirmed
using digestion with Hind III. A 1,300 bp fragment was
released from shuttle-CMV ligated with arresten, by contrast, no
fragment was released from shuttle-CMV without arresten (Fig. 1C). The resultant plasmid was
referred to as shuttle-CMV-arresten, which was identified using DNA
sequencing (21).
The protocols for TRAIL gene cloning and for the
construction of the oncolytic adenovirus CRAd-TRAIL are similar to
those of CRAd-Arresten (21).
Antitumor efficacy of CRAd with dual
expression of TRAIL and arresten in vitro
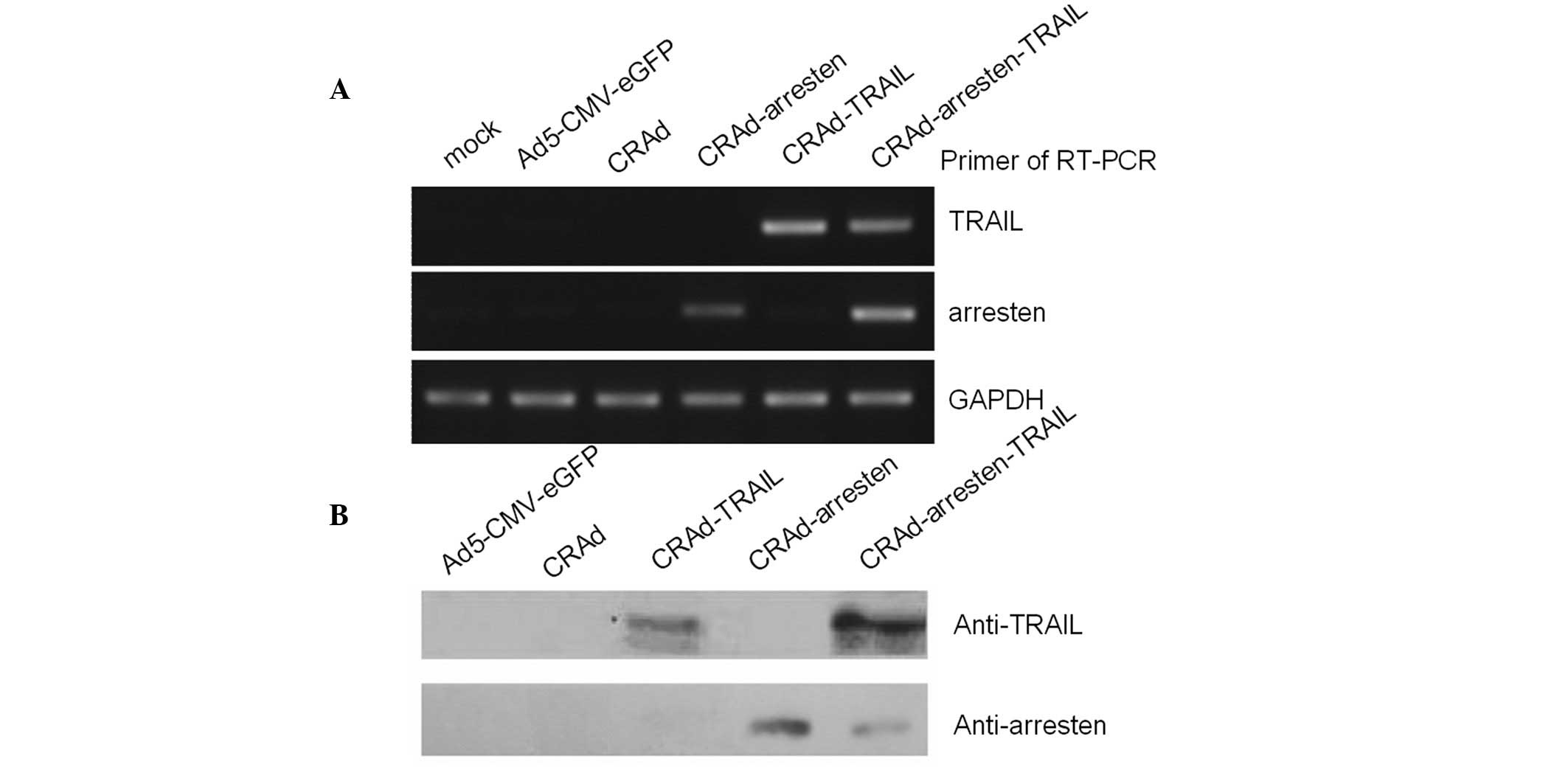
In order to examine the expression patterns of the
infected cells, total RNA was extracted from A549 cell lines
infected with the five adenovirus vectors. RT-PCR assays were
performed and the results suggested that arresten (217 bp) was
expressed in CRAd-arresten- and CRAd-arresten-TRAIL-infected A549
lung carcinoma cells. By contrast it was not expressed in
Ad5-CMV-eGFP-(negative control), CRAd- or CRAd-TRAIL-infected A549
cells. TRAIL was expressed in CRAd-TRAIL- and
CRAd-arresten-TRAIL-infected A549 cells. By contrast, TRAIL was not
expressed in Ad5-CMC-eGFP- (negative control), CRAd- or
CRAd-arresten-infected A549 cell lines (Fig. 2A).
Western blot analysis was also performed.
Anti-arresten was detected in CRAd-arresten- and
CRAd-arresten-TRAIL-infected A549 cells (Fig. 2B). Anti-TRAIL was detected in
CRAd-TRAIL- and CRAd-Arresten-TRAIL-infected A549 cells.
Anti-arresten and anti-TRAIL were not detected in Ad5-CMV-eGFP- or
CRAd-infected A549 cell lines.
The influence of the viruses on cell viability was
evaluated using an MTT assay (Fig.
3). The results of the present study demonstrated that cell
proliferation was significantly inhibited following infection with
the CRAd adenoviruses compared with the cells treated with the
control virus (P<0.01). The results also suggested that CRAd
viruses are capable of replicating in tumor cells but not in
healthy cells (MRC-5 cell line). Furthermore, A549 cell
proliferation was inhibited to a greater degree following infection
with CRAd-arresten ssssand CRAd-TRAIL, which may contribute to the
replication of these viruses in cancer cells. Cells infected with
CRAd-Arresten-TRAIL exhibited the lowest percentage cell viability.
However, there were no significant differences in cell viability
among the CRAd virus infected groups (P>0.05).
 | Figure 3Growth inhibitory effects of
adenoviruses infection in lung cancer cells in vitro. Cells
were infected with Ad5-CMV-eGFP, CRAd, CRAd-arresten, CRAd-TRAIL
and CRAd-arresten-TRAIL at a multiplicity of infection of five. At
48 h post infection, cell survival was measured using a
3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide
assay. Results are expressed as a percentage of the control group’s
cell viability. The data represent the mean ± standard deviation of
three independent experiments. *P<0.05, as compared with the
control group. CRAd, conditionally replicating adenovirus; TRAIL,
tumor necrosis factor-related apoptosis-inducing ligand; Ad5,
adendovirus 5; CMV, cytomegalovirus; eGFP enhanced green
fluorescent protein. |
Lung cancer xenografts
The antitumor effects of adenoviruses were analyzed
in vivo using A549 cancer subcutaneous xenografts. Tumor
growth was significantly inhibited following infection with CRAd,
CRAd-arresten, CRAd-TRAIL and CRAd-arresten-TRAIL adenoviruses,
compared with that of the control group (Fig. 4). Tumor growth in mice infected
with the four CRAd adenoviruses was slower than that in mice
infected with the control adenovirus (Ad5-CMV-eGFP infected
cells).
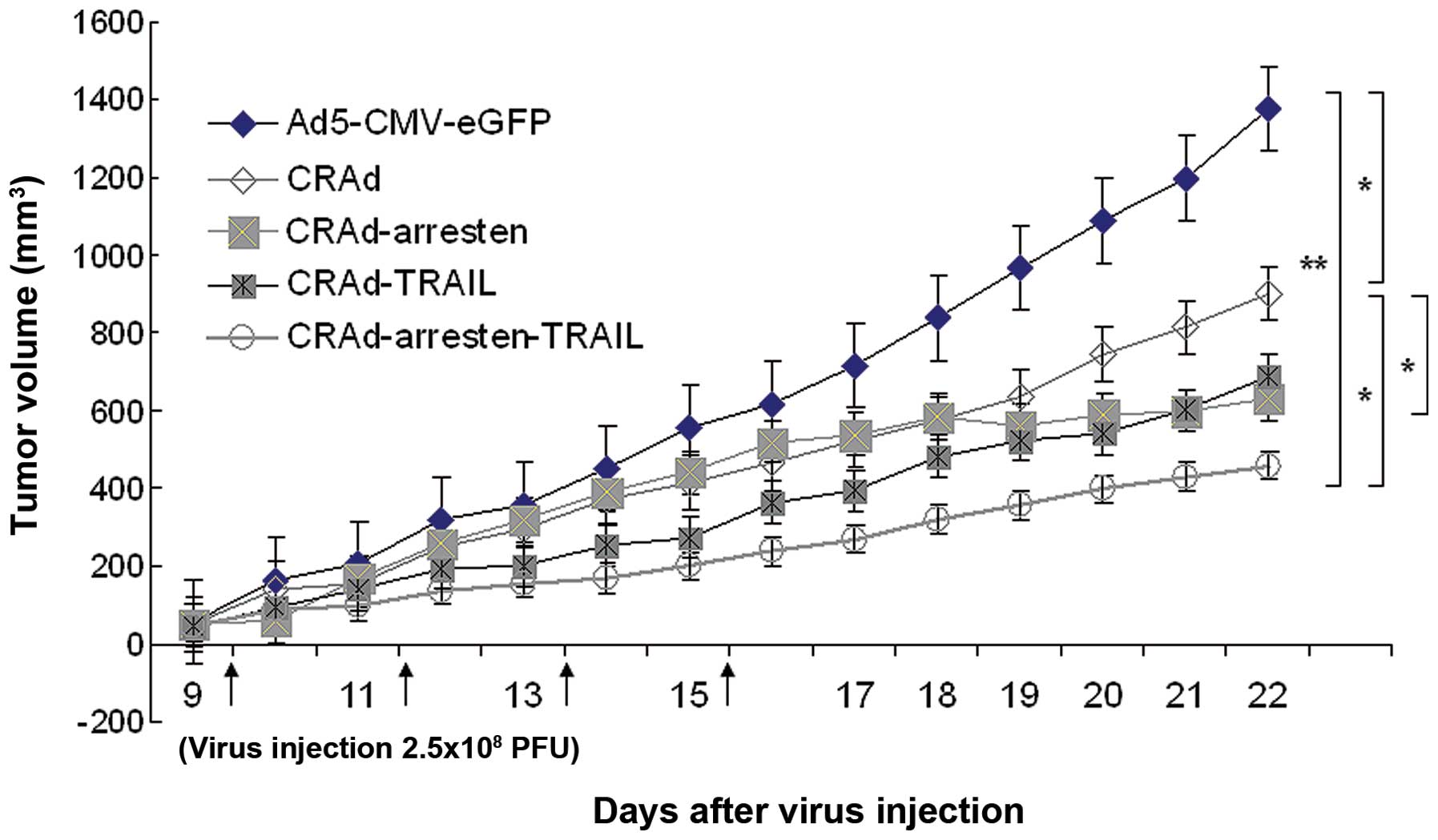 | Figure 4Tumor volume in nude mice bearing
A549 xenograft tumors. When tumor volumes had reached
80-100mm3, mice (n=6 in each group) were intratumorally
injected with 2.5×108 pfu of five adenoviruses;
Ad5-CMV-eGFP (negative control), CRAd, CRAd-arresten, CRAd-TRAIL
and CRAd-arresten-TRAIL) in 100 µl of virus preservation
buffer every other day for eight days. The tumor volumes were
measured everyday. The CRAd-arresten-TRAIL-virus-treated group
exhibited the most significant inhibition of tumor growth. Data are
presented as the mean ± standard deviation. *P<0.05 and
**P<0.01. CRAd, conditionally replicating adenovirus; TRAIL,
tumor necrosis factor-related apoptosis-inducing ligand; pfu,
plaque-forming unit; Ad5, adendovirus 5; CMV, cytomegalovirus;
eGFP, enhanced green fluorescent protein. |
Following four injections of CRAd-arresten, tumor
growth ceased on day 16 (Fig. 4).
In addition, on day 22 tumor volumes in samples treated with
CRAd-arresten (tumor volume 629.75±109.57 mm3) were
significantly smaller than those in the control group (control
cells; 1,477.38±110.23 mm3; P<0.01) and than those in
the CRAd-infected group (902.44±179.61 mm3;
P<0.05).
Mouse mortality was observed in the control group
(Ad5-CMV-eGFP) on day 19. Mortality was observed in the
CRAd-infected group on day 21. By contrast, there were no
mortalities in mice infected with CRAd-arresten-TRAIL. The causes
of mouse mortality were due to heavy tumor burden and consequent
organ failure or cancer cachexia. Autopsies were performed and no
evidence of tumor metastasis was observed.
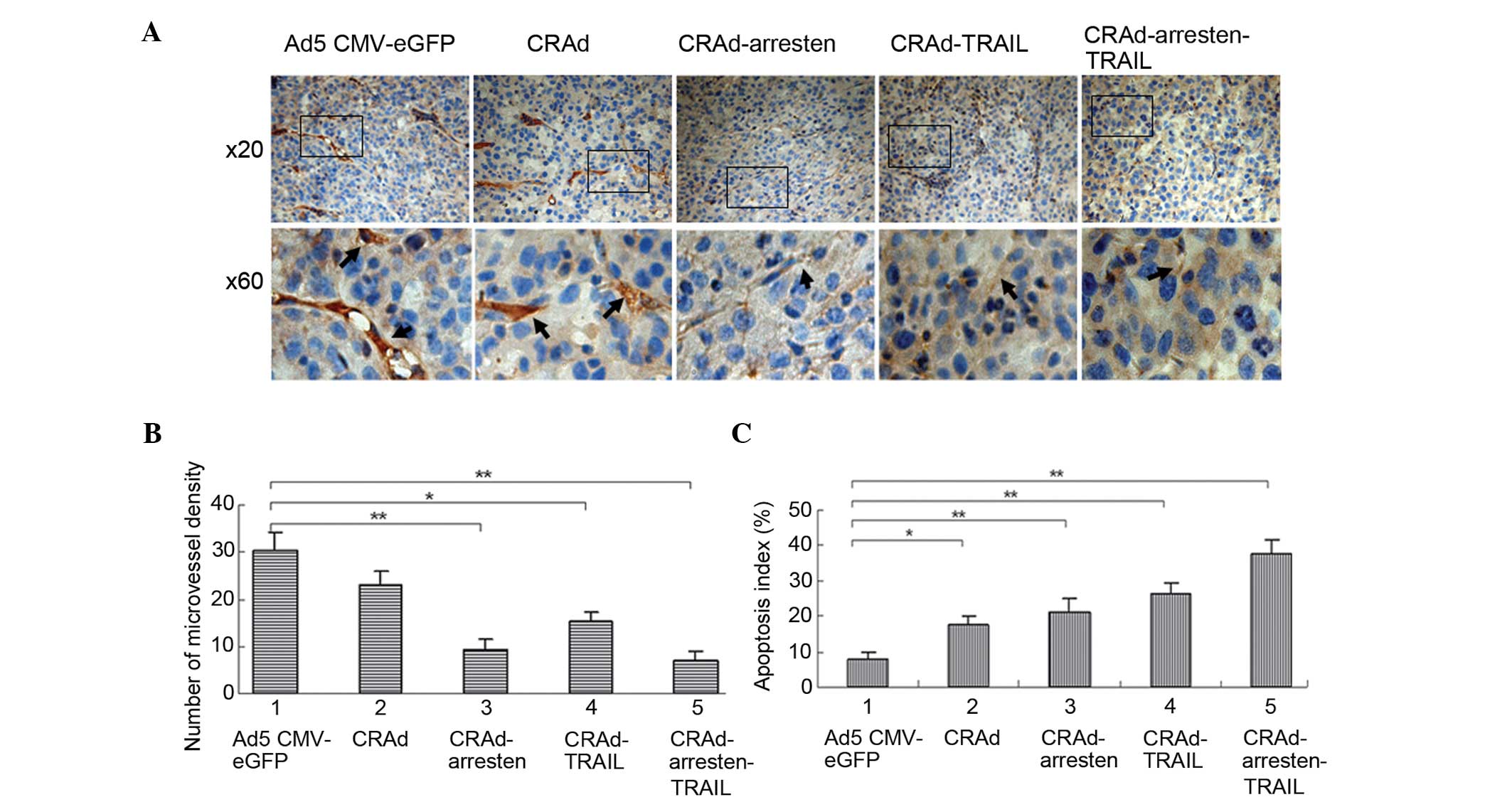
In order to measure angiogenesis and the presence of
apoptotic tumor cells, microvessel density (MVD) and TUNEL analyses
were conducted. Immunostaining with anti-CD31 antibody (Fig. 5A) followed by quantification using
MVD (Fig. 5B), suggested that MVD
in the CRAd-arresten-TRAIL group (6.7±1.4; P<0.01),
CRAd-arresten group (8.6±1.8; P<0.01) and CRAd-TRAIL-group
(15.4±1.3; P<0.05) was significantly lower, as compared with
that of the control virus infected group (Ad5-CMV-eGFP;
29.6±3.4).
TUNEL staining followed by quantification (Fig. 5C) suggested that the apoptotic
indices in the CRAd-infected groups (17.9±2.9%, 21.5±3.2% and
24.6±3.7% and 37.7±3.3% in the CRAd, CRAd-arresten and
CRAd-arresten-TRAIL infected A549 cell line groups, respectively)
exhibited a significantly higher apoptotic index, as compared with
that of the control (Ad5-CMV-eGFP) group (7.7±1.8%)
(**P< 0.01, *P< 0. 05).
Discussion
Previous investigations have suggested that soluble
TRAIL or adenovirus-mediated TRAIL exhibit antitumor properties by
inducing apoptosis in a number of types of cancer, such as lung
cancer (21–23). However, to the best of our
knowledge no studies have examined the influence on cancer cell
growth of two antitumor genes expressed from a single CRAd virus
on. In the present study, in order to establish a
CRAd-arresten-TRAIL adenovirus, two expression cassettes were
inserted into the region between fiber and E4 of CRAd genes, and
were initiated using two promoters (CMV or PGK;Fig. 1A). RT-PCR and western blot analyses
demonstrated that arresten and TRAIL genes were successfully
expressed following in vitro infection of A549 cell lines
with CRAd-arresten-TRAIL.
TRAIL is a member of the tumor necrosis factor
family and is capable of inducing apoptosis in malignant human
cells but not in healthy cells (24). Recently, non-replicative
adenovirus-mediated TRAIL has been used in clinical trials on
malignant glioma (25). In the
present study, TRAIL was expressed by RGD-modified CRAd, which
replicates selectively in tumor cells. The results of the present
study suggested that CRAd did not replicate in a healthy human lung
cell line (MRC-5; Fig. 3) nor in
HUVEC blood vessel epithelium cells (data not provided). However,
cell viability was inhibited in A549, H460 and HeLa cell lines
treated with CRAd, CRAd-arresten, CRAd-arresten TRAIL and
CRAd-TRAIL adenoviruses, although it was not affected in the
control group (Fig. 3). Results of
crystal violet assays confirmed these observations (data not
shown).
Arresten, a 26-kDa, non-collagenous domain involved
in the collagen IV α1 chain, has been shown to inhibit angiogenesis
(26). However, the mechanisms
underlying this process are not fully understood. Previous studies
have demonstrated that arresten is capable of inhibiting vascular
endothelial growth factor-mediated angiogenesis by promoting
apoptosis and caspase-3/poly (ADP-ribose) polymerase-1 activation
(27,28). Furthermore, arresten may inhibit
the phosphorylation of adhesion kinase/p38 mitogen-activated
protein kinase and the expression of B-cell lymphoma 2 and B-cell
lymphoma-extra large, which results in endothelial cell death
(28). Recent research has also
suggested that arresten production is associated with the p53 tumor
suppressor pathway (29). In the
present study, arresten genes were inserted into the CRAd viruses,
to produce CRAd-arresten and CRAd-arresten-TRAIL. CRAd-arresten and
CRAd-arresten-TRAIL infection led to the inhibition of matrigel
neovascularization in HUVEC cell lines in vitro (data not
shown). Xenograph lung tumor growth was inhibited in mice following
subcutaneous injection of CRAd-arresten and CRAd-arresten-TRAIL.
Following four CRAd-arresten adenovirus subcutaneous injections, on
day 16, tumor volumes remained constant until day 22 (Fig. 4). By contrast, tumor growth
continued to increase in mice following four subcutaneous
injections of CRAd, CRAd-TRAIL, CRAd-arresten-TRAIL and
AD5-CMV-eGFP. The results of the present study may contribute to a
better understanding of the mechanisms underlying the inhibition of
endothelial cell growth and its association with arresten secreted
by the CRAd-arresten adenovirus.
In the present study, a oncolytic adenovirus
containing two genes (CRAd-arresten-TRAIL), exhibited greater tumor
inhibitory activity than the CRAd, CRAd-arresten or CRAd-TRAIL
adenoviruses. These observations may be due to a greater level of
viral replication and expression of tumor suppressor genes in cells
infected with CRAd-arresten-TRAIL compared with
CRAd-,CRAd-arresten- or CRAd-TRAIL- adenovirus-infected cells.
However, the relative contributions of these components and the
possible underlying mechanisms remain unclear. According to the
results of in vitro analyses in the present study (Fig. 3), cells infected with CRAd
exhibited cell growth inhibition. Therefore, treatment with CRAd,
without arresten and TRAIL, may be sufficient to inhibit tumor cell
growth. There was a decrease in the relative contribution of the
replicating vector to tumor inhibitory activity in in vivo
analyses (Fig. 4). The mechanisms
underlying the patterns observed are complicated, and may be
associated with the decrease in viral transduction of the tumor
tissue compared with that of the cultured cells.
In conclusion, the present study provides potential
for the development of novel cancer gene therapy. An original
approach is described, involving the treatment of a single viral
vector containing two genes, which may enhance cancer cell
apoptosis. The results may be applicable to lung cancer and to a
number of other types of cancers, such as breast and liver
cancer.
Acknowledgments
The authors would like to thank Dr Xia H for his
support (Department of Life Sciences, Shaanxi Normal University).
This work was supported by the China Postdoctoral Special
Foundation (grant no. 200902585) and Key Project of Social
Development and Science and Technology of Shaanxi Province, China
(grant no. 2015SF-49).
Abbreviations:
|
CMV
|
cytomegalovirus
|
|
FBS
|
fetal bovine serum
|
|
MOI
|
multiplicity of infection
|
|
mRNA
|
messenger RNA
|
|
MTT
|
3,-(4,5-dimethylthiazol-2-yl)-2,5-
diphenyltetrazolium bromide
|
|
pfu
|
plaque forming units
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
|
MVD
|
microvessel density
|
|
TUNEL
|
terminal deoxynucleotidyl transferase
dUTP nick end labeling
|
|
PBS
|
phosphate-buffered saline
|
References
|
1
|
Chen W, Zheng R, Zhang S, Zou X, Zhao P
and He J: Lung cancer incidence and mortality in China, 2009.
Thoracic Cancer. 4:102–108. 2013. View Article : Google Scholar
|
|
2
|
Parkin DM, Bray FI and Devesa SS: Cancer
burden in the year 2000. The global picture. Eur J Cancer. 37(Suppl
8): S4–S66. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ghosh SS, Gopinath P and Ramesh A:
Adenoviral vectors: a promising tool for gene therapy. Appl Biochem
Biotechnol. 133:9–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rein DT, Breidenbach M and Curiel DT:
Current developments in adenovirus-based cancer gene therapy.
Future Oncol. 2:137–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jounaidi Y, Doloff JC and Waxman DJ:
Conditionally replicating adenoviruses for cancer treatment. Curr
Cancer Drug Targets. 7:285–301. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bilsland AE, Merron A, Vassaux G and Keith
WN: Modulation of telomerase promoter tumor selectivity in the
context of oncolytic adenoviruses. Cancer Res. 67:1299–1307. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lanson NA Jr, Friedlander PL,
Schwarzenberger P, et al: Replication of an adenoviral vector
controlled by the human telomerase reverse transcriptase promoter
causes tumor-selective tumor lysis. Cancer Res. 63:7936–7941.
2003.PubMed/NCBI
|
|
8
|
Haviv YS: A simplified in vitro ligation
approach to clone an E1B55k-deleted double-targeted
conditionally-replicative adenovirus. Virol J. 6:182009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen MJ, Green NK, Reynolds GM, et al:
Enhanced efficacy of Escherichia coli nitroreductase/CB1954 prodrug
activation gene therapy using an E1B-55K-deleted oncolytic
adenovirus vector. Gene Ther. 11:1126–1136. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Graat HC, van Beusechem VM, Schagen FH, et
al: Intravenous administration of the conditionally replicative
adenovirus Ad5-Delta24RGD induces regression of osteosarcoma lung
metastases. Mol Cancer. 7:92008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lamfers M, Idema S, Bosscher L, et al:
Differential effects of combined Ad5- delta 24RGD and radiation
therapy in in vitro versus in vivo models of malignant glioma. Clin
Cancer Res. 13:7451–7458. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ries S and Korn WM: ONYX-015: mechanism of
action and clinical potential of replication-selective adenovirus.
Br J Cancer. 86:5–11. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heise C and Kirn DH: Replication-selective
adenoviruses as oncolytic agents. J Clin Invest. 105:847–851. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brown CB and Bell JC: Oncolytic viruses: A
new weapon to fight cancer. J Med Imag Rad Sci. 39:115–127. 2008.
View Article : Google Scholar
|
|
15
|
Vähä-Koskela MJ, Heikkilä JE and Hinkkanen
AE: Oncolytic viruses in cancer therapy. Cancer Lett. 254:178–216.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He XP, Su CQ, Wang XH, et al:
E1B-55kDa-deleted oncolytic adenovirus armed with canstatin gene
yields an enhanced anti-tumor efficacy on pancreatic cancer. Cancer
Lett. 285:89–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fan JK, Xiao T, Gu JF, et al: Increased
suppression of oncolytic adenovirus carrying mutant k5 on
colorectal tumor. Biochemical Biophys Res Commun. 374:198–203.
2008. View Article : Google Scholar
|
|
18
|
Sudhakar A, Nyberg P, Keshamouni VG, et
al: Human alpha1 type IV collagen NC1 domain exhibits distinct
antiangiogenic activity mediated by alpha1beta1 integrin. J Clin
Invest. 115:2801–2810. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chai L, Liu S, Mao Q, Wang D, Li X, Zheng
X and Xia H: A novel conditionally replicating adenoviral vector
with dual expression of IL-24 and arresten inserted in E1 and the
region between E4 and fiber for improved melanoma therapy. Cancer
Gene Ther. 19:247–254. 2012. View Article : Google Scholar
|
|
20
|
National Research Council of The National
Academies: Guide for the care and use of laboratory animals. 8th
edition. The National Academies Press; Washington, DC: 2011
|
|
21
|
Li X, Mao QW, Wang DY, Zhang WF and Xia
HB: A fiber chimeric CRAd vector Ad5/11-D24 double-armed with TRAIL
and arresten for enhanced glioblastoma. Hum Gene Ther. 23:589–596.
2012. View Article : Google Scholar
|
|
22
|
Kim DR, Park MY, Lee CS, et al:
Combination of vorinostat and adenovirus-TRAIL exhibits a
synergistic antitumor effect by increasing transduction and
transcription of TRAIL in lung cancer cells. Cancer Gene Ther.
18:467–477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang H, Sui A, Wang Z, Liu S and Yao R:
Adenovirus-mediated TRAIL expression and downregulation of Bcl-2
expression suppresses non-small cell lung cancer growth in vitro
and in vivo. Int J Mol Med. 30:358–364. 2012.PubMed/NCBI
|
|
24
|
Holoch PA and Griffith TS: TNF-related
apoptosis-inducing ligand (TRAIL): a new path to anti-cancer
therapies. Eur J Pharmacol. 625:63–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim CY, Park SH, Jeong M, et al:
Preclinical studies for pharmacokinetics and biodistribution of
Ad-stTRAIL, an adenovirus delivering secretable trimeric TRAIL for
gene therapy. Exp Mol Med. 43:580–586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Colorado PC, Torre A, Kamphaus G, et al:
Anti-angiogenic cues from vascular basement membrane collagen.
Cancer Res. 60:2520–2526. 2000.PubMed/NCBI
|
|
27
|
Aikio M, Alahuhta I, Nurmenniemi S, et al:
Arresten, a collagen-derived angiogenesis inhibitor, suppresses
invasion of squamous cell carcinoma. PLoS One. 7:e510442012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boosani CS, Nalabothula N, Munugalavadla
V, et al: FAK and p38-MAP kinase-dependent activation of apoptosis
and caspase-3 in retinal endothelial cells by alpha1(IVC1. Invest
Ophthalmol Vis Sci. 50:4567–4575. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Assadian S, El-Assaad W, Wang XQ, et al:
p53 inhibits angiogenesis by inducing the production of Arresten.
Cancer Res. 72:1270–1279. 2012. View Article : Google Scholar : PubMed/NCBI
|