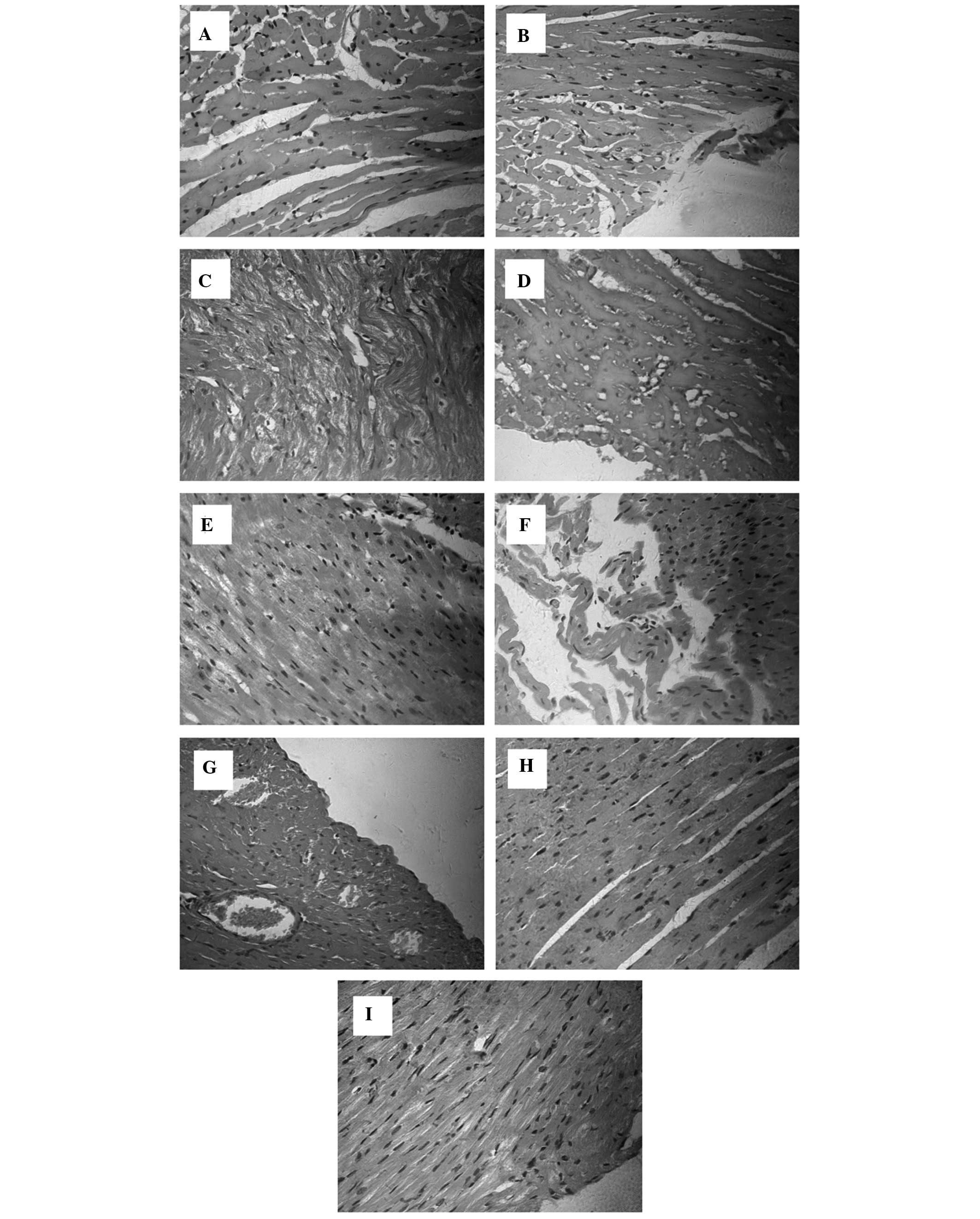
|
1
|
Wheeler DS: Death to sepsis: targeting
apoptosis pathways in sepsis. Crit Care. 13:10102009. View Article : Google Scholar
|
|
2
|
Rudiger A and Singer M: Mechanisms of
sepsis-induced cardiac dysfunction. Crit Care Med. 35:1599–1608.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma AC: Sepsis-induced myocardial
dysfunction. Shock. 28:265–269. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sips PY, Irie T, Zou L, et al: Reduction
of cardiomyocyte S-nitrosylation by S-nitrosoglutathione reductase
protects against sepsis-induced myocardial depression. Am J Physiol
Heart Circ Physiol. 304:H1134–H1146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krishnagopalan S and Kumar A, Parrillo JE
and Kumar A: Myocardial dysfunction in the patient with sepsis.
Curr Opin Crit Care. 8:376–388. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blanco J, Muriel-Bombin A, Sagredo V, et
al: Incidence, organ dysfunction and mortality in severe sepsis: a
Spanish multicentre study. Crit Care. 12:R1582008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Romero-Bermejo FJ, Ruiz-Bailen M,
Gil-Cebrian J and Huertos-Ranchal MJ: Sepsis-induced
cardiomyopathy. Curr Cardiol Rev. 7:163–183. 2011. View Article : Google Scholar
|
|
8
|
Takasu O, Gaut JP, Watanabe E, et al:
Mechanisms of cardiac and renal dysfunction in patients dying of
sepsis. Am J Respir Crit Care Med. 187:509–517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rudiger A and Singer M: The heart in
sepsis: from basic mechanisms to clinical management. Curr Vasc
Pharmacol. 11:187–195. 2013.PubMed/NCBI
|
|
10
|
Aoki Y, Hatakeyama N, Yamamoto S, et al:
Role of ion channels in sepsis-induced atrial tachyarrhythmias in
guinea pigs. Br J Pharmacol. 166:390–400. 2012. View Article : Google Scholar :
|
|
11
|
Levy RJ and Deutschman CS: Cytochrome c
oxidase dysfunction in sepsis. Crit Care Med. 35(Suppl): S468–S475.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao YM, Luan YY, Zhang QH and Sheng ZY:
Pathophysiological aspects of sepsis: an overview. Methods Mol
Biol. 1237:5–15. 2015.
|
|
13
|
Cao J, Xu F, Lin S, et al: IL-27 controls
sepsis-induced impairment of lung antibacterial host defence.
Thorax. 69:926–937. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sharma D, Packiriswamy N, Malik A, et al:
Nonhematopoietic β-Arrestin-1 inhibits inflammation in a murine
model of polymicrobial sepsis. Am J Pathol. 184:2297–2309. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schilling J, Lai L, Sambandam N, Dey CE,
Leone TC and Kelly DP: Toll-like receptor-mediated inflammatory
signaling reprograms cardiac energy metabolism by repressing
peroxisome proliferator-activated receptor γ coactivator-1
signaling. Circ Heart Fail. 4:474–482. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsu BG, Lee RP, Yang FL, Harn HJ and Chen
HI: Post-treatment with N-acetylcysteine ameliorates endotoxin
shock-induced organ damage in conscious rats. Life Sci.
79:2010–2016. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pathan N, Hemingway CA, Alizadeh AA, et
al: Role of interleukin 6 in myocardial dysfunction of
meningococcal septic shock. Lancet. 363:203–209. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kumar A, Kumar A, Paladugu B, Mensing J
and Parrillo JE: Transforming growth factor-beta1 blocks in vitro
cardiac myocyte depression induced by tumor necrosis factor-alpha,
interleukin-1beta, and human septic shock serum. Crit Care Med.
35:358–364. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rainer PP, Hao S, Vanhoutte D, et al:
Cardiomyocyte-specific transforming growth factor beta suppression
blocks neutrophil infiltration, augments multiple cytoprotective
cascades, and reduces early mortality after myocardial infarction.
Circ Res. 114:1246–1257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
ElZarrad MK, Mukhopadhyay P, Mohan N, et
al: Trastuzumab alters the expression of genes essential for
cardiac function and induces ultrastructural changes of
cardiomyocytes in mice. PloS One. 8:e795432013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zang Q, Maass DL, Tsai SJ and Horton JW:
Cardiac mitochondrial damage and inflammation responses in sepsis.
Surg Infect (Larchmt). 8:41–54. 2007. View Article : Google Scholar
|
|
22
|
Bouchier-Hayes L, Lartigue L and Newmeyer
DD: Mitochondria: pharmacological manipulation of cell death. J
Clin Invest. 115:2640–2647. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Groening P, Huang Z, La Gamma EF and Levy
RJ: Glutamine restores myocardial cytochrome C oxidase activity and
improves cardiac function during experimental sepsis. JPEN J
Parenter Enteral Nutr. 35:249–254. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Levy RJ, Vijayasarathy C, Raj NR, Avadhani
NG and Deutschman CS: Competitive and noncompetitive inhibition of
myocardial cytochrome C oxidase in sepsis. Shock. 21:110–114. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Singleton KD, Serkova N, Beckey VE and
Wischmeyer PE: Glutamine attenuates lung injury and improves
survival after sepsis: role of enhanced heat shock protein
expression. Crit Care Med. 33:1206–1213. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chopra M, Golden HB, Mullapudi S, Dowhan
W, Dostal DE and Sharma AC: Modulation of myocardial mitochondrial
mechanisms during severe polymicrobial sepsis in the rat. PLoS One.
6:e212852011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hüttemann M, Lee I, Grossman LI, Doan JW
and Sanderson TH: Phosphorylation of mammalian cytochrome c and
cytochrome c oxidase in the regulation of cell destiny:
respiration, apoptosis, and human disease. Adv Exp Med Biol.
748:237–264. 2012.PubMed/NCBI
|
|
28
|
Sjövall F, Morota S, Persson J, Hansson MJ
and Elmér E: Patients with sepsis exhibit increased mitochondrial
respiratory capacity in peripheral blood immune cells. Crit Care.
17:R1522013. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kantrow SP, Tatro LG and Piantadosi CA:
Oxidative stress and adenine nucleotide control of mitochondrial
permeability transition. Free Radic Biol Med. 28:251–260. 2000.
View Article : Google Scholar
|
|
30
|
Hüttemann M, Lee I, Pecinova A, Pecina P,
Przyklenk K and Doan JW: Regulation of oxidative phosphorylation,
the mitochondrial membrane potential, and their role in human
disease. J Bioenerg Biomembr. 40:445–456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hüttemann M, Helling S, Sanderson TH, et
al: Regulation of mitochondrial respiration and apoptosis through
cell signaling: cytochrome c oxidase and cytochrome c in
ischemia/reperfusion injury and inflammation. Biochim Biophys Acta.
1817:598–609. 2012. View Article : Google Scholar
|
|
32
|
Piel DA, Deutschman CS and Levy RJ:
Exogenous cytochrome C restores myocardial cytochrome oxidase
activity into the late phase of sepsis. Shock. 29:612–616.
2008.PubMed/NCBI
|
|
33
|
Lorente L, Martín MM, López-Gallardo E, et
al: Platelet cytochrome c oxidase activity and quantity in septic
patients. Crit Care Med. 39:1289–1294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Piel DA, Gruber PJ, Weinheimer CJ, et al:
Mitochondrial resuscitation with exogenous cytochrome c in the
septic heart. Crit Care Med. 35:2120–2127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Saas P and Perruche S: Functions of
TGF-β-exposed plasmacytoid dendritic cells. Crit Rev Immunol.
32:529–553. 2012. View Article : Google Scholar
|
|
36
|
Hegarty SV, O’Keeffe GW and Sullivan AM:
BMP-Smad 1/5/8 signalling in the development of the nervous system.
Prog Neurobiol. 109:28–41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Derynck R and Zhang YE: SMAD-dependent and
SMAD-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wrighton KH, Lin X and Feng XH:
Phosphocontrol of TGF-beta superfamily signaling. Cell Res.
19:8–20. 2009. View Article : Google Scholar
|
|
39
|
Voloshenyuk TG, Landesman ES, Khoutorova
E, et al: Induction of cardiac fibroblast lysyl oxidase by TGF-β1
requires PI3K/Akt, Smad3, and MAPK signaling. Cytokine. 55:90–97.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Olieslagers S, Pardali E, Tchaikovski V,
et al: TGF-β1/ALK5-induced monocyte migration involves PI3K and p38
pathways and is not negatively affected by diabetes mellitus.
Cardiovasc Res. 91:510–518. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Euler-Taimor G and Heger J: The complex
pattern of SMAD signaling in the cardiovascular system. Cardiovasc
Res. 69:15–25. 2006. View Article : Google Scholar
|
|
42
|
Yeh YH, Kuo CT, Chang GJ, Qi XY, Nattel S
and Chen WJ: Nicotinamide adenine dinucleotide phosphate oxidase 4
mediates the differential responsiveness of atrial versus
ventricular fibroblasts to transforming growth factor-β. Circ
Arrhythm Electrophysiol. 6:790–798. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sohn EJ, Kim J, Hwang Y, Im S, Moon Y and
Kang DM: TGF-β suppresses the expression of genes related to
mitochondrial function in lung A549 cells. Cell Mol Biol
(Noisy-le-grand). 58(Suppl): OL1763–OL1767. 2012.
|
|
44
|
Corcoran JB, McCarthy S, Griffin B, et al:
IHG-1 must be localised to mitochondria to decrease Smad7
expression and amplify TGF-β1-induced fibrotic responses. Biochim
Biophys Acta. 1833:1969–1978. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Piplani H, Vaish V, Rana C, et al:
Up-regulation of p53 and mitochondrial signaling pathway in
apoptosis by a combination of COX-2 inhibitor, Celecoxib and
Dolastatin 15, a marine mollusk linear peptide in experimental
colon carcinogenesis. Mol Carcinog. 52:845–858. 2013. View Article : Google Scholar
|
|
46
|
Abe Y, Sakairi T, Beeson C and Kopp JB:
TGF-β1 stimulates mitochondrial oxidative phosphorylation and
generation of reactive oxygen species in cultured mouse podocytes,
mediated in part by the mTOR pathway. Am J Physiol Renal Physiol.
305:F1477–F1490. 2013. View Article : Google Scholar : PubMed/NCBI
|