Introduction
Apigenin (4′,5,7-trihydroxyflavone) is a non-toxic
dietary flavonoid present in numerous herbs, including parsley,
thyme, peppermint, chamomile, horsetail herb, lemon balm, perilla,
vervain and yarrow (1). Apigenin
has been demonstrated to exert anti-oxidant (1), anti-inflammatory (2), anti-telomerase (3) and anti-depressant activities
(4). Of note, apigenin also
possesses anti-tumor properties and is therefore of particular
interest in the development of novel drugs for the treatment and/or
prevention of cancer (5,6). It has previously been demonstrated
that apigenin is able to reduce the volume and mass of implanted
androgen-sensitive 22Rv1 and androgen-insensitive PC-3 tumor cells
(7). Furthermore, apigenin
suppresses inducible cyclooxy-genase and nitric oxide synthase in
mouse macrophages (8), and
inhibits ultraviolet light-induced skin carcinogenesis in SKH-1
mice (9). Apigenin has also been
shown to inhibit growth and induce apoptosis in numerous cancer
cell lines, including those of breast (10), lung (11), colon (12,13),
prostate (14), leukemia (15) and pancreatic cancer (16).
Apoptosis, also known as programmed cell death, is a
fundamental physiological process, required for normal development
and tissue homeostasis (17,18).
Apoptotic progression is associated with various caspases, which
comprise a group of aspartate-specific cysteine proteases, which
are members of the interleukin-1-converting enzyme family (17,18).
The caspase cascade signaling pathway has crucial roles in the
induction, transduction and amplification of intracellular
apoptotic signals (17,18). In the majority of tumor cells,
apoptosis is induced via two distinct signaling pathways: The
extrinsic and intrinsic apoptotic pathways. The extrinsic pathway
is associated with the activation of death receptors, including Fas
and the tumor necrosis factor receptors, and the cleavage of
caspase-8 and caspase-3 (19–21).
The intrinsic pathway is associated with the cleavage of caspase-9
and -3, as well as alterations in the mitochondrial membrane
potential and the mitochondrial permeability transition (22). Caspase-3 is responsible for the
cleavage of poly(adenosine diphosphate-ribose) polymerase (PARP)
during cell death in each of these pathways (23).
Overexpression of the human epidermal growth factor
receptor 2 (HER2) tyrosine kinase is associated with the
pathogenesis and aggressive characteristics underlying ~25% of
invasive human breast cancers (24). Clinical and experimental evidence
has suggested that aberrant HER2 signaling may contribute to the
initiation of tumor development and disease progression (24). HER2-positive tumors are associated
with more aggressive phenotypes, characterized by more frequent
recurrence and shorter overall survival, compared with that of
HER2-negative tumor subtypes (25). The recombinant humanized anti-HER2
monoclonal antibody trastuzumab (herceptin) is frequently used in
the treatment of patients with HER-2-overexpressing subtypes of
cancer (26). Trastuzumab induces
downregulation of HER2/Neu, resulting in the disruption of receptor
dimerization and signaling (27).
Trastuzumab also induces cell cycle arrest during G1
phase and inhibits the phosphorylation of p27Kip1, suppressing cdk2
activity and reducing proliferation (28). Trastuzumab suppresses angiogenesis
via the induction of anti-angiogenic factors and the repression of
pro-angiogenic factors. However, numerous patients with breast
cancer are unresponsive to trastuzumab treatment or develop a
resistance to this drug (29).
There have therefore been numerous studies devoted to the
identification of alternative compounds with the ability to
effectively treat HER2-overexpressing subtypes of breast
cancer.
Previously, our group reported that apigenin
promoted apoptosis via the extrinsic pathway, inducing p53 and
inhibiting STAT3 and NFκB signaling in HER2-transfected MCF-7 cells
(30). The present study aimed to
investigate whether apigenin exerted growth-suppressive activity in
natural HER2-overexpressing breast cancer cells, using the SKBR3
cell line. The effects of apigenin on the proliferation and
apoptosis of SKBR3 cells were therefore evaluated. Thyroid cancer
cells were also used for comparison, in order to demonstrate that
the effect of apigenin was not cell-specific. The mechanism
underlying the regulation of SKBR3 cell growth by apigenin was also
investigated, by analysis of the cell cycle and determination of
the expression levels of apoptotic and intracellular signaling
molecules. In addition, whether apigenin was able to inhibit the
STAT3 signaling pathway, resulting in growth suppression of
HER2-overexpressing breast cancer cells was examined.
Materials and methods
Compounds
Apigenin (4′,5,7-trihydroxyflavone) genistein and
quercetin were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Apigenin was dissolved in dimethyl sulfoxide (DMSO), and the final
concentration of DMSO in the controls and each sample did not
exceed 0.1%. It was found that 0.1% DMSO did not influence the cell
growth rate compared with 0% DMSO (no treatment) in the breast
cancer cells (data not shown). The caspase-8 inhibitor
Z-IETD-fmk and the caspase-9 inhibitor Z-LEHD-fmk
were obtained from R&D Systems, Inc. (Minneapolis, MN, USA).
The STAT3 inhibitor S31-201 was obtained from Calbiochem
(Billerica, MA, USA) and an EZ-western chemiluminescent detection
kit was purchased from Daeillab Service Co. (Seoul, Korea).
Cell culture
The human breast cancer cell line SKBR3 (American
Type Culture Collection, Manassas, VA, USA) was cultured in
Dulbecco’s modified Eagle’s medium (Life Technologies Korea LLC,
Seoul, Korea) containing 50 U/ml penicillin, 50 mg/ml streptomycin
and 10% fetal bovine serum (FBS; Welgene, Daegu, Korea) at 37°C in
an atmosphere of 5% CO2. The human thyroid cancer cell
lines SNU790 and SNU80 were obtained from the Korean Cell Line Bank
(Seoul, Korea) and cultured in RPMI (Life Technologies Korea LLC)
containing 50 U/ml penicillin, 50 mg/ml streptomycin (Life
Technologies Korea LLC) and 10% FBS at 37°C in an atmosphere of 5%
CO2.
Antibodies
Primary antibodies against cleaved caspase-8 (9496)
and PARP (9542), were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA). Primary antibodies against B cell lymphoma
2 (BCL2; sc-7382), BCL2-associated X (BAX; sc-7480), p53 (sc-126)
and hypoxia-inducible factor (Hif)-1α (sc-13515) were obtained from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Primary
antibodies against STAT3 (06-596), p-STAT3 (Tyr705; 05-485),
vascular endothelial growth factor (VEGF; 05-1116) and p-janus
kinase 2 (JAK2, Tyr1022/Tyr1023; 06-255) were obtained from
EMD-Millipore (Billerica, MA, USA). The anti-tubulin antibody
(T3526) was from Sigma-Aldrich. Horseradish peroxidase
(HRP)-conjugated secondary antibodies (mouse and rabbit) were
purchased from Calbiochem (San Diego, CA, USA) and the anti-goat
secondary antibody was from Jackson ImmunoResearch Laboratories,
Inc. (West Grove, PA, USA).
Cell proliferation assay
Cells were seeded in 12-well culture plates at a
density of 5×104 cells/well. After the cells were
exposed to various concentrations of apigenin genistein or
quercetin (0, 20, 40, 60, 80 or 100 μM) and incubated at
37°C for three days, the cells were harvested by trypsinization
(Sigma-Aldrich), resuspended in 1–2 ml medium, and counted using a
hemocytometer (Sigma-Aldrich).
MTT assay
Cells were seeded in 96-multiwell culture plates at
a density of 2×103−3×103 cells/well and
incubated for 24 h at 37°C. Subsequently, the cells were treated
with different concentrations of apigenin (0–80 μM) for 24,
48 or 72 h. Following incubation, MTT reagent (0.5 mg/ml) was added
to each well, and the plates were incubated in the dark at 37°C for
2 h. At the end of the incubation, the medium was removed, the
resulting formazan was dissolved in DMSO, and the optical density
was measured at 570 nm using an ELISA plate reader (Gemini EM
Microplate reader, Versa Max; Molecular Devices, Sunnyvale, CA,
USA).
Cell cycle analysis by flow
cytometry
Cells were harvested with 0.25% trypsin and washed
once with phosphate-buffered saline (PBS). Following
centrifugation, the cells were fixed in cold 95% ethanol with 0.5%
Tween-20 and stored at −20°C for at least 30 min. The cells were
incubated in 50 μg/ml propidium iodide [PI (Sigma-Aldrich),
including 1% sodium citrate (Sigma-Aldrich) and 50 μg/ml
RNase A (Sigma-Aldrich)] at room temperature in the dark for 30
min. Analysis of the apoptotic cells was performed with a FACScan
flow cytometer (Becton Dickinson, Mountain View, CA, USA), and the
data were analyzed using CellQuest software (BD Biosciences, San
Jose, CA, USA).
Immunocytochemistry
Cells (4×104 cells/well) were seeded in
eight-well chamber slides, incubated for 24 h at 37°C and treated
with apigenin (40 μM) in the presence or absence of
CoCl2 (Sigma-Aldrich) for a further 24 h. The cells were
fixed with 4% paraformaldehyde (Sigma-Aldrich) for 30 min and
treated with 3% hydrogen peroxide (H2O2;
Sigma-Aldrich) in methanol for 20 min to quench the endogenous
peroxidase activity. The cells were washed with PBS, blocked with
5% bovine serum albumin in PBS for 1 h and incubated with the
anti-STAT3 primary antibody (1:200 dilution) overnight at 4°C.
After washing with PBS, the cells were incubated with the
anti-rabbit biotin-conjugated secondary antibody for 1 h at room
temperature. Subsequently, the cells were treated with Vectastain
ABC reagent (Vector Laboratories, Inc. Burlingame, CA, USA) for 30
min at 4°C and stained with diaminobenzidine tetrachloride (DAB;
Thermo Fisher Scientific, Waltham, MA, USA) and hematoxylin
(Sigma-Aldrich). The cells were mounted with mounting medium
(Vector Laboratories, Inc., Burlingame, CA, USA) and subsequently
analyzed by microscopy (CKX41; Olympus America Inc., Center Valley,
PA, USA).
Measurement of VEGF secreted from SKBR3
cells by ELISA
To assess the level of VEGF in the SKBR3 cell
supernatants, the cells were treated with apigenin (0–80 μM)
in the presence or absence of CoCl2 (100 μM) to
mimic hypoxia. After 24 h, the media were collected, centrifuged at
15,000 x g at room temperature to remove the cellular debris, and
stored at −70°C until assayed for VEGF. The amount of VEGF secreted
into the culture medium was measured by ELISA according to the
manufacturer’s instructions (Human VEGF Quantikine ELISA kit;
R&D Systems, Minneapolis, MN, USA). Briefly, 96-well plates
were coated with capture antibody in ELISA coating buffer and
incubated overnight at 4°C. The plates were then washed with PBS
with 0.05% Tween 20 (PBS-T) and subsequently blocked with 10% FBS
in PBS for 1 h at 20°C. Serial dilutions of standard antigen or
sample in dilution buffer (10% FBS in PBS) were added to the
plates, and the plates were incubated for 2 h at 20°C. Following
washing, biotin-conjugated anti-mouse immunoglobulin E and
streptavidin-conjugated horseradish peroxidase (SAv-HRP) (R&D
Systems, Inc.) were added to the plates, and the plates were
incubated for 1 h at 20°C. Finally, the tetramethylbenzidine (TMB)
substrate was added to the plates, and after 15 min of incubation
in the dark, 2 N H2SO4 was added to stop the
reaction. The optical density was measured at 450 nm on the
automated ELISA reader (Molecular Devices).
Western blot analysis
Cells were lysed in modified
radioim-munoprecipitation assay buffer [150 mM NaCl, 1% NP-40, 0.5%
deoxycholate, 0.1% SDS, 50 mM Tris (pH 8.0), 1 mM EDTA, 1 mM
phenylmethylsulfonyl fluoride, 1 mM NaF, 1 mM
Na3VO4 and a protease inhibitor mixture]
(Life Technologies Korea LLC). The lysates were cleared by
centrifugation at 10,000 x g for 15 min, and the supernatants were
collected. The protein concentration was quantified using a
Bradford Protein Assay (Bio-Rad, Hercules, CA, USA). Equal amounts
of protein lysates were used for western blot analysis with the
indicated antibodies (primary antibody, 1:1,000 dilution, 4°C;
secondary antibody, 1:3,000 dilution, room temperature). The
immunoreactive protein bands were detected using an EZ-Western
Detection kit (Daeillab Service Co., Seoul, Korea).
Statistical analysis
All experiments were performed in triplicate.
Results of the cell proliferation assay and MTT assay are expressed
as the mean ± standard deviation. The standard deviations for all
of the measured biological parameters are displayed in the
appropriate figures. A Student’s t-test (Microsoft Excel,
Albuquerque, NM, USA) was used for single variable comparisons, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Apigenin suppresses the growth of SKBR3
cells
The growth suppressive activity of three
phytoestrogens (apigenin, genistein and quercetin) was evaluated in
SKBR3 cells using a cell proliferation assay. As indicated in
Fig. 1A, apigenin, genistein and
quercetin significantly inhibited SKBR3 cell proliferation in a
dose-dependent manner (0–100 μM) following 72 h of
treatment. Among the three phytoestrogens, apigenin exerted the
greatest growth suppressive activity in the SKBR3 cells. Therefore,
apigenin was selected for use in the present study. In addition,
the time-dependent growth suppressive activity of apigenin was
measured by MTT assay, as shown in Fig. 1B. It was demonstrated that the
proliferation assay was more sensitive than the MTT assay with
respect to measuring the extent of cell growth inhibition, as
indicated by the comparison between Fig. 1A and B. Furthermore, the growth
inhibition exerted by apigenin was confirmed by microscopic
cellular evaluation. The results in Fig. 1C demonstrated that apigenin was
able to effectively attenuate the growth of SKBR3 monolayer cells
following 72 h of treatment. Of note, apigenin also induced
morphological changes in these cells (Fig. 1C). Furthermore, the growth
suppressive activity of apigenin was not limited to breast cancer
cells. The results presented in Fig.
1D demonstrated that apigenin also inhibited the growth of
papillary and anaplastic thyroid cancer cells (SNU790 and
SNU80).
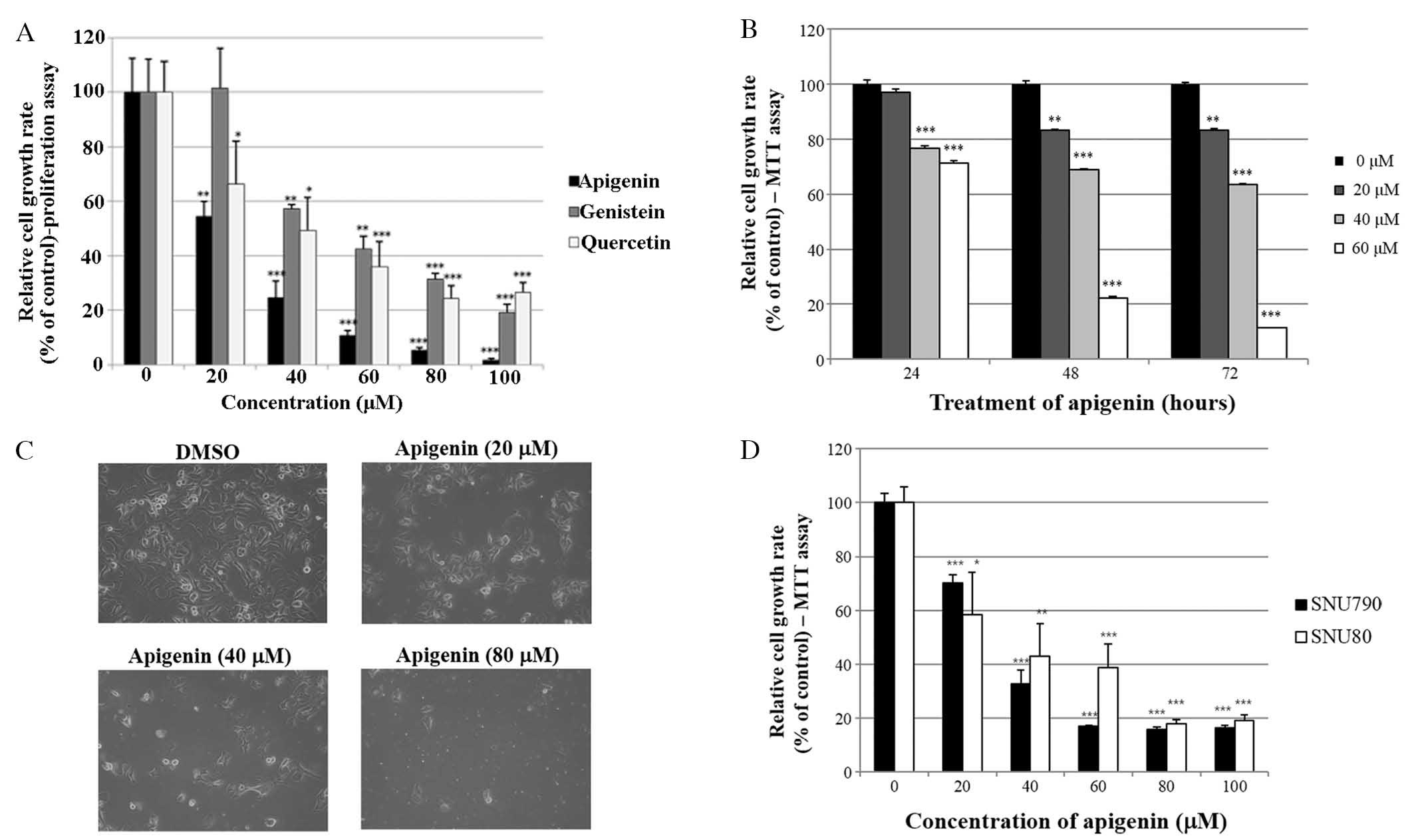 | Figure 1Effect of apigenin on cancer cell
growth. (A) SKBR3 cells were treated with various doses of
apigenin, genistein and quercetin (0, 20, 40, 60, 80 or 100
μM). Following 72 h of culture, cell viability was assessed
using a cell proliferation assay. (B) SKBR3 cells were treated with
various doses of apigenin (0, 20, 40 or 60 μM) and the
relative cell growth rate was measured by MTT assay following 24,
48 and 72 h of culture. The growth rate of the vehicle-treated
cells was set to 100%, and the relative decrease in cell viability
resulting from the apigenin treatment was expressed as a percentage
of the control. (C) SKBR3 cells were treated with various doses of
apigenin (0, 20, 40 or 80 μM) for 72 h and photographed by
phase contrast microscopy (magnification, x40). Control cells were
treated with DMSO alone. (D) SNU790 and SNU80 human thyroid cancer
cell lines were treated with various doses of apigenin (0, 20, 40,
60, 80 or 100 μM). The relative cell growth rate was
measured by MTT assay following 72 h of culture. The growth rate of
the vehicle-treated cells was set to 100%, and the relative
decrease in cell viability resulting from the apigenin treatment
was expressed as a percentage of the control. Values are presented
as the mean ± standard deviation of three independent experiments
(*P<0.05, **P<0.01,
***P<0.001, as compared with 0 μM treated
cells). DMSO, dimethyl sulfoxide. |
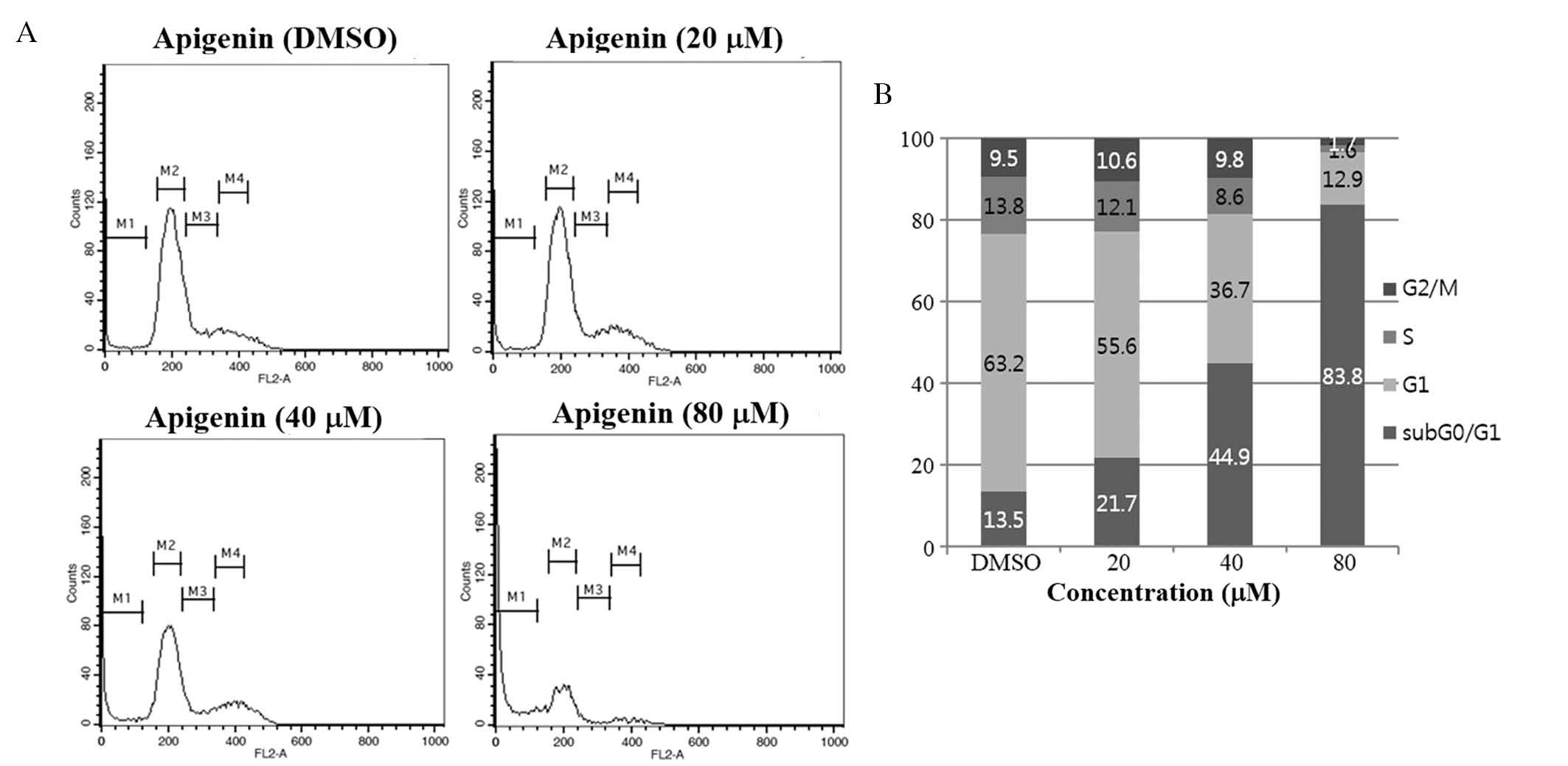
Growth-suppressive activity of apigenin
is accompanied by an increase in the sub
G0/G1 apoptotic population in SKBR3
cells
To investigate whether apigenin inhibited cell
proliferation via the induction of alterations in cell cycle
progression, the effects of apigenin on the cell cycle were
evaluated in SKBR3 cells. Cells were treated with apigenin (0–80
μM) for 72 h and cell cycle distribution was determined by
flow cytometric analysis. The results demonstrated that apigenin
induced an increase in the sub G0/G1
apoptotic population in SKBR3 cells (Fig. 2).
Apigenin induces apoptosis via
caspase-dependent pathways in SKBR3 cells
Whether apigenin activated caspase-dependent
apoptosis was assessed via evaluation of the expression levels of
caspase-8, caspase-3 and PARP. It was revealed that apigenin
upregulated the expression levels of cleaved caspase-8 and -3, and
induced PARP cleavage in SKBR3 cells (Fig. 3A). It was also demonstrated that
the cleavage of caspase-8, caspase-3 and PARP was inhibited by the
caspase-8 inhibitor Z-IETD-fmk and the caspase-9 inhibitor
Z-LEHD-fmk (Fig. 3B).
However, apigenin abrogated this inhibition and induced the
cleavage of caspase-8, caspase-3 and PARP in the presence of
Z-IETD-fmk and Z-LEHD-fmk (Fig. 3B). Furthermore, the caspase-8 and
caspase-9 inhibitors did not suppress cell growth, but apigenin was
still able to induce apoptosis in their presence (Fig. 3C). These results confirmed that
apigenin promoted apoptosis via caspase-dependent mechanisms.
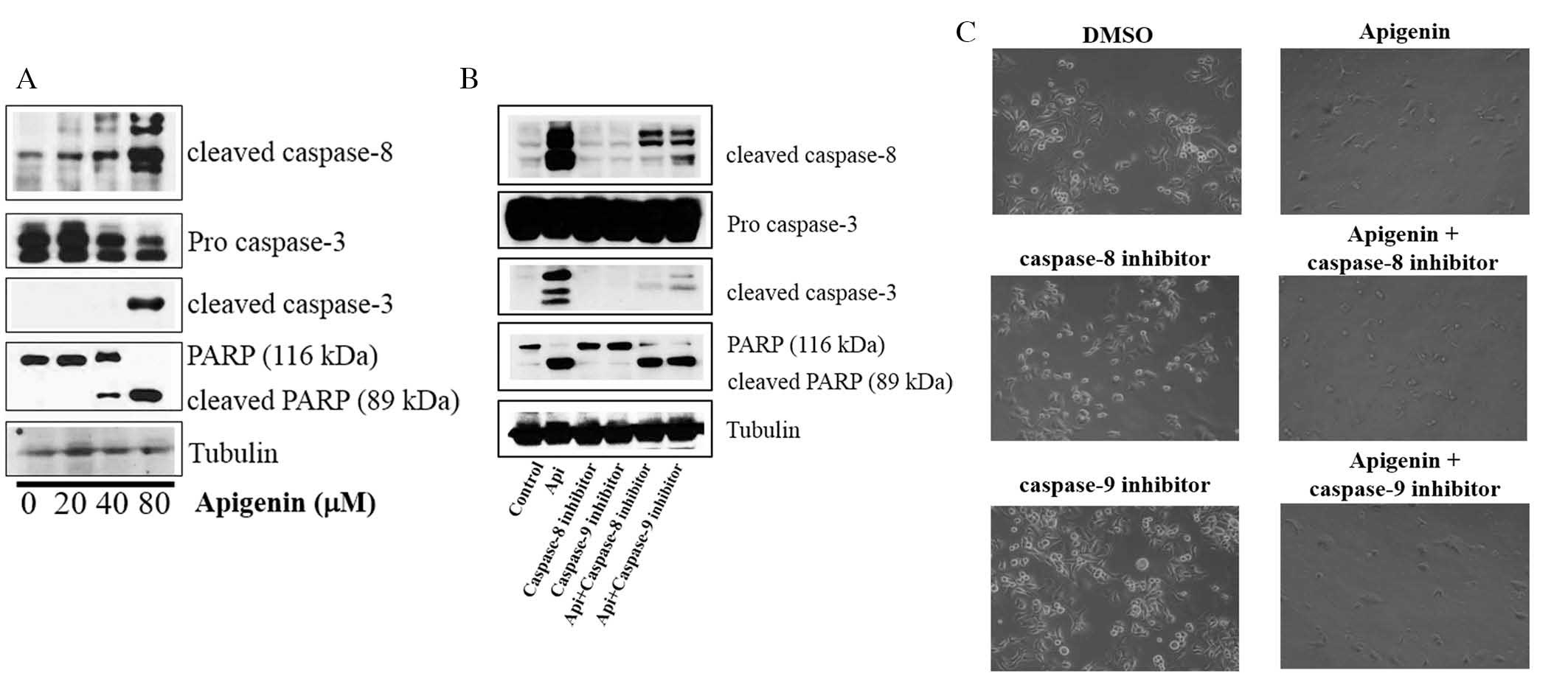 | Figure 3Effect of apigenin on the expression
of apoptotic molecules in SKBR3 cells. (A) Apigenin induces
apoptosis via a caspase-dependent apoptotic pathway in SKBR3 cells.
SKBR3 cells were treated with apigenin (0, 20, 40 or 80 μM)
for 24 h. Whole-cell lysates were evaluated by western blot
analysis with anti-cleaved caspase-8, anti-cleaved caspase-3,
anti-PARP and anti-tubulin antibodies. Blots are representatives of
three independent experiments that produced similar results. (B)
Effect of caspase-8 and caspase-9 inhibitors on apigenin-induced
apoptosis in SKBR3 cells. SKBR3 cells were exposed to 80 μM
apigenin with or without caspase-8 inhibitor (40 μM) or
caspase-9 inhibitor (40 μM) for 24 h, the cell lysates were
separated by SDS-PAGE, and western blot analysis with specific
antibodies was performed (anti-cleaved caspase-8, anti-cleaved
caspase-3, anti-cleaved PARP and anti-tubulin). Results displayed
are representative of three independent experiments that produced
similar results. (C) Effect of caspase-8 and caspase-9 inhibitors
on SKBR3 cell proliferation. SKBR3 cells were exposed to 80
μM apigenin in the presence or absence of caspase-8
inhibitor (40 μM) or caspase-9 inhibitor (40 μM) for
72 h and photographed by phase contrast microscopy (magnification,
x40). DMSO, dimethyl sulfoxide; PARP, poly(adenosine
diphosphate-ribose) polymerase; Api, apigenin. |
Apigenin decreases cell growth rate of
SKBR3 cells via the JAK2-STAT3-VEGF signaling pathway
Whether apigenin regulated the levels of p53, BCL2
and BAX was subsequently evaluated. As indicated in Fig. 4A, apigenin did not influence the
expression of p53, BCL2 or BAX. However, apigenin downregulated the
expression levels of p-STAT3, p-JAK2 (an upstream kinase of STAT3)
and VEGF in SKBR3 cells (Fig. 4B).
In addition, apigenin suppressed the expression of p-STAT3 and
Hif-1α, which were upregulated by hypoxia mimic CoCl2
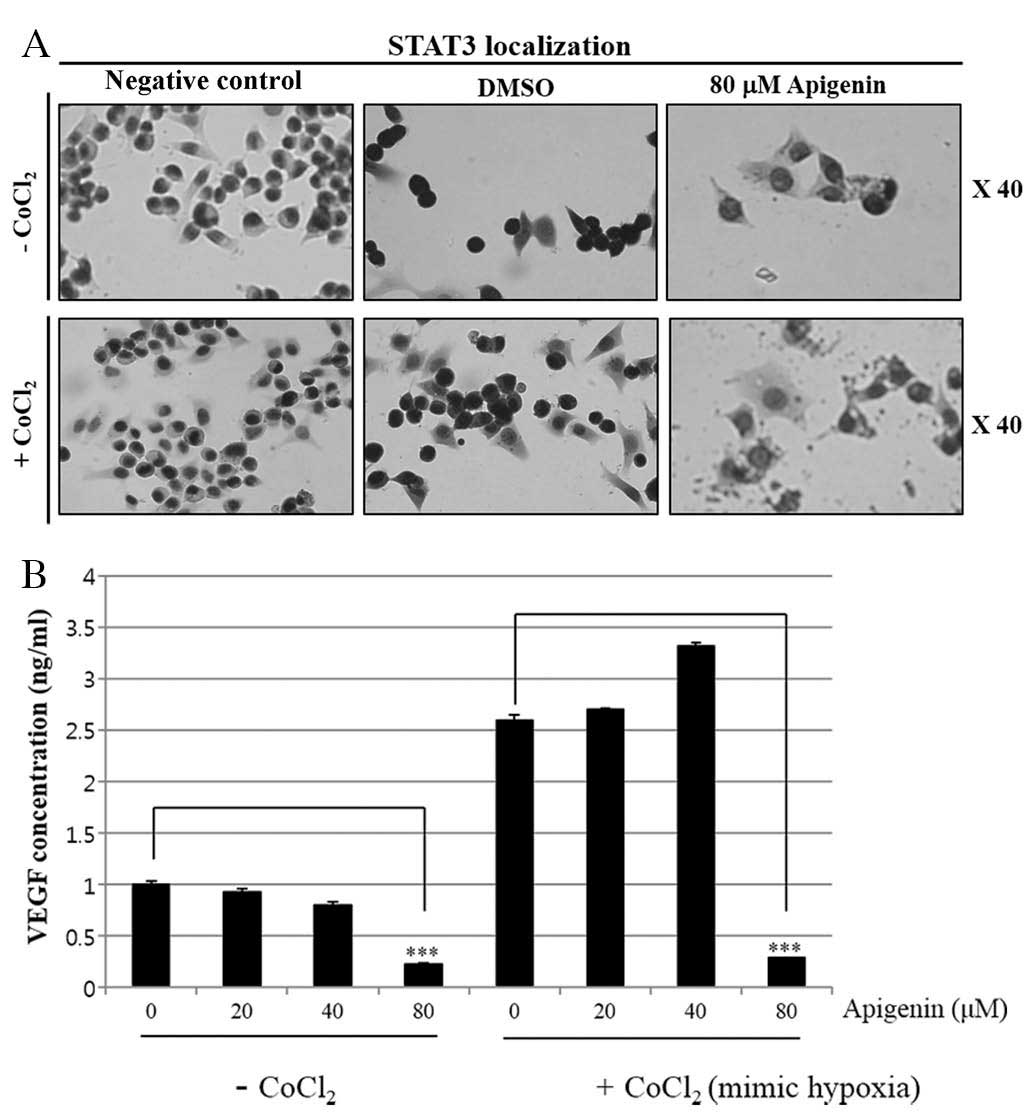
(Fig. 4C). Immunocytochemical
staining indicated that apigenin decreased the nuclear localization
of STAT3 in the presence and absence of CoCl2 (Fig. 5A). Of note, apigenin significantly
attenuated the CoCl2-induced upregulation of VEGF
(Fig. 5B). These results suggested
that apigenin decreased the cell growth rate via inhibition of the
JAK2-STAT3-VEGF signaling pathway.
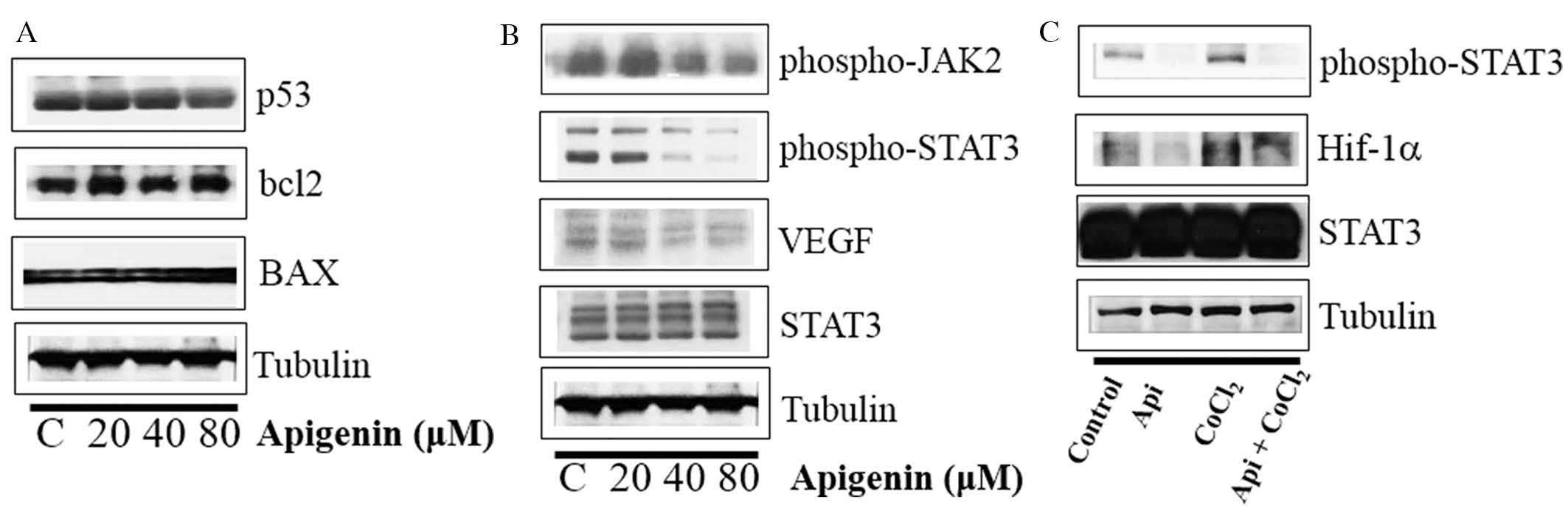 | Figure 4Effect of apigenin on STAT3
activation in SKBR3 cells. (A) SKBR3 cells were treated with
apigenin (0–80 μM) for 24 h. Whole-cell lysates were
analyzed by western blotting with anti-p53, anti-bcl2, anti-BAX and
anti-tubulin antibodies. (B) SKBR3 cells were treated with apigenin
(0–80 μM) for 24 h. Whole-cell lysates were analyzed by
western blotting with anti-phospho-JAK2, anti-phospho-STAT3,
anti-VEGF, anti-STAT3, and anti-tubulin antibodies. (C) SKBR3 cells
were treated with apigenin (80 μM) for 24 h in the presence
or absence of CoCl2 (4 h). Whole-cell lysates were
analyzed by western blotting with anti-phospho-STAT3, anti-HIF-1α,
anti-STAT3, and anti-tubulin antibodies. Blots shown are
representative of three independent experiments that gave similar
results. STAT3, signal transducer and activator of transcription 3;
VEGF, vascular endothelial growth factor; bcl2, B-cell lymphoma 2;
BAX, bcl2-like protein 4; JAK2, janus kinase 2; HIF-1α, hypoxia
inducible factor-1α. |
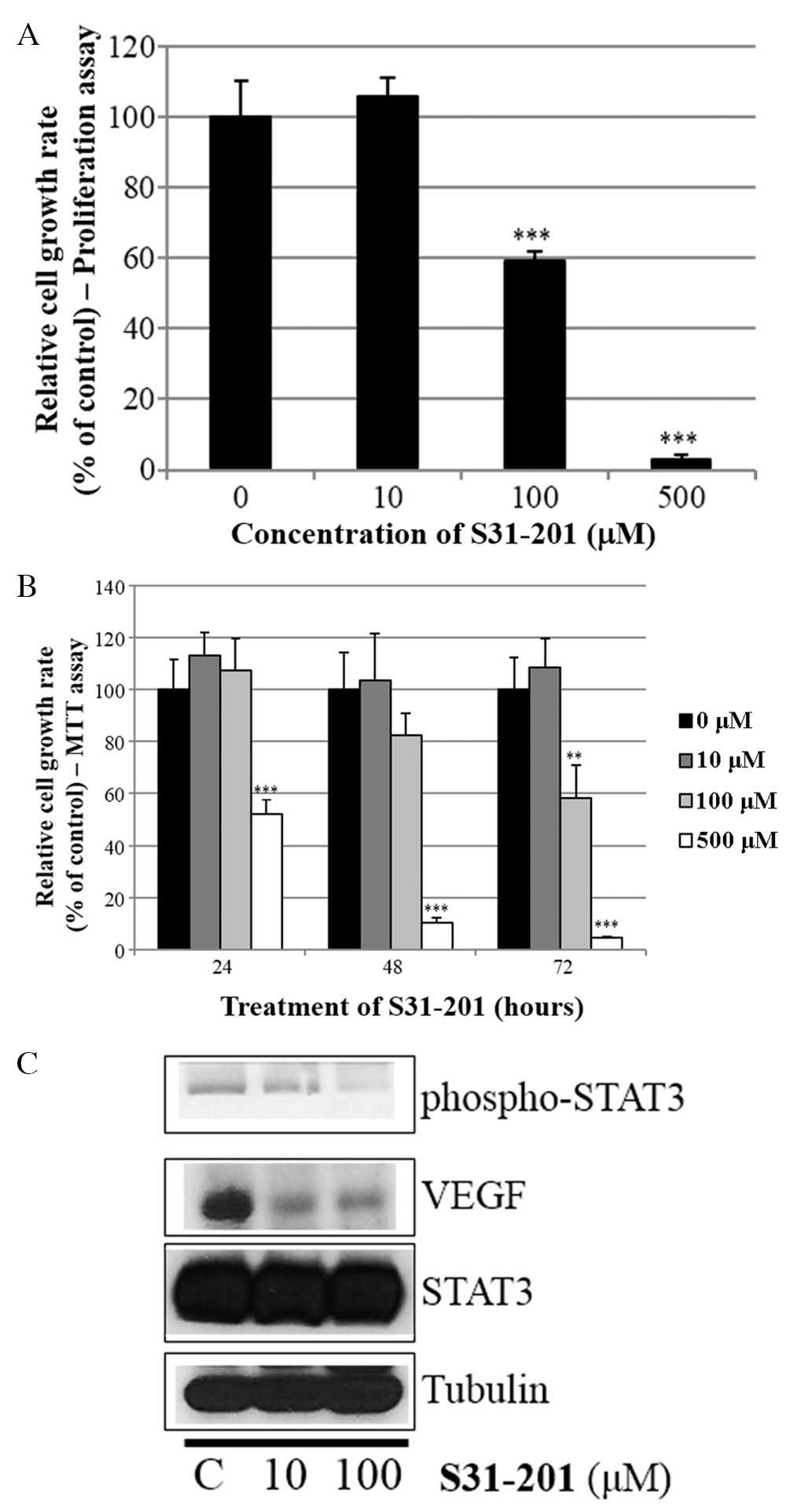
S31-201 inhibits cell growth and
expression of oncogenic molecules in SKBR3 cells
Whether the STAT3 inhibitor S31-201 inhibited cell
proliferation and STAT3 activation in SKBR3 cells was also
investigated. As indicated in Fig. 6A
and B, S31-201 decreased cell growth in a dose- and
time-dependent manner. Furthermore, S31-201 reduced the expression
levels of p-STAT3 and VEGF (Fig.
6C). These results demonstrated that STAT3 inhibition induced
cell growth inhibition and suppressed the expression of oncogenic
molecules.
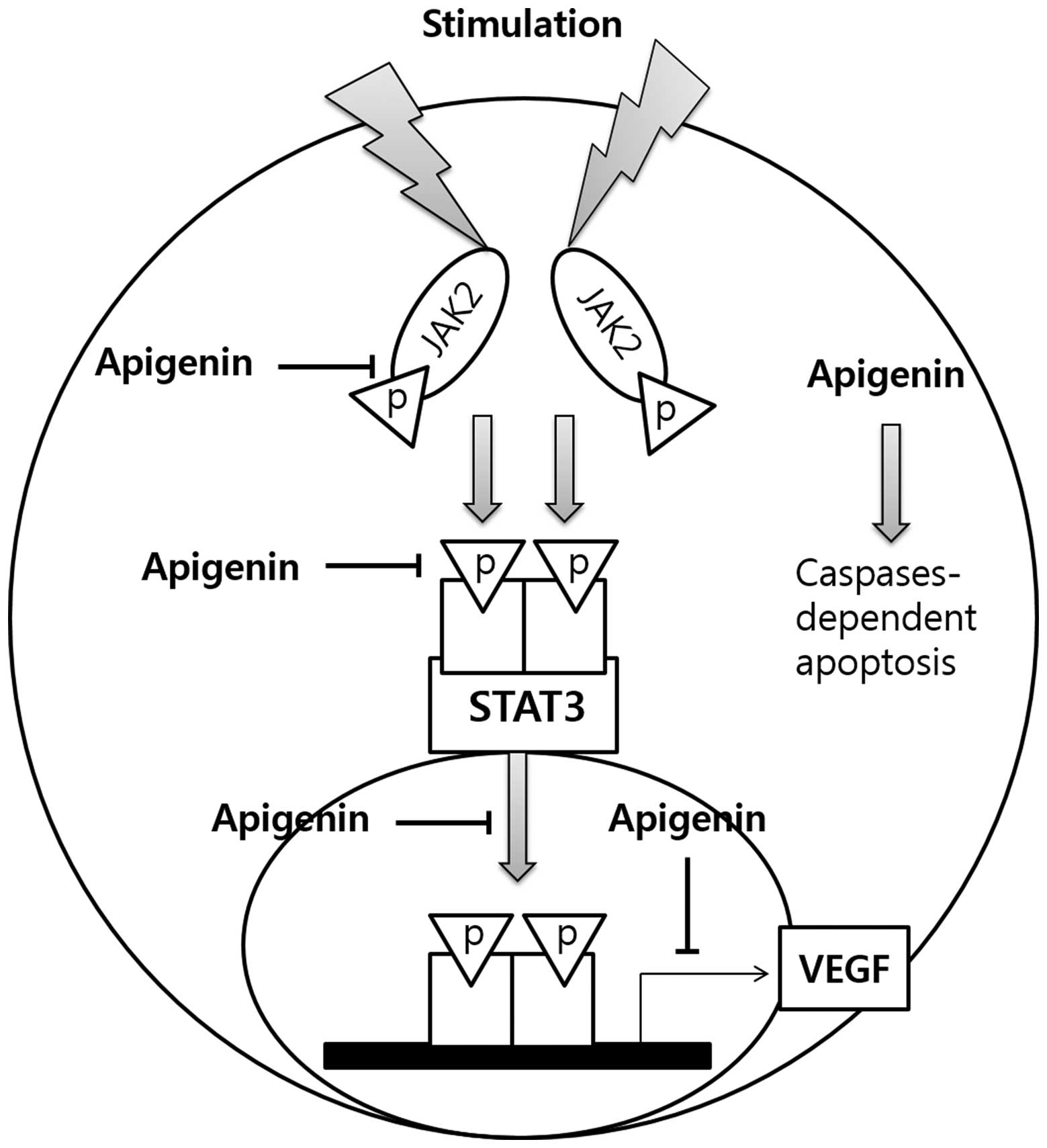
Discussion
The present study aimed to evaluate the potential
anti-proliferative activity of apigenin in SKBR3
HER2-overexpressing breast cancer cells, and elucidate its
mechanism of action (Fig. 7).
Apigenin suppressed the growth of SKBR3 cells in a dose- and
time-dependent manner. Apigenin also inhibited the growth of
papillary and anaplastic thyroid cancer cells (SNU790 and SNU80) in
a dose-dependent manner, which suggested that the
anti-proliferative effect of apigenin was not limited to breast
cancer cells.
The results of the present study indicated that the
growth inhibition induced by apigenin was associated with an
increase in the sub-G0/G1 apoptotic
population of SKBR3 cells. Apigenin increased the number of
apoptotic cells in a dose-dependent manner, as revealed by
fluorescence-assisted cell sorting analysis. Of note, apigenin
induced apoptosis via caspase-dependent pathways by enhancing the
cleavage of caspase-8, caspase-3 and PARP. In order to determine
whether apigenin-induced apoptosis occured via a caspase-8- and
caspase-3-dependent pathway, SKBR3 cells were treated with
caspase-8 inhibitor Z-IETD-fmk and the caspase-9 inhibitor
Z-LEHD-fmk and the expression of caspase-pathway associated
factors was evaluated by western blot analysis. It was revealed
that the cleavage of caspase-8, caspase-3 and PARP was inhibited by
the caspase-8 and caspase-9 inhibitors. However, this inhibition
was abrogated by apigenin, suggesting that it is a potent inducer
of apoptosis.
Caspases are members of a cysteine-dependent
aspartate-regulated protease family, frequently associated with
cell death (31). Caspases are
initially synthesized as relatively inactive zymogens, which are
subsequently activated by scaffold-mediated transactivation or
cleavage by upstream proteases in the relevant intracellular
cascade (31). Following
activation, the caspases cleave various intracellular polypeptides,
including major cytoplasmic and nucleic structural elements,
components of the DNA repair machinery and numerous protein kinases
(31).
The results of the western blot analyses in the
present study revealed that apigenin did not influence the
expression levels of apoptotic molecules, including p53, BCL2 and
BAX, which suggested that apigenin may not be able to regulate the
levels of p53 (32). However,
apigenin reduced the expression of p-STAT3 and p-JAK2 in SKBR3
cells. The VEGF promoter contains various transcription factor
binding sites, including sites for STAT3 (33) and Hif-1 (34). The physical interaction between
STAT3 and Hif-1 regulates the transcriptional activation of VEGF
via their binding to the VEGF promoter (35). In the present study, it was
demonstrated that apigenin inhibited VEGF expression and
production, as well as p-STAT3 expression and nuclear localization
in the presence or absence of CoCl2. The STAT3 inhibitor
S31-201 decreased the expression of p-STAT3 and VEGF. Culturing of
SKBR3 cells under conditions that mimicked hypoxia or normoxia did
not induce the expression or activation of MMP-2 or MMP-9 (data not
shown), as indicated in a previous study (36). Conversely, the co-culture of SKBR3
with another cell line or tumor-associated macrophages induces the
expression and activation of MMP-2 and MMP-9 (36). The results of the present study
demonstrated that apigenin suppressed cell growth via inhibition of
the STAT3-VEGF signaling pathway in SKBR3 cells (Fig. 7).
STAT3 is a transcription factor which modulates gene
expression in response to certain cellular stimuli and has
significant roles in the mediation of cell growth and apoptosis.
STAT3 frequently functions as a tumor promoter; however, a
tumor-suppressor role for STAT3 has also been reported (37,38).
STAT3 enhances cellular proliferation and angiogenesis, inhibits
apoptosis and promotes invasion and metastasis (39–41).
The expression of STAT3 in melanoma tumors is associated with poor
prognosis (39–41). Constitutive STAT3 phosphorylation
is regulated by upstream kinases (Jak and Src) and has been
suggested to be a crucial stage in oncogenesis (42,43).
Resveratrol (a phytoestrogen) has been shown to inhibit STAT3
signaling and induce apoptosis in malignant cells expressing
activated STAT3 (44).
HER2 overexpression is associated with ~20–25% of
invasive breast carcinomas (45).
A normal, healthy breast cell has 20,000 HER2 receptors, compared
with up to 1.5 million in a breast cancer cell. HER2 is a member of
the HER/ErbB2/Neu protein family, which comprises multiple
receptors, including HER1/EGFR, HER3 and HER4. Crosstalk between
the HER2 and estrogen receptor (ER) signal transduction pathways
has been identified (46), and ER
is able to modulate HER2 expression levels. In the present study,
it was demonstrated that apigenin was able to significantly inhibit
the growth of HER2-overexpressing breast cancer cells. This result
revealed that apigenin may represent a potential natural
therapeutic for the treatment and prevention of HER2-overexpressing
breast cancer.
Acknowledgments
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education, Science and Technology
(NRF-2012R1A1A3004797). This study was also supported by a grant
from the Korean Medicine R&D project of the Ministry of Health
and Welfare (B120014).
References
|
1
|
Shukla S and Gupta S: Apigenin: A
promising molecule for cancer prevention. Pharm Res. 27:962–978.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rezai-Zadeh K, Ehrhart J, Bai Y, Sanberg
PR, Bickford P, Tan J and Shytle RD: Apigenin and luteolin modulate
microglial activation via inhibition of STAT1-induced CD40
expression. J Neuroinflammation. 5:412008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moon DO, Kim MO, Choi YH, Lee HG, Kim ND
and Kim GY: Gossypol suppresses telomerase activity in human
leukemia cells via regulating hTERT. FEBS Lett. 582:367–373. 2008.
View Article : Google Scholar
|
|
4
|
Nakazawa T, Yasuda T, Ueda J and Ohsawa K:
Antidepressant-like effects of apigenin and
2,4,5-trimethoxycinnamic acid from Perilla frutescens in the forced
swimming test. Biol Pharm Bull. 26:474–480. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hashemi M, Nouri Long M, Entezari M,
Nafisi S and Nowroozii H: Anti-mutagenic and pro-apoptotic effects
of apigenin on human chronic lymphocytic leukemia cells. Acta Med
Iran. 48:283–288. 2010.
|
|
6
|
Patel D, Shukla S and Gupta S: Apigenin
and cancer chemoprevention: progress, potential and promise
(review). Int J Oncol. 30:233–245. 2007.
|
|
7
|
Shukla S and Gupta S: Molecular targets
for apigenin-induced cell cycle arrest and apoptosis in prostate
cancer cell xenograft. Mol Cancer Ther. 5:843–852. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang YC, Huang YT, Tsai SH, Lin-Shiau SY,
Chen CF and Lin JK: Suppression of inducible cyclooxygenase and
inducible nitric oxide synthase by apigenin and related flavonoids
in mouse macrophages. Carcinogenesis. 20:1945–1952. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Birt DF, Mitchell D, Gold B, Pour P and
Pinch HC: Inhibition of ultraviolet light induced skin
carcinogenesis in SKH-1 mice by apigenin, a plant flavonoid.
Anticancer Res. 17:85–91. 1997.PubMed/NCBI
|
|
10
|
Seo HS, Ju JH, Jang K and Shin I:
Induction of apoptotic cell death by phytoestrogens by
up-regulating the levels of phospho-p53 and p21 in normal and
malignant estrogen receptor α-negative breast cells. Nutrition Res.
31:139–146. 2011. View Article : Google Scholar
|
|
11
|
Lu HF, Chie YJ, Yang MS, Lee CS, Fu JJ,
Yang JS, Tan TW, Wu SH, Ma YS, Ip SW and Chung JG: Apigenin induces
caspase-dependent apoptosis in human lung cancer A549 cells through
Bax- and Bcl-2-triggered mitochondrial pathway. Int J Oncol.
36:1477–1484. 2010.PubMed/NCBI
|
|
12
|
Wang W, Heideman L, Chung CS, Pelling JC,
Koehler KJ and Birt DF: Cell-cycle arrest at G2/M and growth
inhibition by apigenin in human colon carcinoma cell lines. Mol
Carcinog. 28:102–110. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Turktekin M, Konac E, Onen HI, Alp E,
Yilmaz A and Menevse S: Evaluation of the effects of the flavonoid
apigenin on apoptotic pathway gene expression on the colon cancer
cell line (HT29). J Med Food. 14:1107–1117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gupta S, Afaq F and Mukhtar H: Involvement
of nuclear factor-kappa B, Bax and Bcl-2 in induction of cell cycle
arrest and apoptosis by apigenin in human prostate carcinoma cells.
Oncogene. 21:3727–3738. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruela-de-Sousa RR, Fuhler GM, Blom N,
Ferreira CV, Aoyama H and Peppelenbosch MP: Cytotoxicity of
apigenin on leukemia cell lines: implications for prevention and
therapy. Cell Death Dis. 1:e192010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ujiki MB, Ding XZ, Salabat MR, Bentrem DJ,
Golkar L, Milam B, Talamonti MS, Bell RH Hr, Iwamura T and Adrian
TE: Apigenin inhibits pancreatic cancer cell proliferation through
G2/M cell cycle arrest. Mol Cancer. 5:762006. View Article : Google Scholar
|
|
17
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 37:719–727. 2005. View Article : Google Scholar
|
|
18
|
Bosch M, Poulter NS, Vatovec S and
Franklin-Ton VE: Initiation of programmed cell death in
self-incompatibility: role for cytoskeleton modifications and
several caspase-like activities. Mol Plant. 1:879–887. 2008.
View Article : Google Scholar
|
|
19
|
Zhang A, Wu Y, Lai HWL and Yew DT:
Apoptosis-a brief review. Neuroembryology. 3:47–59. 2004.
View Article : Google Scholar
|
|
20
|
Waring P and Mullbacher A: Cell death
induced by the Fas/Fas ligand pathway and its role in pathology.
Immunol Cell Biol. 77:312–317. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gupta S: Molecular signaling in death
receptor and mitochondrial pathways of apoptosis (review). Int J
Oncol. 22:15–20. 2003.
|
|
22
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boulares AH, Yakovlev AG, Ivanova V,
Stoica BA, Wang G, Iyer S and Smulson M: Role of poly(ADP-ribose)
polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP
mutant increases rates of apoptosis in transfected cells. J Biol
Chem. 274:22932–22940. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wilson CA, Cajulis EE, Green JL, Olsen TM,
Chung YA, Damore MA, Dering J, Calzone FJ and Slamon DJ: HER-2
over-expression differentially alters transforming growth
factor-beta responses in luminal versus mesenchymal human breast
cancer cells. Breast Cancer Res. 7:R1058–R1079. 2005. View Article : Google Scholar
|
|
25
|
Prat A, Carey LA, Adamo B, Vidal M,
Tabernero J, Cortés J, Parker JS, Perou CM and Baselga J: Molecular
features and survival outcomes of the intrinsic subtypes within
HER2-positive breast cancer. J Natl Cancer Inst. 106:dju1522014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Joshi JP, Brown NE, Griner SE and Nahta R:
Growth differentiation factor 15 (GDF15)-mediated HER2
phosphorylation reduces trastuzumab sensitivity of
HER2-overexpressing breast cancer cells. Biochem Pharmacol.
82:1090–1099. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Favoni RE, Daga A, Malatesta P and Florio
T: Preclinical studies identify novel targeted pharmacological
strategies for treatment of human malignant pleural mesothelioma.
Br J Pharmacol. 166:532–553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tokunaga E, Oki E, Nishida K, Koga T,
Egashira A, Morita M, Kakeji Y and Maehara Y: Trastuzumab and
breast cancer: Developments and current status. Int J Clin Oncol.
11:199–208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dean-Colomb W and Esteva FJ: Her2-positive
breast cancer: Herceptin and beyond. Eur J Cancer. 44:2806–2812.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Seo HS, Choi HS, Kim SR, Choi YK, Woo SM,
Shin I, Woo JK, Park SY, Shin YC and Ko SG: Apigenin induces
apoptosis via extrinsic pathway, inducing p53 and inhibiting STAT3
and NFκB signaling in HER2-overexpressing breast cancer cells. Mol
Cell Biochem. 366:319–334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Earnshaw WC, Martins LM and Kaufmann SH:
Mammalian caspases: structure, activation, substrates and functions
during apoptosis. Annu Rev Biochem. 68:383–424. 1999. View Article : Google Scholar
|
|
32
|
Blagosklonny MV, An WG, Romanova LY,
Trepel J, Fojo T and Neckers L: p53 inhibits hypoxia-inducible
factor-stimulated transcription. J Biol Chem. 273:11995–11998.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Niu G, Wright KL, Huang M, Song L, Haura
E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R,
Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R and Yu H:
Constitutive Stat3 activity up-regulates VEGF expression and tumor
angiogenesis. Oncogene. 21:2000–2008. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Forsythe JA, Jiang BH, Iyer NV, Agani F,
Leung SW, Koos RD and Semenza GL: Activation of vascular
endothelial growth factor gene transcription by Hypoxia-inducible
factor 1. Mol Cell Biol. 16:4604–4613. 1996.PubMed/NCBI
|
|
35
|
Jung JE, Lee HG, Cho IH, Chung DH, Yoon
SH, Yang YM, Lee JW, Choi S, Park JW, Ye SK and Chung MH: STAT3 is
a potential modulator of HIF-1-mediated VEGF expression in human
renal carcinoma cells. FASEB J. 19:1296–1298. 2005.PubMed/NCBI
|
|
36
|
Hagemann T, Robinson SC, Schulz M, Trümper
L, Balkwill FR and Binder C: Enhanced invasiveness of breast cancer
cell lines upon co-cultivation with macrophages is due to TNF-alpha
dependent up-regulation of matrix metalloproteases. Carcinogenesis.
25:1543–1549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
de la Iglesia N, Konopka G, Puram SV, Chan
JA, Bachoo RM, You MJ, Levy DE, DEPinho RA and Bonni A:
Identification of a PTEN-regulated STAT3 brain tumor suppressor
pathway. Genes Dev. 22:449–462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lewis HD, Winter A, Murphy TF, Tripathi S,
Pandey VN and Barton BE: STAT3 inhibition in prostate and
pancreatic cancer lines by STAT3 binding sequence oligonucleotides:
Differential activity between 5′ and 3′ ends. Mol Cancer Ther.
7:1543–1550. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kortylewski M, Jove R and Yu H: Targeting
STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev.
24:315–327. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Niu G, Bowman T, Huang M, Shivers S,
Reintgen D, Daud A, Chang A, Kraker A, Jove R and Yu H: Roles of
activated Src and Stat3 signaling in melanoma tumor cell growth.
Oncogene. 21:7001–7010. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xie TX, Huang FJ, Aldape KD, Kang SH, Liu
M, Gershenwald JE, Xie K, Sawaya R and Huang S: Activation of stat3
in human melanoma promotes brain metastasis. Cancer Res.
66:3188–3196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sellers LA, Feniuk W, Humphrey PP and
Lauder H: Activated G proteincoupled receptor induces tyrosine
phosphorylation of STAT3 and agonist-selective serine
phosphorylation via sustained stimulation of mitogen-activated
protein kinase. Resultant effects on cell proliferation. J Biol
Chem. 274:16423–16430. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Y, Turkson J, Carter-Su C, Smithgall
T, Levitzki A, Kraker A, Krolewski JJ, Medveczky P and Jove R:
Activation of Stat3 in v-Src-transformed fibroblasts requires
cooperation of Jak1 kinase activity. J Biol Chem. 275:24935–24944.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kotha A, Sekharam M, Cilenti L, Siddiquee
K, Khaled A, Zervos AS, Carter B, Turkson J and Jove R: Resveratrol
inhibits src and Stat3 signaling and induces the apoptosis of
malignant cells containing activated Stat3 protein. Mol Cancer
Ther. 5:621–629. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tolaney SM and Krop IE: Mechanisms of
trastuzumab resistance in breast cancer. Anticancer Agents Med
Chem. 9:348–355. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Buzdar AU: Role of biologic therapy and
chemotherapy in hormone receptor- and HER2-positive breast cancer.
Ann Oncol. 20:993–999. 2009. View Article : Google Scholar : PubMed/NCBI
|