Introduction
Stroke is the third leading cause of mortality in
industrialized countries (1).
Acute ischemic stroke, resulting from sudden blood vessel occlusion
by a thrombus or embolism, is the most common form of stroke
(1). Different mechanisms are
involved in the pathogenesis of ischemic stroke, and ischemic
injury and post-ischemia/reperfusion (I/R), induced by thrombolytic
therapy, always leads to disruption in the blood-brain barrier
(BBB), resulting in the development of brain edema and subsequent
damage (2). Thus, methods to
protect the BBB may assist in developing therapeutic strategies for
the treatment of ischemic stroke.
The BBB is formed by endothelial cells, tight
junctions (TJs), pericytes, astrocytes and other extracellular
matrix components (3). BBB is
essential for maintaining appropriate neural function, and for
protecting the central nervous system from injury and disease,
which tightly regulates the movement of molecules, ions and cells
between the blood and the central nervous system (4,5).
TJs are important BBB structural components, which
seal the gaps between adjacent endothelial cells and thus maintain
paracellular permeability (6).
Claudin and occludin are key transmembrane proteins forming this
seal (7). Alterations in the
distribution or loss of TJ proteins is frequently observed in
ischemic cerebral microvessels, resulting in compromised BBB
integrity (8,9).
Caveolae are small vesicular invaginations of the
plasmamembrane, which have been implicated in endocytosis,
vesicular trafficking and signal transduction (10). The principal structural proteins of
caveolae are the caveolins, which consist of three distinct
proteins, caveolin-1, -2 and -3. Caveolin-1 is particularly
abundant in endothelial cells and has been implicated in the
pathogenesis of cerebral I/R injury (11). However, the phosphorylation of
caveolin-1 at tyrosine14 is required for the regulation of caveolae
formation and function (12–14).
Furthermore, it has been suggested that phosphorylation of
caveolin-1 may be one of the factors associated with early BBB
breakdown and brain edema in brain injury (15).
In Asia, and particularly China, electroacupuncture
(EA), a novel therapy based on traditional acupuncture combined
with modern electrotherapy, is a general method used for the
treatment of cerebrovascular diseases (16). Several experimental and clinical
studies have discussed the effect of EA on regulating different
brain and heart diseases (17).
There is evidence that EA significantly promotes the recovery of
neurological function and, thus, improves patient quality of life
(18,19). However, the exact mechanisms
underlying the effects of EA pretreatment on BBB require
elucidation.
Therefore, the present study was designed to
investigate the role of EA pretreatment in BBB disruption following
I/R, with a focus on caveolae and TJs. This was investigated using
a rat stroke model of middle cerebral artery occlusion (MCAO), and
the effect of EA pretreatment on BBB permeability and on the
expression levels of caveolin-1, phosphorylated (p-) caveolin-1,
claudin-5 and occludin were determined.
Materials and methods
Animals
The experimental procedure used in the present study
was approved by the Ethics Committee for Animal Experimentation of
Nanjing University of Chinese Medicine (Nanjing, China) and all
procedures were performed in accordance with the National
Institutes of Health Guidelines for Animal Research (20). Male Sprague-Dawley rats weighing
between 280 and 300 g were provided by the Experimental Animal
Center of Nanjing Medical University. The rats were housed in cages
under controlled conditions, with a 12 h light/dark cycle,
temperature at 22±2°C and humidity at 60–70% for at least one week
prior to surgery, and received food and water ad libitum.
The rats were fasted 12 h prior to surgery, but were allowed free
access to water. A total of 140 rats were randomly divided into
five groups: Sham group (S); 3 h post-I/R group (I/R3 h), 24 h
post-I/R group (I/R24 h); EA pretreated 3 h post-I/R group (EA+I/R3
h); and EA pretreated 24 h post-I/R group (EA+I/R24 h). Each group
contained 28 rats All the rats were assessed between the point of
ischemia and 24 h post-reperfusion.
MCAO model
Transient focal cerebral ischemia was induced by
MCAO. Anesthetization was induced using pentobarbital sodium (40
mg/kg body weight; Sigma-Aldrich, St. Louis, MO, USA)
intraperitoneally. The right common carotid artery and the right
external carotid artery were exposed through a ventral midline neck
incision, and were ligated proximally and temporarily. A 2.0
monofilament nylon suture (Doccol Corporation, Redlands, CA, USA),
with its tip rounded through heating in a flame, was inserted into
the common carotid artery through an arteriectomy, just beneath the
carotid bifurcation, and was advanced into the internal carotid
artery ~18-20 mm distal to the carotid bifurcation, until mild
resistance indicated occlusion of the origin of the anterior
cerebral artery and the MCA Following 2 h of ischemia, reperfusion
was accomplished by withdrawing the suture. The incision was closed
and the animals were allowed to recover from the surgery. In the
sham group, the same surgery was performed, but without occlusion
of the MCA. The rectal temperature was maintained at 38±0.5°C
throughout the procedure using a thermostat-controlled heating pad
(Doccol Corporation).
Cerebral blood flow (CBF) of the MCA was measured
using laser Doppler flowmetry. A flexible fiber-optic probe was
affixed to the skull over the cortex supplied by the proximal part
of the MCA (2 mm caudal to the bregma and 6 mm lateral to the
middle). Rats exhibiting <80% reduction in CBF in the core of
the MCA area were excluded from further investigation. Besides the
sham group (S), the other four groups all had rats excluded (I/R3
h, n=3; I/R24 h, n=2; EA+I/R3 h, n=2; EA+I/R24 h, n=2).
EA pretreatment
According to the Experimental Animals Meridians
Mapping, Baihui (GV20), which is located at the vertex of the
parietal bone, that is, the midpoint of the connecting line between
the auricular apices, was selected (18,19).
The rats in EA pretreatment groups were anesthetized and stimulated
using a 1 mA current, with a density-sparse wave of 2/15 Hz, for 30
min/day for five consecutive days. This was performed using a Hwato
Electronic Acupuncture Treatment instrument (SDZ-V; Suzhou Medical
Applicances Co., Ltd., Suzhou, China).
Neurobehavioral evaluation
A modified neurologic deficit score, described by
Longa et al (21) was used
for neurological assessment and was scored as follows: 0, no
deficit; 1, failure to extend left forepaw fully; 2, circling to
the left; 3, falling to the left; 4, no spontaneous walking with a
depressed level of consciousness. Rats scoring between 2 and 3 were
included in the subsequent experiments.
Brain water content
Following neurobehavioral evaluation, the rats (n=5)
were sacrificed and decapitated after an intraperitoneal injection
of 40 mg/kg pentobarbital sodium, followed by removal of the
brains. The brains were rapidly separated into left and right
cerebral hemispheres, and the right cerebral hemispheres were
weighed (wet weight) using an electronic balance (Ohaus
Corporation, Parisippany, NY, USA). Subsequently, the brains were
dried for 24 h at 100°C in order to obtain the dry weight. The
brain water content was calculated as follows: Brain water content
= [(wet weight - dry weight) / wet weight] × 100. This was used as
an index for brain edema (22).
Infarct volume assessment
Following decapitation, the brains (n=8) were
removed and frozen at −20°C for 15 min. The brains were were then
sliced using a plastic matrix (2 mm thickness; Sigma-Aldrich) and
stained using 2% 2,3,5-triphenyltetrazolium chloride (TTC;
Sigma-Aldrich) in 0.1 mol/l phosphate buffer (Sigma-Aldrich) for 30
min at 37°C to evaluate the infarct volume (23). The infarcted tissue remained
unstained (white), whereas normal tissues was stained red. The
infarct volume was calculated as follows: Infarct volume =
contralateral hemisphere volume - non-infarcted volume of the
ipsilateral hemisphere) / contralateral hemisphere volume (24).
Ultrastructure examination
The rats (n=5) were anesthetized with 40 mg/kg
intraperitoneal pentobarbital sodium, then were perfused with
pre-cooled phosphate-buffered saline (PBS; pH 7.4), followed by PBS
containing 4% para-formaldehyde (Sigma-Aldrich) and 0.25%
glutaraldehyde (Sigma-Aldrich). The brain was sectioned into three
slices, starting 3 mm from the anterior tip of the frontal lobe in
the coronal plane. The slices were 3, 4, and 3 mm thick from front
to back, respectively. The middle slice was cut longitudinally in
the ischemic hemisphere 2 mm from the midline then a transverse
diagonal cut was made at the 2 o’clock position to separate the
core from the penumbra. A 1 mm thick coronal slice from the cortex
penumbra area was removed. The slice was placed in fresh prepared
2.5% glutaraldehyde overnight at 4°C. Following rinsing with 0.1
mol/l PBS three times, the slice was post-fixed in 1% osmium
tetroxide (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for
1 h, dehydrated in graded ethanol (Sigma-Aldrich), and embedded in
epoxy resin (Sigma-Aldrich). Polymerization was performed at 80°C
for 24 h. Blocks were cut from the slice using a Reichert/Leica
Ultracut S Ultramicrotome (Leica Microsystems GmbH, Wetzlar,
Germany) into ultrathin sections (60–70 nm), which were then
post-stained with uranyl acetate (Sigma-Aldrich) and lead citrate
(Sigma-Aldrich), and examined using a Hitachi 7100 electron
microscope (Nikon, Corporation, Toyko, Japan).
Western blot analysis
To analyze the expression of proteins, the rats
(n=5) were decapitated and brains were removed. The ischemic
cortices, and corresponding cortices of the sham rats, were rapidly
dissected and frozen on dry ice (Sigma-Aldrich). Total protein
samples were extracted using radioimmunoprecipitation assay (RIPA;
Sigma-Aldrich) buffer for determination of the expression of
claudin-5, occludin, caveolin-1 and Akt. To determine the
expression of p-caveolin-1 and p-Akt, protease inhibitor cocktail
tablets (Sigma-Aldrich) were added, at a ratio of 1 tablet/10 ml
RIPA buffer. The protein concentrations were determined using a
spectrophotometer (UV-2540; Shimadzu Corporation, Kyoto, Japan).
Equivalent quantities of proteins (70 µg) from each sample
were separated using 10% SDS-PAGE (Sigma-Aldrich) and subsequently
transferred onto a nitrocellulose membrane (Sigma-Aldrich). The
membranes were then incubated overnight at 4°C with the following
rabbit primary antibodies: Claudin-5 (1:1,000; cat.no. ab53765;
Abcam), occludin (1:1,000; cat.no. ab31721, Abcam), caveolin-1
(1:1,000; cat. no. 3238; Cell signaling Technology, Inc., Danvers,
MA, USA), Akt (1:1,000; cat. no. 9272; Cell signaling Technology,
Inc.), p-caveolin-1 (1:1,000; cat. no. 3251; Cell signaling
Technology, Inc.) and p-Akt (1:1,000; cat. no. 9275; Cell signaling
Technology, Inc.), followed by incubation with the respective
horseradish peroxidase-conjugated anti-rabbit secondary antibodies
(1:5,000; Jackson Immunoresearch Laboratories, Inc., West Grove,
PA, USA) for 60 min at room temperature. Immunoreactivity was
detected using an enhanced chemiluminescent autoradiography system
(sc-2048 Western Blotting Luminol Reagent; Santa Cruz
Biotechnology, Inc.). Each blot was reprobed with β-actin (1:5,000;
cat. no. sc-130657; Santa Cruz Biotechnology, Inc.), following
stripping by heat and detergent to remove the antibodies from the
membrane, to provide a control for the load variations between the
samples. Autoradiographic films (Santa Cruz Biotechnology, Inc.)
were used for the final determination of protein expression using
SigmaScan 5.0 (Sigma-Aldrich) and normalized to the relative
optical density obtained for β-actin.
Statistical analysis
SPSS 19.0 for Windows (IBM SPSS, Armonk, NY, USA)
was used to performed statistical analysis. All values are
expressed as the mean ±standard deviation. Data were analyzed using
one-way analysis of variance, and inter-group differences were
detected using Student-Newman-Keuls post-hoc analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
EA pretreatment reduces infarction volume
and neurological deficits
A modified neurologic deficit score, described by
Longa et al (21), was used
for neurological assessment of the different groups. The rats in
the I/R3 h group exhibited severe neurological deficits compared
with the sham group. Following 24 h reperfusion without any
pretreatment, the neurological deficits remained unchanged, while
EA pretreatment significantly improved the neurological deficits
induced by I/R.
TTC staining was used to reveal cerebral infarcts,
with normal brain tissues stained red, and infarct lesions
remaining unstained (white). Compared with the sham group, the
brain infarct volume increased significantly in the I/R3 h and
I/R24 h groups. In the EA+I/R groups, these I/R-induced cerebral
infarcts were reduced significantly and dose-dependently (Fig. 1).
 | Figure 1EA pretreatment reduces brain injury
and improves neurological outcome. (A) Representative images of rat
brain 2,3,5-triphenyltetrazolium chloride staining in different
groups, red stain, normal brain tissues, white stain, infarct
lesions (n=8). (B) Quantitative analysis of infarct size in
different groups (n=8). (C) Neurological scores of animals in
different groups (n=5). Values are expressed as the mean ± standard
deviation. *P<0.05, vs. sham group;
#P<0.05, vs. I/R3 h group; †P<0.05, vs.
I/R24 h group. EA, electroaccupuncture; I/R.
ischemia/reperfusion. |
EA pretreatment improves brain water
content
The brain water content was significantly increased
in the I/R groups, compared with the sham group. In addition, EA
pretreatment significantly reduced the brain water content,
compared with the I/R3 h and I/R24 h groups. (P<0.05; Fig. 2).
Taken together, these findings demonstrated that EA
pretreatment attenuated the I/R-induced increase in BBB
permeability.
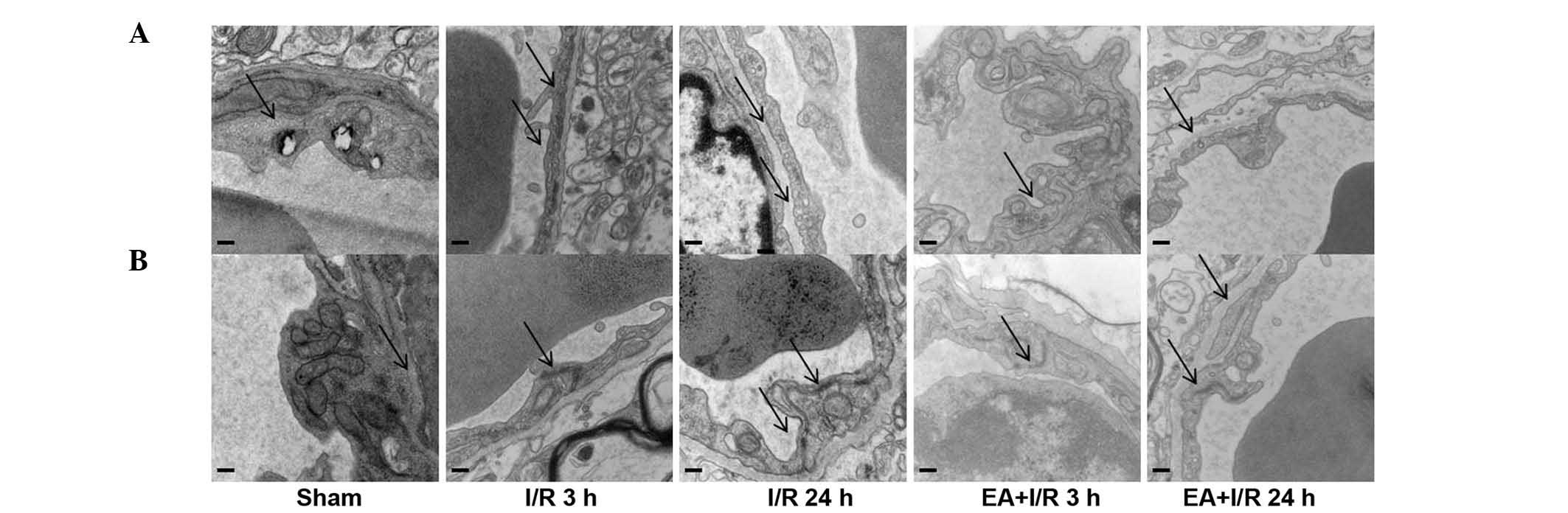
EA pretreatment ameliorates cerebral
microvasculature
In order to determine the mechanisms underlying the
role of EA pretreatment in maintaining BBB permeability,
transmission electron microscopy was used to identify the
morphological changes of cerebral microvasculature in the cortex in
all the groups. In the sham group, the cerebral microvasculature
was relatively normal, with normal caveolae, and the TJs localized
between endothelial cells as continuous lines. By contrast, in the
I/R groups, the caveolae in the endothelial cells were increased,
and the continuous lines of the TJs became ill-defined, appearing
as dotted lines, indicating degradation of the TJ proteins.
However, these observations were improved by EA pretreatment. These
results were also confirmed using western blotting (Fig. 3).
EA pretreatment alleviates the
degradation of TJ proteins
Western blotting was used to evaluate the expression
levels of claudin-5 and occludin. Compared with the sham group, I/R
induced a significant decrease in claudin-5 and occludin. Following
EA pretreatment, the expression of these two TJ proteins increased
significantly. No significant differences were observed between the
EA+I/R3 h group and the EA+I/R24 h group (Fig. 4).
EA pretreatment alleviates the increase
of p-caveolin-1
Western blotting was used to evaluate the expression
of caveolin-1 and p-caveolin-1. In terms of caveolin-1, no
significant differences were identified among the treatment groups.
However, the expression of p-caveolin-1 was significantly increased
following I/R compared with that of the sham group, while EA
pretreatment significantly relieved this effect (Fig. 5).
EA pretreatment alleviates the increase
of p-Akt
Western blotting was used to evaluate the expression
of Akt and p-Akt. Similar to caveolin-1, no significant differences
were observed among the groups for Akt. However, the expression of
p-Akt was significantly increased following I/R compared with that
of the sham group, while EA pretreatment significantly relieved
this effect (Fig. 6).
Discussion
In the present study, the effects and possible
mechanisms of EA pretreatment on BBB permeability following focal
cerebral I/R were investigated. Initially, EA pretreatment was
observed to effectively reduce cerebral infarct volume and improved
the neurobehavioral scores, alleviating the ischemic damage. EA
pretreatment also ameliorated brain water content and cerebral
microvasculature, and reduced the degradation of TJ proteins,
including claudin-5 and occludin. Furthermore, EA pretreatment
reduced the increased expression of p-caveolin-1 and p-Akt in the
endothelial cells.
In countries, including China, EA at Baihui (GV 20)
(25–27), an acupoint of the DU series, has
been commonly used for treating cerebrovascular diseases. A number
of studies have demonstrated the protective effects of EA
pretreatment in cerebral ischemia (18). The present study and previous
studies have reported that EA pretreatment reduced the cerebral
infarct volume and improved neurobehavioral scores following
transient MCAO. However, the effects of EA pretreatment on BBB
disruption associated with cerebral I/R remain to be fully
elucidated.
Ischemic stroke and subsequent reperfusion cause
severe clinical complications, including brain edema (28). In the present study, following 3 h
of reperfusion and 2 h subsequent ischemia, marked brain edema was
observed on examination of brain water content, which was
maintained until 24 h of reperfusion. In addition, EA pretreatment
significantly alleviated brain edema, indicating EA pretreatment as
a therapeutic strategy for ischemia.
It is well-known that the BBB is a highly
specialized structure between the blood circulation and the CNS,
maintaining the appropriate environment for appropriate neural
function and protecting the CNS from injury and disease (29). Following a period of ischemia, the
BBB is broken down, resulting in vasogenic brain edema. It is
suggested that two pathways are involved in endothelial cells,
which affect BBB permeability: Tight junctions, which mediate
paraendothelial transport, and caveolae, which mediate
transcellular traffic (30,31).
The present study demonstrated, using western blotting and electron
microscopy, that these two pathways may be involved in I/R induced
BBB interruption, and was alleviated by EA pretreatment.
During BBB breakdown associated with cerebral I/R,
TJ protein degradation is a critical step. Occludin, the first
integral transmembrane protein, may act as a primary
shock-absorber, mediating TJ responses to acute vascular dynamics
changes (32). Claudin-5, a TJ
protein with four transmembrane domains, is particularly important
in regulating paracellular permeability for small solutes across
the BBB (33). In the present
study, western blotting demonstrated that the protein expression
levels of occludin and claudin-5 decreased following cerebral I/R,
and were partly relieved by EA pretreatment. In addition, electron
microscopy revealed that the appearance of TJs were ill-defined
following cerebral I/R, and this was relieved by EA pretreatment.
Therefore, the protective action of EA pretreatment on BBB
permeability is possibly associated with paraendothelial
transport.
Caveolin-1 is known to be important in vesicular
trafficking by transcytosis, endocytosis and potocytosis (10). Previous studies have demonstrated
that early BBB breakdown may be associated with increased or
decreased expression of caveolin-1, using several experimental
models (34–37). In the present study, the
association between EA pretreatment and caveolin-1 was examined.
Therefore, the effects of EA pretreatment on the expression of
caveolin-1 following cerebral I/R were investigated. However, no
significant differences in caveolin-1 were observed among the
groups.
The phosphorylation of caveolin-1 at tyrosine14 is
required to regulate caveolae formation and function (12,15),
and the present study demonstrated that the expression of
p-caveolin-1 was significantly increased following I/R compared
with that in the sham group, while EA pretreatment significantly
relieved this effect. These results suggested that p-caveolin-1
signaling increased the density of caveolae and caused transcytosis
of proteins, leading to BBB breakdown and brain edema following
cerebral I/R. These effects were reversed by EA pretreatment.
In the endothelium, caveolin-1 regulates nitric
oxide signaling by binding to and inhibiting endothelial nitric
oxide synthase (eNOS). Activation of the Akt kinase leads to eNOS
activation and its dissociation from caveolin-1 (10). Therefore, the expression of Akt and
p-Akt were also investigated in the present study. Similar to
caveolin-1, no significant differences were observed among the
groups for Akt. However, the expression of p-Akt was significantly
increased following I/R compared with that of the sham rats, while
EA pretreatment significantly relieved this effect. It was
hypothesized that the increased expression of p-Akt may be due to
the phosphorylation of caveolin-1, leading to the downstream
activation of phosphatidylinositol 3-kinase and Akt. There may be a
correlation between p-caveolin-1 and p-Akt.
In conclusion, the present study demonstrated that
EA pretreatment was capable of protecting against I/R-induced BBB
disruption in rats, partly by interference in the degradation of TJ
protein and caveolin-1-mediated signal transmission in vascular
endothelial cells. These findings suggested that EA pretreatment
may provide novel strategies for the clinical treatment of I/R
injury, as an alternative approach to alleviate severe brain edema.
This requires further investigation.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81202802); and by
the Jiangsu Province Hospital of Traditional Chinese Medicine and
the Affiliated Hospital of Nanjing University of Traditional
Chinese Medicine (grant no. Y13034).
References
|
1
|
Lo EH, Dalkara T and Moskowitz MA:
Mechanisms, challenges and opportunities in stroke. Nat Rev
Neurosci. 4:399–415. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jung JE, Kim GS, Chen H, et al:
Reperfusion and neurovascular dysfunction in stroke: from basic
mechanisms to potential strategies for neuroprotection. Mol
Neurobiol. 41:172–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rubin LL and Staddon JM: The cell biology
of the blood-brain barrier. Annu Rev Neurosci. 22:11–28. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saunders NR, Ek CJ, Habgood MD and
Dziegielewska KM: Barriers in the brain: a renaissance? Trends
Neurosci. 31:279–286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zlokovic BV: The blood-brain barrier in
health and chronic neurodegenerative disorders. Neuron. 57:178–201.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wolburg H and Lippoldt A: Tight junctions
of the blood-brain barrier: development, composition and
regulation. Vascul Pharmacol. 38:323–337. 2002. View Article : Google Scholar
|
|
7
|
Forster C: Tight junctions and the
modulation of barrier function in disease. Histochem Cell Biol.
130:55–70. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu W, Hendren J, Qin XJ, Shen J and Liu
KJ: Normobaric hyperoxia attenuates early blood-brain barrier
disruption by inhibiting MMP-9-mediated occludin degradation in
focal cerebral ischemia. J Neurochem. 108:811–820. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
ElAli A, Doeppner TR, Zechariah A and
Hermann DM: Increased blood-brain barrier permeability and brain
edema after focal cerebral ischemia induced by hyperlipidemia: role
of lipid peroxidation and calpain-1/2, matrix metalloproteinase-2/9
and RhoA overactivation. Stroke. 42:3238–3244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Minshall RD, Sessa WC, Stan RV, Anderson
RG and Malik AB: Caveolin regulation of endothelial function. Am J
Physiol Lung Cell Mol Physiol. 285:L1179–L1183. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jasmin JF, Malhotra S, Singh Dhallu M,
Mercier I, Rosenbaum DM and Lisanti MP: Caveolin-1 deficiency
increases cerebral ischemic injury. Circ Res. 100:721–729. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li S, Seitz R and Lisanti MP:
Phosphorylation of caveolin by src tyrosine kinases. The
alpha-isoform of caveolin is selectively phosphorylated by v-Src. J
Biol Chem. 271:3863–3868. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee H, Volonte D, Galbiati F, et al:
Constitutive and growth factor-regulated phosphorylation of
caveolin-1 occurs at the same site (Tyr-14) in vivo: Identification
of a c-Src/Cav-1/Grb7 signalling cassette. Mol Endocrinol.
14:1750–1775. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Labrecque L, Nyalendo C, Langlois S, et
al: Src-mediated tyrosine phosphorylation of caveolin-1 induces its
association with membrane type 1 matrix metalloproteinase. J Biol
Chem. 279:52132–52140. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nag S, Manias JL and Stewart DJ:
Expression of endothelial phosphorylated caveolin-1 is increased in
brain injury. Neuropath Appl Neuro. 35:417–426. 2009. View Article : Google Scholar
|
|
16
|
Chou P, Chu H and Lin JG: Effects of
electroacupuncture treatment on impaired cognition and quality of
life in Taiwanese stroke patients. J Altern Complement Med.
15:1067–1073. 2009.
|
|
17
|
Xiong L, Lu Z, Hou L, Zheng H, Zhu Z, Wang
Q and Chen S: Pretreatment with repeated electroacupuncture
attenuates transient focal cerebral ischemic injury in rats. Chin
Med J (Engl). 116:108–111. 2003.
|
|
18
|
Wang Q, Xiong L, Chen S, Liu Y and Zhu X:
Rapid tolerance to focal cerebral ischemia in rats is induced by
preconditioning with electroacupuncture: window of protection and
the role of adenosine. Neurosci Lett. 381:158–162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Q, Peng Y, Chen S, et al:
Pretreatment with electroacupuncture induces rapid tolerance to
focal cerebral ischemia through regulation of endocannabinoid
system. Stroke. 40:2157–2164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US); Washington, DC: 2011
|
|
21
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu Q, Ma Q, Zhan Y, He Z, Tang J, Zhou C
and Zhang J: Isoflurane enhanced hemorrhagic transformation by
impairing antioxidant enzymes in hyperglycemic rats with middle
cerebral artery occlusion. Stroke. 42:1750–1756. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsubokawa T, Jadhav V, Solaroglu I,
Shiokawa Y, Konishi Y and Zhang JH: Lecithinized superoxide
dismutase improves outcomes and attenuates focal cerebral ischemic
injury via anti-apoptotic mechanisms in rats. Stroke. 38:1057–1062.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Swanson RA, Morton MT, Tsao-Wu GJ, Savalos
RA, Davidson C and Sharp FR: A semiautomated method for measuring
brain infarct volume. Cereb Blood Flow Metab. 10:290–293. 1990.
View Article : Google Scholar
|
|
25
|
Zhang C: The brain-resuscitation
acupuncture method for treatment of post wind-stroke mental
depression - a report of 45 cases. J Tradit Chin Med. 25:243–246.
2005.
|
|
26
|
Zhang H, Zhao L, He CQ, Hu KM and Liu J:
Clinically multi-central randomized controlled study on scalp
electroacupuncture for treatment of vascular dementia. Zhongguo
Zhen Jiu. 28:783–787. 2008.In Chinese. PubMed/NCBI
|
|
27
|
Zhou Y, Zhou GY, Li SK and Jin J: Clinical
observation on the therapeutic effect of electroacupuncture
combined with cupping on post-stroke fatigue. Zhen Ci Yan Jiu.
35:380–383. 2010.In Chinese.
|
|
28
|
Jung JE, Kim GS, Chen H, et al:
Reperfusion and neurovascular dysfunction in stroke: from basic
mechanisms to potential strategies for neuroprotection. Mol
Neurobiol. 41:172–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ballabh P, Braun A and Nedergaard M: The
blood-brain barrier: an overview: structure, regulation and
clinical implications. Neurobiol Dis. 16:1–13. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bazzoni G and Dejana E: Endothelial
cell-to-cell junctions: molecular organization and role in vascular
homeostasis. Physiol Rev. 84:869–901. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schubert W, Frank PG, Razani B, Park DS,
Chow CW and Lisanti MP: Caveolae-deficient endothelial cells show
defects in the uptake and transport of albumin in vivo. J Biol
Chem. 276:48619–48622. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Furuse M, Hirase T, Itoh M, Nagafuchi A,
Yonemura S and Tsukita S: Occludin: a novel integral membrane
protein localizing at tight junctions. J Cell Biol. 123:1777–1788.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nitta T, Hata M, Gotoh S, et al:
Size-selective loosening of the blood-brain barrier in
claudin-5-deficient mice. J Cell Biol. 161:653–660. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shen J, Ma S, Chan P, et al: Nitric oxide
down-regulates caveolin-1 expression in rat brains during focal
cerebral ischemia and reperfusion injury. J Neurochem.
96:1078–1089. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gu Y, Zheng G, Xu M, et al: Caveolin-1
regulates nitric oxide-mediated matrix metalloproteinases activity
and blood-brain barrier permeability in focal cerebral ischemia and
reperfusion injury. J Neurochem. 120:147–156. 2012. View Article : Google Scholar
|
|
36
|
Huang P, Zhou CM, Qin Hu, et al:
Cerebralcare Granule attenuates blood-brain barrier disruption
after middle cerebral artery occlusion in rats. Exp Neurol.
237:453–463. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang S, Liu Y, Zhao Z and Xue Y: Effects
of green tea polyphenols on Caveolin-1 of microvessel fragments in
rats with cerebral ischemia. Neurol Res. 32:963–970. 2010.
View Article : Google Scholar : PubMed/NCBI
|