Introduction
Guillain-Barré syndrome (GBS) is an autoimmune
disorder, characterized by demyelination and infiltration of the
peripheral nervous system by inflammatory cells, causing
progressive weakness of the extremities. GBS consists of acute
inflammatory demyelinating polyneuropathy, acute motor axonal
neuropathy, acute motor sensory axonal neuropathy and Miller Fisher
syndrome (MFS). The incidence of GBS has been reported to be 0.75–2
cases per 100,000 individuals per year (1). In recent years, studies have reported
that vaccines, such as the influenza A (H1N1) 2009 monovalent
inactivated vaccine, are associated with a mildly increased risk of
GBS (2,3). However, the pathogenesis of GBS
remains to be elucidated. To date, numerous hypotheses have been
suggested to explain the pathogenesis of GBS, including the
involvement of molecular mimicry, cytokines, anti-ganglioside
antibodies and Toll-like receptors (TLRs). The present study was
performed to investigate the association between TLRs and GBS.
TLRs are pattern recognition receptors, which
recognize conserved motifs on pathogens via pathogen-associated
molecular patterns (4). To date,
11 members of the TLR family have been identified in humans.
Ligands of TLRs consist of endogenous and exogenous ligands. Each
sub-type is able to recognize a specific ligand. For example, TLR2
recognizes peptidoglycan (PGN) (5)
and TLR4 recognizes lipopolysaccharide (LPS) (6). After recognizing specific ligands,
TLRs signal through myeloid differentiation factor
(MyD)88-dependent or -independent pathways. TLR2 signals
predominantly through a MyD88-dependent pathway, whereas TLR4
signals through the two types of pathway. Nuclear factor (NF)-κB is
a downstream signaling molecule of the MyD88-dependent pathway.
Following signal transduction through the MyD88-dependent pathway,
increased levels of NF-κB are generated. MyD88 and NF-κB are
important in TLR signal transduction.
The major function of TLRs is to promoe the
production of cytokines (7),
including interleukin (IL)-1β, IL-6, IL-12, tumor necrosis factor
(TNF)-α and interferon (IFN)-γ. Cytokines are thought to be
important in the pathogenesis of GBS (8), which suggests that TLRs may be
involved in the pathogenesis of GBS. TLRs are also able to promote
the differentiation and maturation of immune cells, which may also
be associated with the pathogenesis of GBS. TNF-α and IL-1β are
major pro-inflammatory cytokines secreted by T-helper (Th)1 cells.
Numerous studies have demonstrated that the levels of TNF-α and
IL-1β are significantly increased in the plasma and cerebrospinal
fluid (CSF) of patients with GBS (9,10).
The current hypothesis states that TLRs are involved in the
initiation of GBS. Following TLR activation, they increase NF-κB
levels through MyD88-dependent or -independent pathways, and
finally induce the secretion of pro-inflammatory cytokines. To
investigate the association between TLR2 and TLR4 and GBS
pathogenesis, TNF-α and IL-1β levels were measured in the culture
supernatant of peripheral blood mononuclear cells (PBMCs) from
patients with GBS and healthy controls in the present study.
Gangliosides have important biological functions,
including cellular growth and differentiation, modulation of signal
transduction and immune responses. GBS arises as a result of an
autoimmune attack due to structural similarities between certain
LPS molecules from Campylobacter jejuni and human nerve
tissue gangliosides (11,12). LPS is recognized by TLRs (13) and therefore, TLRs may be involved
in the pathogenesis of GBS. Recent evidence has suggested that
anti-ganglioside antibodies are associated with the pathogenesis of
GBS (14).
Excessive activation of TLRs is capable of
eliminating auto-immune tolerance and may lead to autoimmune
diseases (15), including systemic
lupus erythematosus (SLE) and autoimmune hepatitis. Numerous
studies have demonstrated that TLRs are involved in the
pathogenesis of experimental autoimmune neuritis (EAN), an animal
model of GBS (16,17), and may be important in the
pathogenesis of GBS.
To demonstrate the involvement of TLR2 and TLR4, as
well as their signal transduction pathways, in the progression and
pathogenesis of GBS, the mRNA, cytokines and anti-ganglioside
antibodies of patients with GBS were analyzed.
Patients and methods
Patients and healthy controls
A total of 18 patients with GBS (11 patients with
mild GBS and 7 patients with severe GBS) and 20 healthy controls
were enrolled in the present study from the Health Examination
Center of Beijing Tiantan Hospital (Beijing, China) between October
2012 and December 2013. All patients met the diagnostic criteria of
GBS, and were classified into mild (1–3 points) and severe (4–6
points, including dysphagia and dyspnea) groups according to their
Hughes scores (18). The exclusion
criteria included patients with a fever or infection. The study was
approved by the Ethics Committee of Beijing Tiantan Hospital,
Capital Medical University (Beijing, China), and written informed
consent was obtained from all participants.
Preparation of PBMCs
Approximately 15 ml [3 ml for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
12 ml for cell culture] of venous blood was collected from each
subject into EDTA-anticoagulated vacuum tubes and PBMCs were
isolated by Ficoll gradient separation with lymphocyte isolation
agent (Sigma-Aldrich, St. Louis, MO, USA). Centrifugation was
conducted for 30 min at 400 × g.
RNA extraction
PBMCs were immediately snap-frozen in TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) following washing
three times in phosphate-buffered saline (PBS; Invitrogen Life
Technologies). Total RNA from PBMCs was extracted using TRIzol,
absolute ethanol and isopropanol. The RNA concentration and quality
were measured using the NanoDrop-1000 spectrophotometer (NanoDrop
Technologies, Thermo Fisher Scientific, Waltham, MA, USA). An
optical density of 1.8–2.1 at 260nm/280nm indicated high-quality
RNA.
RT
A total of 20 μl cDNA was prepared using a
First Strand cDNA synthesis kit (Fermentas, Vilnius, Lithuania)
from 1 μg RNA. RT was performed according to the
manufacturer’s instructions. To ensure the success of RT, a general
PCR was performed prior to RT-qPCR, which was performed under the
same conditions. The general PCR was performed using 2X EasyTaq PCR
Supermix (TransGen Biotech Co., Ltd., Beijing, China) using an ABI
9600 thermocycler (Applied Biosystems, Thermo Fisher Scientific).
The amplification and detection stages of general PCR were
performed as follows: 94°C for 5 min followed by 30 cycles of 94°C
for 30 sec, 58°C for 30 sec and 72°C for 30 sec, followed by 72°C
for 5 min. The 20-μl reaction mix consisted of RNase-free
water (7.8 μl), 0.6 μl 10 μmol/l
GAPDH1 forward/reverse primer, 1 μl cDNA and 10
μl PCR mix. All primers were synthesized by Sangon Biotech
(Shanghai, China) and are listed in Table I. The products were analyzed using
agarose gel electrophoresis. The agarose gels were purchased from
TransGen Biotech Co., Ltd., the electrophoresis meter used was the
EPS 300 from Tanon Science & Technology Co., Ltd. (Shanghai,
China) and the BioSpectrum Imaging System was from UVP, LLC
(Upland, CA, USA).
 | Table IPrimers for TLR2, TLR4, MyD88, NF-κB,
GAPDH1 and GAPDH2. |
Table I
Primers for TLR2, TLR4, MyD88, NF-κB,
GAPDH1 and GAPDH2.
| Genes | Forward
primers | Reverse
primers | Product length
(bp) |
|---|
| TLR2 |
5′-GCCTCTCCAAGGAAGAATCC-3′ |
5′-TCCTGTTGTTGGACAGGTCA-3′ | 144 |
| TLR4 |
5′-AAGCCGAAAGGTGATTGTTG-3′ |
5′-CTGAGCAGGGTCTTCTCCAC-3′ | 153 |
| MyD88 |
5′-CATCACCACACTTGATGACCC-3′ |
5′-TGCACAAACTGGATGTCGC-3′ | 90 |
| NF-κB |
5′-AGCCTGGTAGACACGTACCG-3′ |
5′-CCGTACGCACTGTCTTCCTT-3′ | 200 |
|
GAPDH1 |
5′-GTCACCAGGGCTGCTTTT-3′ |
5′-CATCACGCCACAGTTTCC-3′ | 544 |
|
GAPDH2 |
5′-TCGGAGTCAACGGATTTGG-3′ |
5′-GCAACAATATCCACTTACCAGAGTTAA-3′ | 79 |
qPCR
qPCR was performed using the ABI Step-One Plus
Real-Time PCR system (Applied Biosystems) using Power SYBR Green
PCR Master mix (Applied Biosystems) according to the manufacturer’s
instructions. The sequences of the primers are shown in Table I. The amplification and detection
stages of qPCR were performed as follows: 95°C for 10 min followed
by 40 cycles of 95°C for 15 sec, 58°C for 30 sec and 72°C for 30
sec. The 20-μl reaction system consisted of SYBR mix (10
μl), 0.4 μl 10 μmol/l forward/reverse primer,
1 μl cDNA and 8.2 μl RNase-free water. Relative
quantification of RNA expression was calculated using the
2−∆CT method using the following equation: ∆CT =
CTtarget gene - CTGAPDH2. GAPDH2 was used as an internal
control.
PBMC culture and cytokine analysis
PBMCs were isolated from 12 ml venous blood from
patients with GBS or healthy controls and were cultured with
ligands of TLR2 (PGN; Sigma-Aldrich; 100 μg/ml) or TLR4
(LPS; Sigma-Aldrich; 10 μg/ml). PBMCs cultured without
ligands were used as blank controls. Subsequently, 500 μl
PBS was added to dilute the PBMCs. The cell concentration was
determined using the Sysmex XT-4000i hematology analyzer (Sysmex,
Kobe, Japan). PBMCs were cultured in 24 well-plates and each well
consisted of RPMI 1640 medium supplemented with 10% fetal calf
serum (Invitrogen Life Technologies), 106 PBMCs and the
respective agonist. Following incubation for 24 h in an SS-23062
carbon dioxide incubator (SHEL LAB, Cornelius, OR, USA) with 5%
CO2, the supernatant was harvested, and TNF-α and IL-1β
were measured using the IMMULITE 1000 immunoassay system (Siemens
Healthcare Diagnostics, Erlangen, Germany) according to the
manufacturer’s instructions.
Anti-ganglioside antibody detection
Serum anti-ganglioside antibodies were assessed
using an immunoblot assay in 18 patients with GBS and 20 healthy
controls using EUROLINE anti-ganglioside profile 2: IgG and IgM
(EUROIMMUN Medizinische Labordiagnostika AG, Lübeck, Germany).
Statistical analysis
All values are expressed as the mean ± standard
error. Comparisons of mRNA levels between patients with GBS and
healthy controls were performed using Student’s t-test. The Fisher
exact probability test was used to examine the correlation between
positive anti-ganglioside antibody rate and mild or severe GBS.
SPSS software, version 17.0 (SPSS, Inc., Chicago, IL, USA) was used
for statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Analysis of TLR2, TLR4, MyD88 and NF-κB
mRNA
A previous study demonstrated that TLRs and their
signal transduction pathway may be involved in the pathogenesis of
GBS (19). In the present study,
PCR assays were performed using PBMCs from patients with GBS and
healthy controls to analyze the mRNA levels of TLR2, TLR4, MyD88
and NF-κB. As shown in Fig. 1,
TLR2, TLR4, MyD88 and NF-κB mRNA levels were significantly
upregulated in patients with GBS compared with those in healthy
controls (P<0.05).
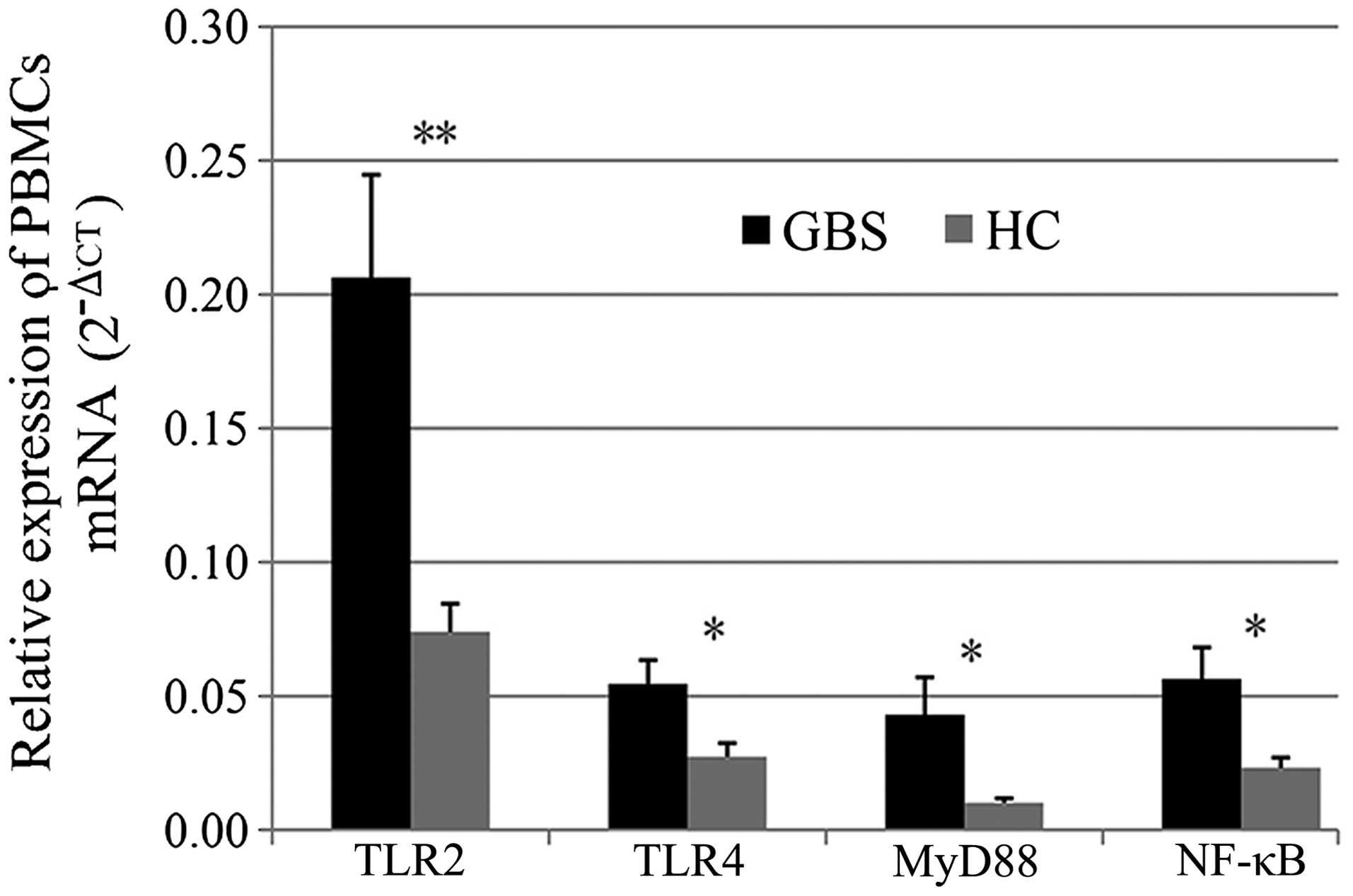 | Figure 1Relative expression of TLR2, TLR4,
MyD88 and NF-κB mRNA. Values are expressed as the mean ± standard
error. Expression levels of TLR2, TLR4, MyD88 and NF-κB mRNA
increased significantly in patients with GBS compared with those in
healthy controls (*P<0.05; **P<0.01 for
comparison between GBS and HC groups). PTLR2=0.003,
PTLR4=0.017, PMyD88=0.032 and PNF-κB=0.015.
GBS, Guillain-Barré syndrome; HC, healthy controls; TLR, Toll-like
receptor; NF, nuclear factor; MyD, myeloid differentiation factor;
PBMCs, peripheral blood mononuclear cells; CT, cycle threshold. |
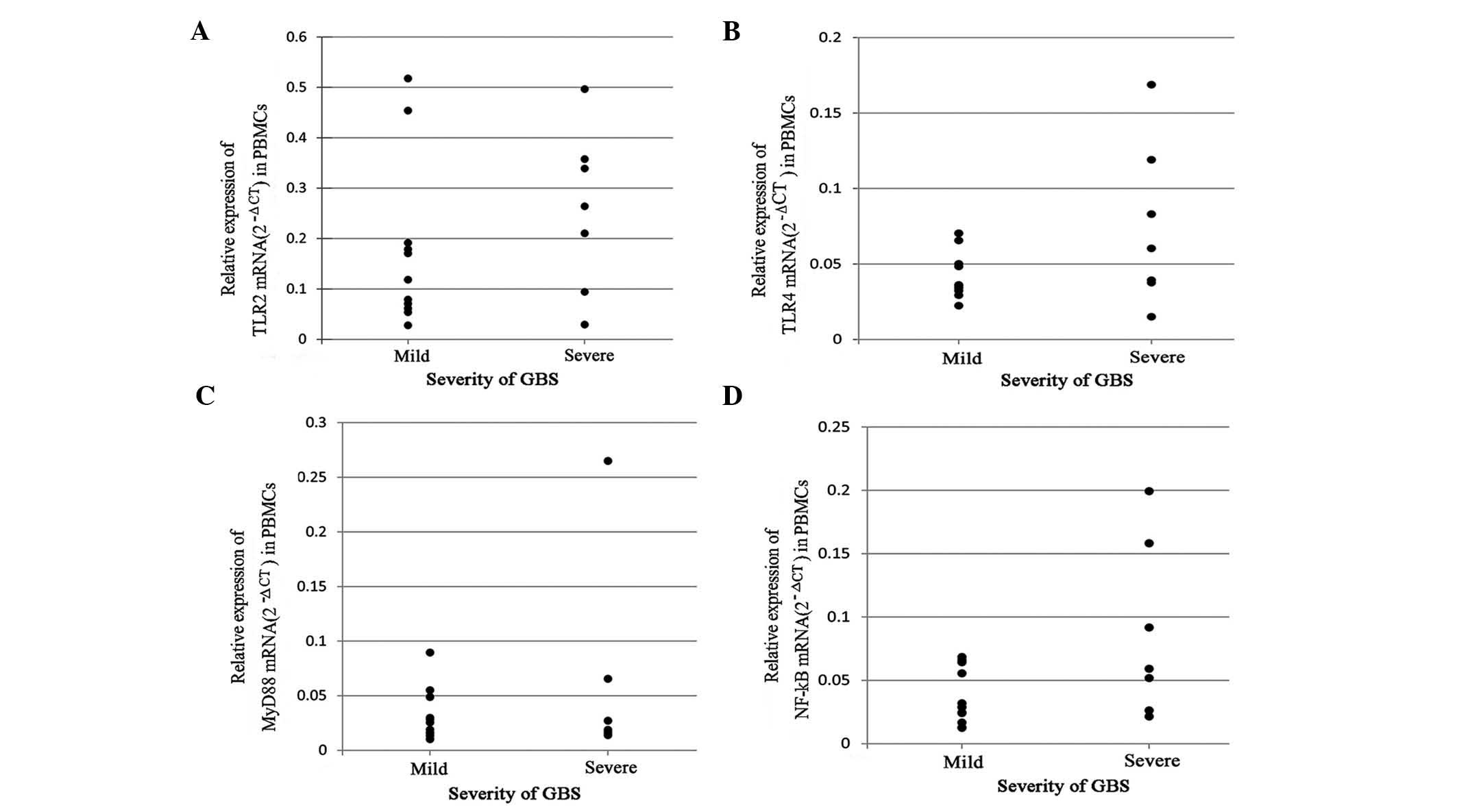
The correlation of mRNA expression and the severity
of GBS was evaluated using Student’s t-test. As shown in Fig. 2, no significant difference was
identified in TLR2, TLR4 and NF-κB mRNA expression between the two
groups, although the levels tended to be higher in patients with
severe GBS (P>0.05). In addition, no significant difference was
identified in MyD88 levels between patients with mild or severe
GBS.
Analysis of cytokines secreted by
stimulated PBMCs
GBS is a T-helper (Th)1-type autoimmune disease.
TNF-α and IL-1β are major pro-inflammatory cytokines released by
Th1 cells. It was hypothesized that TLRs are involved in the
initiation of GBS, and that following activation, TLRs are able to
promote increased NF-κB levels through MyD88-dependent or
-independent pathways, to induce the secretion of pro-inflammatory
cytokines, including TNF-α and IL-1β. To further investigate the
association between TLR2 and TLR4, and GBS pathogenesis, TNF-α and
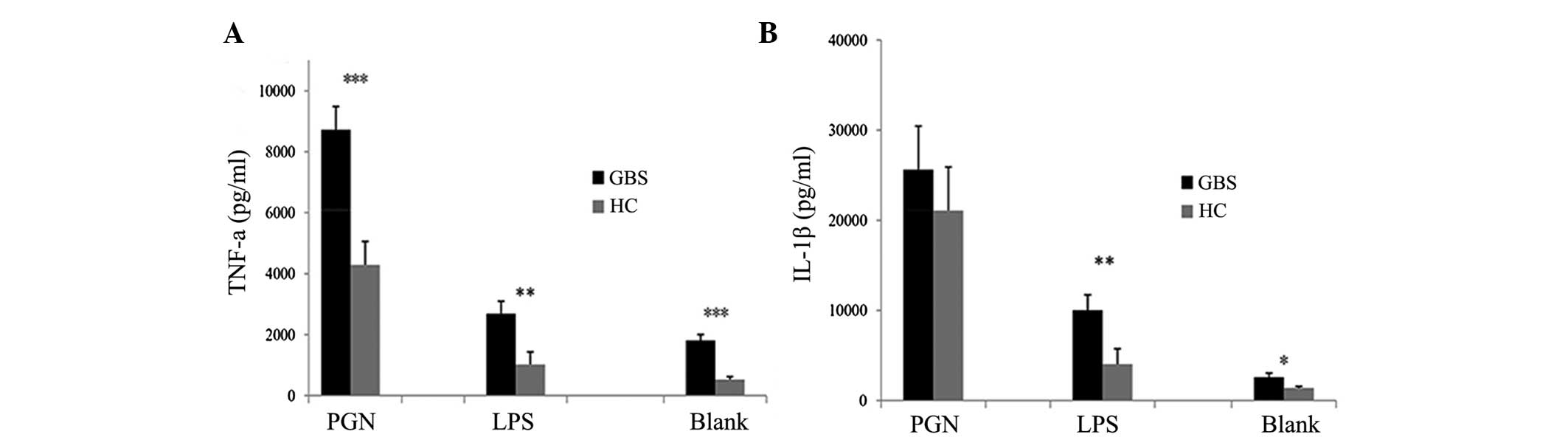
IL-1β levels were measured in PBMC culture supernatants. PBMCs from
patients with GBS secreted higher levels of TNF-α when stimulated
with LPS or PGN compared with those of healthy controls (Fig. 3A). IL-1β secretion by PBMCs from
patients with GBS was significantly higher in the unstimulated or
LPS-stimulated groups compared with that of healthy controls
(P<0.05 and P<0.01, respectively) (Fig. 3B). However, no significant
difference was identified between patients with GBS and healthy
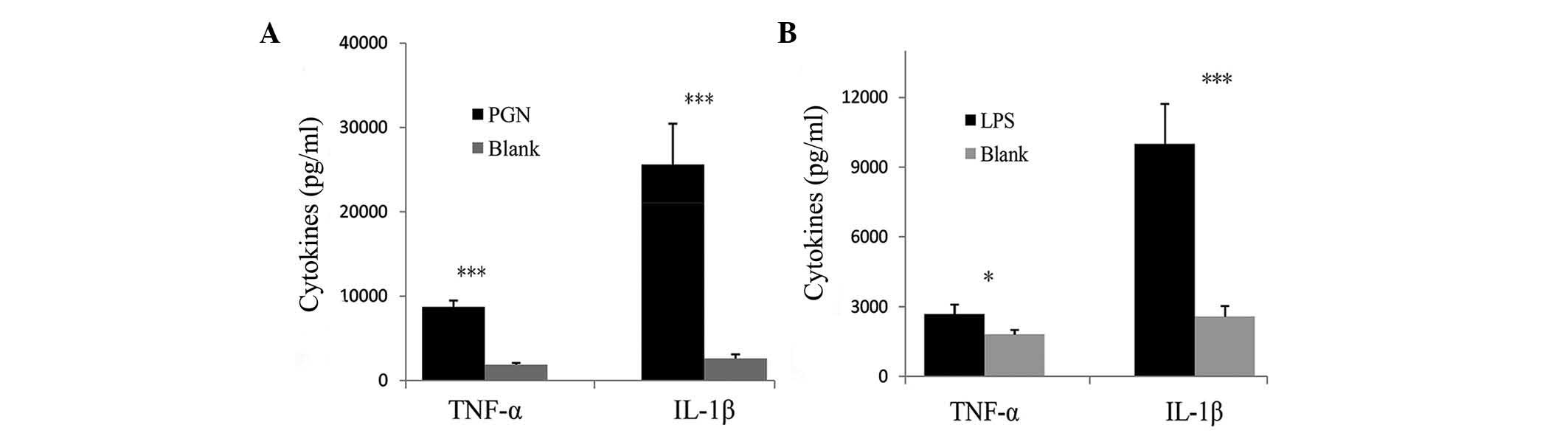
controls when PBMCs were stimulated with PGN (Fig. 3B). Compared with the blank control,
the secretion of TNF-α and IL-1β was significantly increased in
PBMCs from patients with GBS when stimulated with PGN (P<0.001)
(Fig. 4A). Similarly, stimulation
with LPS significantly increased the secretion of TNF-α and IL-1β
by PBMCs compared with those in the control group (P<0.05 and
P<0.001, respectively) (Fig.
4B). The results revealed an increased activation of TLR2 and
TLR4 in patients with GBS. As shown in Fig. 3, the release of the cytokines TNF-α
and IL-1β was increased in the PBMCs of patients with GBS under
basal conditions (blank) compared with that in healthy controls,
which suggests an enhanced sensitivity of the PBMCs in patients
with GBS. The increased activation and enhanced sensitivity of the
PBMCs may be somewhat important for the induction and/or
progression of GBS.
Anti-ganglioside antibody
measurement
To examine the association between TLRs and GBS
indirectly, serum anti-ganglioside antibodies were measured using
the EUROLINE anti-ganglioside profile 2. The total positive rate of
patients with GBS was significantly higher than that of healthy
controls (P<0.05; Table II),
which is in line with a previous study (20). Among 11 patients with mild GBS, 1
patient was IgG-positive, while 2 other patients were IgM-positive.
By contrast, there were 3 IgG-positive and 3 IgM-positive patients
in the group of patients with severe GBS. In addition, 2 of the 3
IgG-positive GBS patients were additionally IgM-positive. As shown
in Table III, IgG and IgM
anti-ganglioside antibody-positive rates were higher in patients
with severe GBS than those in patients with mild GBS, although no
statistically significant difference was identified. These results
were consistent with the results regarding the expression of TLR2,
TLR4, MyD88 and NF-κB, which indicated that TLRs and their
signaling pathways may be involved in the pathogenesis of GBS.
 | Table IIPositive anti-ganglioside antibody
rates in patients with GBS and healthy controls. |
Table II
Positive anti-ganglioside antibody
rates in patients with GBS and healthy controls.
| Group (n) | IgG (%)
| IgM (%)
| Positive rate
(%) |
|---|
| GQ1b | GT1b | GD1b | GD1a | GM3 | GM2 | GM1 | GQ1b | GT1b | GD1b | GD1a | GM3 | GM2 | GM1 |
|---|
| GBS (18) | 0.00 | 11.11 | 11.11 | 11.11 | 11.11 | 5.56 | 27.78 | 0.00 | 16.67 | 0.00 | 5.56 | 22.22 | 0.00 | 5.56 | 38.89 |
| HC (20) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 5.00 | 5.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 10.00 |
 | Table IIIAnti-ganglioside antibody-positive
rate in patients with mild (n=11) or severe (n=7) Guillain-Barré
syndrome. |
Table III
Anti-ganglioside antibody-positive
rate in patients with mild (n=11) or severe (n=7) Guillain-Barré
syndrome.
| Severity | IgG (%) | IgM (%) |
|---|
| Mild | 9.09 | 18.18 |
| Severe | 42.86 | 42.86 |
Discussion
GBS presents as a weakness of the extremities and
may progress to weakness of the trunk, cervical area, facial
muscles and even respiratory muscles, causing respiratory failure
in severe cases (21). Patients
with GBS are often treated with hormones or plasma exchange.
However, the effects of treatments remain poor. Thus, it is
important to investigate the pathogenesis of GBS, in order to
develop beneficial and efficacious treatments for patients with
GBS. Previous studies indicated that TLRs, such as SLE, are
significant in autoimmune diseases (22,23),
primary biliary cirrhosis and autoimmune hepatitis (22). Several studies also demonstrated
that the expression of TLRs was increased in EAN rats and patients
with GBS. The present study identified that TLR2 and TLR4 and their
signaling pathways are functionally involved in the pathogenesis of
GBS.
Gries et al (24) systematically examined the
expression patterns of TLRs in sciatic nerves and lymphoid organs
during the course of murine EAN, and observed a pronounced
upreg-ulation of TLR2, TLR6 and TLR11 on T cells and TLR4 and TLR6
on antigen-presenting cells. Another study reported that MyD88
(−/−) mice were completely EAN-resistant and TLR4 (−/−) and TLR9
(−/−) mice exhibited markedly more severe EAN symptoms than
wild-type mice (17). This
indicated that TLR4, TLR9 and MyD88 are important in the
pathogenesis of EAN. In patients with GBS, Wang et al
(19) found that TLR2, TLR4 and
TLR9 mRNA levels were significantly increased. In accordance with
the results of studies using the murine EAN model, it was suggested
that TLR2, TLR4 and TLR9 may be involved in the pathogenesis of
GBS.
In line with investigations into EAN and GBS, it was
demonstrated that TLR2 and TLR4 mRNA expression was significantly
elevated in PBMCs from patients with GBS. To further investigate
the association between TLRs and GBS, the mRNA levels of MyD88 and
NF-κB were measured using RT-qPCR. MyD88 and NF-κB are two key
molecules of the TLR signaling pathways. As expected, marked
increases in MyD88 and NF-κB mRNA levels were observed in patients
with GBS compared with those in healthy controls. This indicated
that TLR2, TLR4 and their signaling pathways are functionally
involved in the pathogenesis of GBS.
Previous studies have described contradictory
results regarding the association between TLRs and GBS severity.
Wang et al (19) indicated
that TLR mRNA levels had a marked positive correlation with the
disability scores of patients with GBS. However, another study
reported that the severity of clinical symptoms had a negative
correlation with TLR2, TLR4 and TLR6 mRNA expression levels
(24). Patients with GBS were
divided into two groups (mild and severe) according to their
clinical manifestation, and their mRNA levels were compared using
Student’s t-test. In accordance with the findings of Wang et
al (19), the results of the
current study demonstrated the same trend of alterations between
TLR2, TLR4 and NF-κB mRNA and the condition of the patients with
GBS. However, no significant difference was identified in MyD88
mRNA levels between patients with mild and severe GBS. This may be
the result of an insufficient sample size. A larger cohort of
patients with GBS is required to investigate this condition
further.
It was hypothesized that GBS is a Th1-type
autoimmune disease, and that Th1 cytokines, including IL-1β, TNF-α
and IFN-γ, are significantly correlated with GBS pathogenesis.
Matsui et al (25) reported
that IL-1β, TNF-α and IFN-γ were increased in the spinal cord of
EAN rats, whereas anti-inflammatory cytokines, including IL-4 and
IL-10, were decreased (25). Serum
IFN-γ was also elevated in patients with GBS (26). TNF-α is able to inhibit Schwann
cell proliferation and mediate Schwann cell death (27). TNF-α was elevated and associated
with disease severity in EAN (28)
and GBS (29). In a further study,
Zhang et al (30) observed
that the onset of EAN in TNF-α gene-deficient mice was markedly
later than that in wild type mice. In GBS, an increased expression
of IL-1β was immunolocalized on Schwann cell membranes in sural
nerve biopsies (31) and IL-1β was
detected in the cerebrospinal fluid (32). These findings indicated that TNF-α,
IL-1β and IFN-γ are involved in the pathogenesis of GBS. Therefore,
in the present study, PBMCs from patients with GBS and healthy
controls were cultured and TNF-α and IL-1β levels were measured in
the supernatants. The results indicated that, LPS-stimulated PBMCs
from patients with GBS secreted more TNF-α and IL-1β than those of
healthy controls. This indicated that excessive activation of TLRs
was involved in the pathogenesis of GBS. When stimulated by PGN or
LPS, PBMCs from patients with GBS secreted more TNF-α and IL-1β
than blank controls. Therefore, the present study provided evidence
supporting the hypothesis that TLR2 and TLR4 are functionally
involved in the pathogenesis of GBS.
In the majority of previous studies,
anti-ganglioside antibodies have been found in autoimmune
neuropathies, particularly GBS (33,34).
It was hypothesized that different sub-types of anti-ganglioside
antibodies are associated with different GBS subtypes (35). Anti-GM1 antibodies are commonly
associated with a pure motor variant of GBS (36), and GQ1b may be a target antigen
responsible for patients with MFS (37). A study reported that
anti-ganglioside antibodies are able to inhibit axon regeneration
through neuronal gangliosides independent of endogenous
regeneration inhibitors (38).
Gangliosides have biological functions, including cellular growth
and differentiation, modulation of signal transduction and immune
reactions. Sheikh and Zhang (39)
found that anti-ganglioside antibodies induced damage to intact
nerve fibers and inhibited axon regeneration via an Fcγ receptor.
Based on these studies, it was hypothesized that anti-ganglioside
antibodies may be associated with the severity of GBS. The present
results revealed that positive anti-ganglioside antibody rates were
significantly increased in patients with GBS. In addition, positive
anti-ganglioside antibody rates were higher in patients with severe
GBS compared with those with mild GBS, although no significant
difference was identified. This revealed that anti-ganglioside
antibodies may be associated with the severity of GBS; however,
larger numbers of patients with GBS are required to verify these
results in for future studies. The antibody-positive rates of the
mild and severe patients with GBS are in accordance with the
results regarding the mRNA levels of TLR2, TLR4, MyD88 and NF-κB,
which further suggests that TLR2, TLR4 and their signaling pathways
are involved in the pathogenesis of GBS.
The results of the present study revealed that TLR2,
TLR4, MyD88 and NF-κB mRNA increased significantly in GBS, and that
activated TLR2 and TLR4 are able to promote the abnormal secretion
of TNF-α and IL-1β. However, anti-ganglioside antibodies produced
due to molecular mimicry were shown to be significantly associated
with GBS and the severity of GBS. In conclusion, the present study
further supported the hypothesis that TLR2, TLR4 and their
signaling pathways are functionally involved in the pathogenesis of
GBS. The present study suggested that TLR2, TLR4 and their
signaling pathways may be potential treatment targets, which
requires further investigation.
Acknowledgments
The present study was supported by Natural Science
Foundation of Beijing (grant no. 7142051), the High Level Technical
Talent Development of Beijing Health System (grant no. 2013-3-052)
and the National Key Technology Research and Development Program of
the Ministry of Science and Technology of China (grant no.
2013BAI09B03).
References
|
1
|
McGrogan A, Madle GC, Seaman HE and de
Vries CS: The epidemiology of Guillain-Barŕe syndrome worldwide. A
systematic literature review. Neuroepidemiology. 32:150–163. 2009.
View Article : Google Scholar
|
|
2
|
Huang WT, Yang HW, Liao TL, et al: Safety
of pandemic (H1N1) 2009 monovalent vaccines in taiwan: a
self-controlled case series study. PLoS One. 8:e588272013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salmon DA, Proschan M, Forshee R, et al:
Association between Guillain-Barré syndrome and influenza A (H1N1)
2009 monovalent inactivated vaccines in the USA: a meta-analysis.
Lancet. 381:1461–1468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang D, Zhang G, Hayden MS, et al: A
toll-like receptor that prevents infection by uropathogenic
bacteria. Science. 303:1522–1526. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eom SH, Gu GJ, Suh CW, et al: Suppression
of inducible nitric oxide synthase expression induced by Toll-like
receptor agonists by (E)-1-(2-(2-nitrovinyl)phenyl) pyrrolidine.
Int Immunopharmacol. 17:205–209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Santaolalla R, Sussman DA, Ruiz JR, et al:
TLR4 activates the β-catenin pathway to cause intestinal neoplasia.
PLoS One. 8:e632982013. View Article : Google Scholar
|
|
7
|
Arbour NC, Lorenz E, Schutte BC, et al:
TLR4 mutations are associated with endotoxin hyporesponsiveness in
humans. Nat Genet. 25:187–191. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang ZY, Zhang Z and Schluesener HJ:
FTY720 attenuates lesional interleukin-17 (+) cell accumulation in
rat experimental autoimmune neuritis. Neuropathol Appl Neurobiol.
35:487–495. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang ZY, Zhang Z and Schluesener HJ:
Toll-like receptor-2, CD14 and heat-shock protein 70 in
inflammatory lesions of rat experimental autoimmune neuritis.
Neuroscience. 159:136–142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reynolds JM, Pappu BP, Peng J, et al:
Toll-like receptor 2 signaling in CD4(+) T lymphocytes promotes T
helper 17 responses and regulates the pathogenesis of autoimmune
disease. Immunity. 32:692–702. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Islam Z, Gilbert M, Mohammad QD, et al:
Guillain-Barré syndrome-related Campylobacter jejuni in Bangladesh:
ganglioside mimicry and cross-reactive antibodies. PLoS One.
7:e439762012. View Article : Google Scholar
|
|
12
|
Heikema AP, Koning RI, Duarte dos Santos
Rico S, et al: Enhanced sialoadhesin-dependent uptake of
Guillain-Barre syndrome-associated Campylobacter jejuni strains by
human macrophages. Infect Immun. 81:2095–2103. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakamura M, Funami K, Komori A, et al:
Increased expression of Toll-like receptor 3 in intrahepatic
biliary epithelial cells at sites of ductular reaction in diseased
livers. Hepatol Int. 2:222–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaida K, Ariga T and Yu RK:
Antiganglioside antibodies and their pathophysiological effects on
Guillain-Barr e syndrome and related disorders-A review.
Glycobiology. 19:676–692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hans M and Hans VM: Toll-like receptors
and their dual role in periodontitis: a review. J Oral Sci.
53:263–271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng YN and Zhou WB: Expression of TLR4
and TLR9 mRNA in Lewis rats with experimental allergic neuritis.
Neuroimmunomodulation. 14:337–343. 2007. View Article : Google Scholar
|
|
17
|
Marta M, Andersson A, Isaksson M, et al:
Unexpected regulatory roles of TLR4 and TLR9 in experimental
autoimmune encephalomyelitis. Eur J Immunol. 38:565–575. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Koningsveld R, Steyerberg EW, Hughes
RA, et al: A clinical prognostic scoring system for Guillain-Barré
syndrome. Lancet Neurol. 6:589–594. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang YZ, Liang QH, Ramkalawan H, et al:
Expression of Toll-like receptors 2, 4 and 9 in patients with
Guillain-Barré syndrome. Neuroimmunomodulation. 19:60–68. 2012.
View Article : Google Scholar
|
|
20
|
Kusunoki S: Antiglycolipid antibodies in
Guillain–Barré syndrome and autoimmune neuropathies. Am J Med Sci.
319:234–239. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arcila-Londono X and Lewis RA:
Guillain-Barré syndrome. Semin Neurol. 32:179–186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Komatsuda A, Wakui H, Iwamoto K, et al:
Up-regulated expression of Toll-like receptors mRNAs in peripheral
blood mononuclear cells from patients with systemic lupus
erythe-matosus. Clin Exp Immunol. 152:482–487. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Papadimitraki ED, Choulaki C, Koutala E,
et al: Expansion of toll-like receptor 9-expressing B cells in
active systemic lupus erythematosus: implications for the induction
and maintenance of the autoimmune process. Arthritis Rheum.
54:3601–3611. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gries M, Davies L, Liu Y, Bachhuber A, et
al: Response of Toll-like receptors in experimental Guillain-Barré
syndrome: a kinetic analysis. Neurosci Lett. 518:154–160. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matsui H, Ohgomori T, Natori T, et al:
Keratan sulfate expression in microglia is diminished in the spinal
cord in experimental autoimmune neuritis. Cell Death Dis.
4:e9462013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Orlikowski D, Chazaud B, Plonquet A, et
al: Monocyte chemoat-tractant protein 1 and chemokine receptor CCR2
productions in Guillain-Barré syndrome and experimental autoimmune
neuritis. J Neuroimmunol. 134:118–127. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boyle K, Azari MF, Cheema SS and Petrato
SS: TNF alpha mediates Schwann cell death by upregulating p75NTR
expression without sustained activation of NFkappaB. Neurobiol Dis.
20:412–427. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kurz M, Pischel H, Hartung HP and Kieseier
BC: Tumor necrosis factor-alpha-converting enzyme is expressed in
the inflamed peripheral nervous system. J Peripher Nerv Syst.
10:311–318. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang J, Dong H, Li B, et al: Association
of tumor necrosis factor polymorphisms with Guillain-Barré
syndrome. Eur Neurol. 58:21–25. 2007. View Article : Google Scholar
|
|
30
|
Zhang HL, Hassan MY, Zheng XY, et al:
Attenuated EAN in TNF-α deficient mice is associated with an
altered balance of M1/M2 macrophages. PLoS One. 7:e381572012.
View Article : Google Scholar
|
|
31
|
Hayashi R, Xiao W, Kawamoto M, et al:
Systemic glucocorticoid therapy reduces pain and the number of
endoneurial tumor necrosis factor-alpha (TNF alpha)-positive mast
cells in rats with a painful peripheral neuropathy. J Pharmacol
Sci. 106:559–565. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sivieris, Ferrarini AM, Lolli F, et al:
Cytokine pattern in the cerebrospinal fluid from patients with GBS
and CIDP. J Neurol Sci. 147:93–95. 1997. View Article : Google Scholar
|
|
33
|
Vaishnavi C, Behura C, Prabhakar S, Sharma
A and Kharbanda P: Anti-ganglioside antibodies in patients with
Guillain Barré syndrome and other neurological disorders. Indian J
Med Microbiol. 31:177–179. 2013.PubMed/NCBI
|
|
34
|
Chatani H, Tanaka M, Nagata T, et al:
Guillain-Barré syndrome-like-onset neurosarcoidosis positive for
immuno-globulin G anti-N-acetylgalactosaminyl-GD1a antibody. J Clin
Neurosci. 21:170–172. 2014. View Article : Google Scholar
|
|
35
|
Kaida K, Ariga T and Yu RK:
Antiganglioside antibodies and their pathophysiological effects on
Guillain-Barré syndrome and related disorders-a review.
Glycobiology. 19:676–692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jacobs BC, van Doorn PA, Schmitz PI, et
al: Campylobacter jejuni infections and anti-GM1 antibodies in
Guillain-Barré syndrome. Ann Neurol. 40:181–187. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koga M, Gilbert M, Takahashi M, et al:
GQ1b-seronegative Fisher syndrome: clinical features and new
serological markers. J Neurol. 259:1366–1374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lehmann HC, Lopez PH, Zhang G, Ngyuen T,
Zhang J, et al: Passive immunization with anti-ganglioside
antibodies directly inhibits axon regeneration in an animal model.
J Neurosci. 27:27–34. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sheikh KA and Zhang G: An update on
pathobiologic roles of anti-glycan antibodies in Guillain-Barré
syndrome. F1000 Biol Rep. 2:212010.
|