Introduction
Systemic lupus erythematosus (SLE) is a chronic
autoimmune disease, characterized by the development of
autoantibodies. SLE can damage every physiological system,
including the skin, joints and kidneys. Lupus nephritis is a major
cause of mortality in patients with SLE, and ~60% of patients with
SLE develop clinically relevant lupus nephritis during the course
of the disease (1,2).
CD4+/CD8+ T cells, antigen presenting cell,
dendritic cells and macrophages are involved in the development of
SLE. Previous studies have demonstrated that the Notch signaling
pathway is involved in the function, proliferation and
differentiation of these cells (3–9). It
has been reported that miR-23b promotes the tolerogenic properties
of ovalbumin-challenged dendritic cells (DCs) in vitro via
inhibition of the Notch1/nuclear factor κB signaling pathways
(10). Notch signaling also
facilitates the CD8+ T cell lineage (11). Naive CD4+ T cells can differentiate
into Th1, Th2, Th17 or regulatory T cells (12), and the expression of Notch ligands
on antigen-presenting cells (APCs) is induced by Th1- or
Th2-promoting stimuli (13).
Accumulating evidence indicates that Notch and its ligands may be
important mediators of T cell differentiation in SLE (9,14).
Notch signaling is a well-conserved signaling
pathway in evolutionary terms. It includes four cell surface
reporters (Notch1–4), which regulate the differentiation,
maturation and function of a wide array of cell types (15). Whilst Notch signaling is involved
in tissue and organ formation during embryonic development,
evidence indicates that it continues to affect developmental
processes postnatally and is implicated in the pathogenesis of
human disorders, including cancer and autoimmune diseases (16).
Previous studies have demonstrated that the
inhibition of Notch1 signaling is a potential therapeutic approach
for SLE (17). Chemical inhibition
of all four Notch receptors in response to nonspecific γ-secretase
inhibitors suppresses Th1- and Th17-type differentiation and
ameliorates signs of autoimmunity and renal damage in lupus-prone
MRL-lpr mice (18). A previous
study demonstrated significantly lower levels of Notch-1 in T cells
from patients with clinically active SLE, compared with healthy
patients, at the mRNA and protein levels (19).
The present study aimed to investigate the effects
of the Notch signaling inhibitor, DAPT, and glucocorticoids, in
combination and alone on lupus nephritis in BABL/C-lpr mice.
Recognition of self nucleic acids by Toll-like receptors (TLR9) on
B cells and plasmacytoid dendritic cells (PDCs) is an important
step in the pathogenesis of SLE (20). Therefore, he present study used
immunohistochemical and western blot analyses in order to examine
changes in the expression of TLR9 in the kidneys of BABL/C-lpr
mice, following treatment with glucocorticoids and DAPT.
Materials and methods
Ethical approval
The procedures used in the present study were
performed in accordance with the Regulations for the administration
of affairs concerning experimental animals (1998, China). The
procedures were approved by the Committee on the Ethics of Animal
Experiments of Central South University (Changsha, China).
Mice
A total of 28 4 week-old female BABL/C-lpr mice
(SYXK (xiang) 2011–0001) were obtained from the Department of
Animal Experiments, Central South University. The mice were housed
in a specific pathogen-free room under controlled temperature
(22°C) and humidity, and underwent a 12 h light/dark cycle. The
experiments were performed according to the Guide for the Care and
Use of Medical Laboratory Animals (Ministry of Health, People’s
Republic of China, 1998), with approval from the Shanghai Medical
Laboratory Animal Care and Use Committee and the Ethical Committee
of Central South University (Changsha, China).
Generation of the SLE murine model
The mice were randomly divided into four groups
(n=7): Model 1, BABL/C-lpr mice without treatment; Model 2,
BABL/C-lpr mice treated with DAPT (Selleck Chemicals, Houston, TX,
USA); Model 3, BABL/C-lpr mice treated with prednisone (Xianju
Company, Xianju, China); and Model 4, BABL/C-lpr mice treated with
prednisone following treatment with DAPT. BABL/C-lpr mice develop
nephritis, which is similar to that observed in humans with SLE
(21). At 8 weeks of age, all of
the mice were injected with 0.5 ml pristane
(2,6,10,14-tetramethylpen-tadecane; Sigma-Aldrich, St. Louis, MO,
USA). Following 24 weeks of pristane treatment, serum (20 μl) was
obtained from the mice via retro-orbital bleeding. The serum
samples were then frozen at the time of collection and were
analyzed for mouse anti-double stranded DNA (anti-dsDNA)
immunoglobulin (Ig)G-specific antibodies and anti-nuclear antibody
IgM using a 96-well quantitative enzyme-linked immunosorbent assay
(ELISA) kits from Alpha Diagnostic International (Department of
Rheumatism, Xiangya Hospital, Changsha, China).
DAPT treatment
DAPT was prepared using reconstituting DAPT powder
with 100% ethanol (EtOH) and stored as a stock solutions, at −20°C.
Each day, fresh stock solution was diluted in a mixture of corn oil
and EtOH to a final concentration of 5% EtOH and 95% corn oil,
according to previously described methods (21). The experiments were performed under
an Institutional Animal Care and use Committee-approved
protocol.
Following pristane treatment for 24 weeks, the Model
4 mice were treated with DAPT (5 mg/kg day−1; 5
days/week) for 8 weeks, Model 3 mice were treated with prednisone
(9 mg/kg day−1) for 4 weeks. After 4 weeks, the
treatment in Model 4 was changed to prednisone for a further 4
weeks (9 mg/kg day−1). All drugs were administered
intragastrically.
The levels of urine protein were measured prior to
and following 8 weeks of treatment. Urinary collections (24 h) were
obtained at weeks 24 and 32. Albuminuria was measured in the urine
collections using a Mouse Albumin ELISA Quantification kit (Lanpai
Company, Shanghai, China).
Histological renal injury
Following completion of the treatment period, the
mice were sacrificed by cervical dislocation and the kidneys were
removed and fixed in 4% formalin. The kidney sections (1×1
cm2) were stained using hematoxylin and eosin (Sbjbio
Company, Nanjing, China) and the histopathology was assessed by a
pathologist in a blinded-manner.
Glomerular TLR9 immunostaining
The levels of glomerular TLR9 were assessed in 6-μm
frozen kidney sections using rabbit polyclonal antibody conjugated
to TLR9 IgG (1:400; Abcam, Shanghai, China). Scores were assigned
based on the intensity of the expression of IgG.
Western blot analysis
Whole, cytoplasmic and nuclear protein extractions
and western blot analyses were performed, according to previously
described methods. The antibodies used were Rabbit polyclonal TLR9
IgG (2 μg/ml; 1:200; cat. no. ab13928; Abcam) and goat anti-mouse
IgG-horseradish peroxidase (cat. no. sc-2970; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA).
Statistical analysis
All data are presented as the mean ± standard
deviation. Comparative analysis was performed for the protein
expression levels of TLR9. A two-tailed Mann Whitney T-test was
used to determine the presence of significant differences between
the Models. P<0.05 was considered to indicate a statistically
significant difference. Analysis of the western blotting was
performed using GraphPad Prism (Version 5) software (GraphPad
Software, Inc., La Jolla, CA, USA) and the immunostaining was
analyzed using Image-Pro® Plus software (Media
Cybernetics, Inc., Rockville, MD, USA) following microscopy (CKX41;
Olympus Corporation, Tokyo, Japan).
Results
Notch inhibition improves nephritis in
BABL/C-lpr mice
BABL/C-lpr mice develop nephritis in a similar way
to humans with SLE (22). In order
to determine the effect of inhibition of Notch on lupus nephritis,
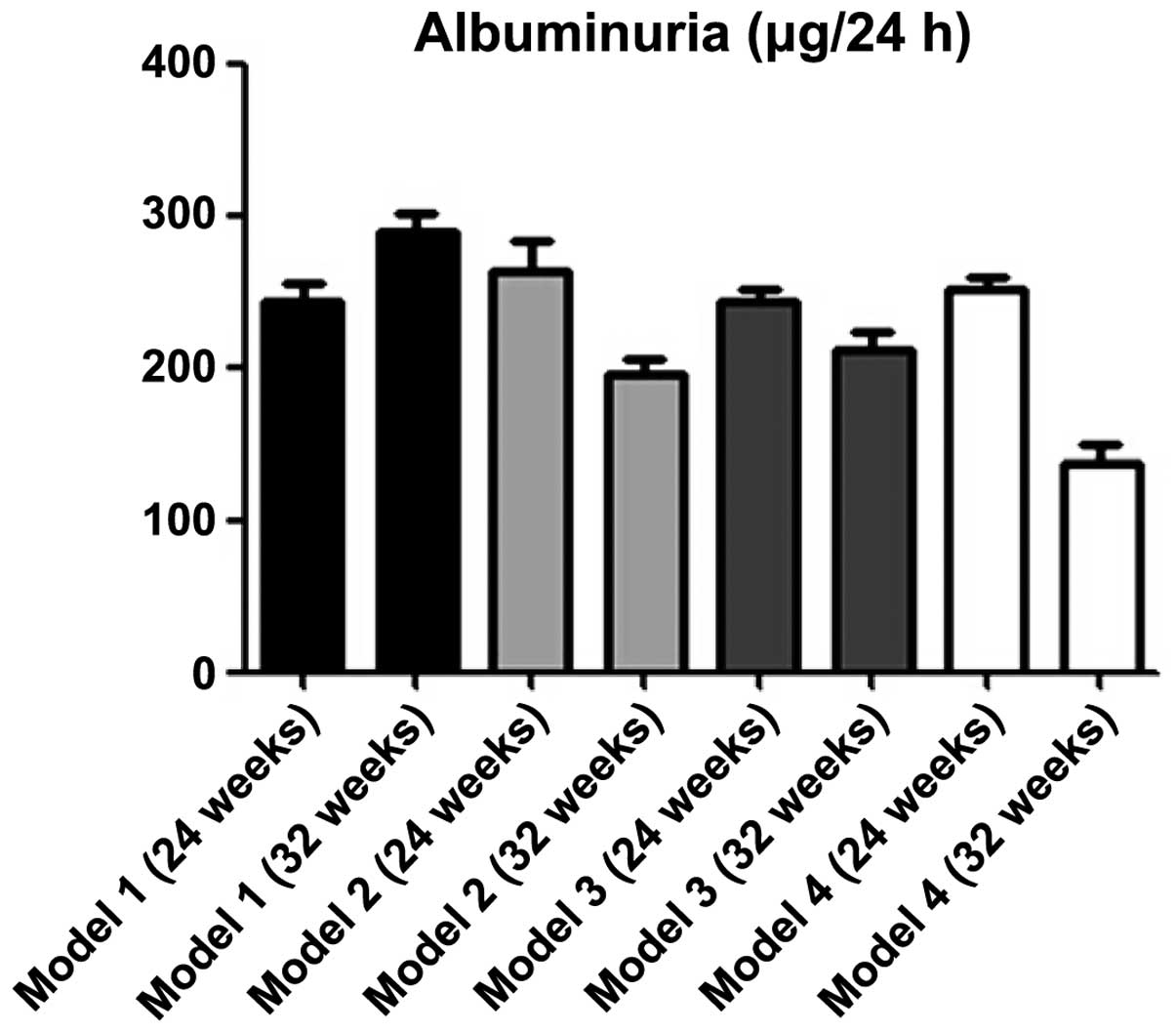
pristane-induced BABL/C-lpr mice were treated with DAPT for 8 weeks
and urine protein levels were measured and compared between 12
treated and 12 control mice. The urine protein levels of the mice
treated with DAPT decreased significantly compared with the control
mice, according to ANOVA (P<0.001; Fig. 1).
Renal pathobiology was identified as a diagnostic
factor for lupus nephritis (Fig.
2). The pathological results demonstrated a marked improvement
in the renal structures of the mice treated with DAPT, indicating
that DAPT treatment effectively inhibited lupus nephritis in the
BABL/C-lpr mice. The response to glucocorticoids increased
following Notch inhibition. Following DAPT treatment, the effect of
glucocorticoid treatment increased, improving lupus nephritis in
the BABL/C-lpr mice. The urine protein levels in the mice treated
with DAPT and glucocorticoid were lower than those in other Model
groups. The pathological results suggested that the kidneys from
the DAPT and glucocorticoid-treated mice exhibited structural
improvement, compared with the other Model groups.
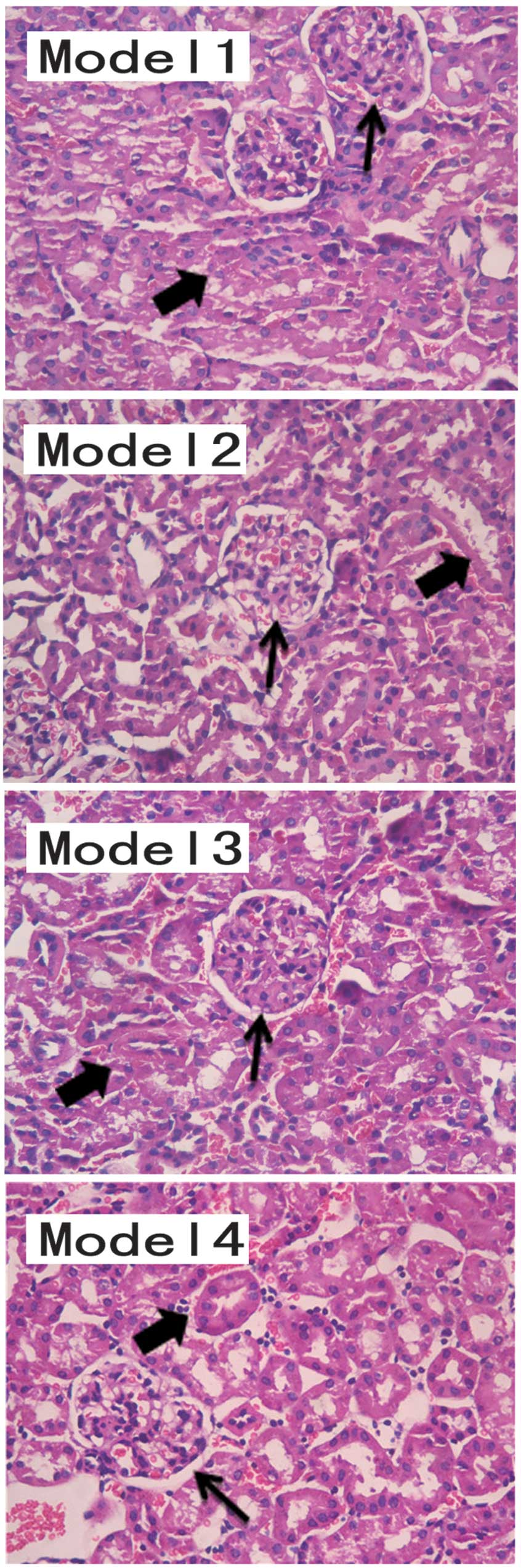 | Figure 2Model 1, diffuse thickening of the
glomerular capillary (thin arrows; proliferation of mesangial cells
and endothelial cells), glomerular basement membrane thickened
irregular, tubular, interstitial infiltration of inflammatory
cells, renal tubule degeneration and luminal stenosis (thick
arrows). Model 2 and Model 3, the glomerular lesion is improved
compared with that in Model 1. However, renal tubules are present.
Model 4, fewer pathological histological changes were observed in
the structure of the kidney sample, compared with the other models.
The results were evaluated by three pathologists. Hematoxylin and
eosin staining (magnification, ×200). |
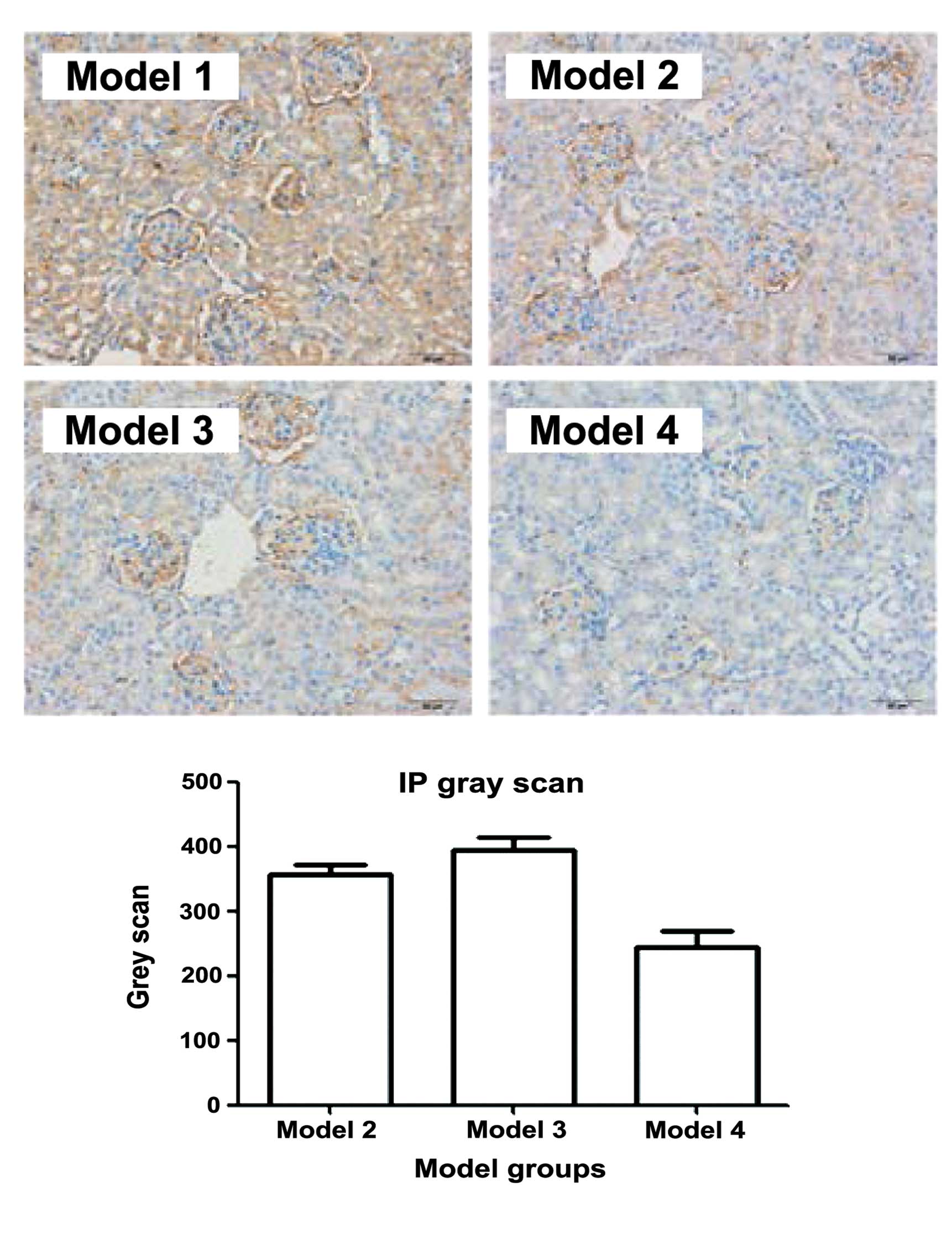
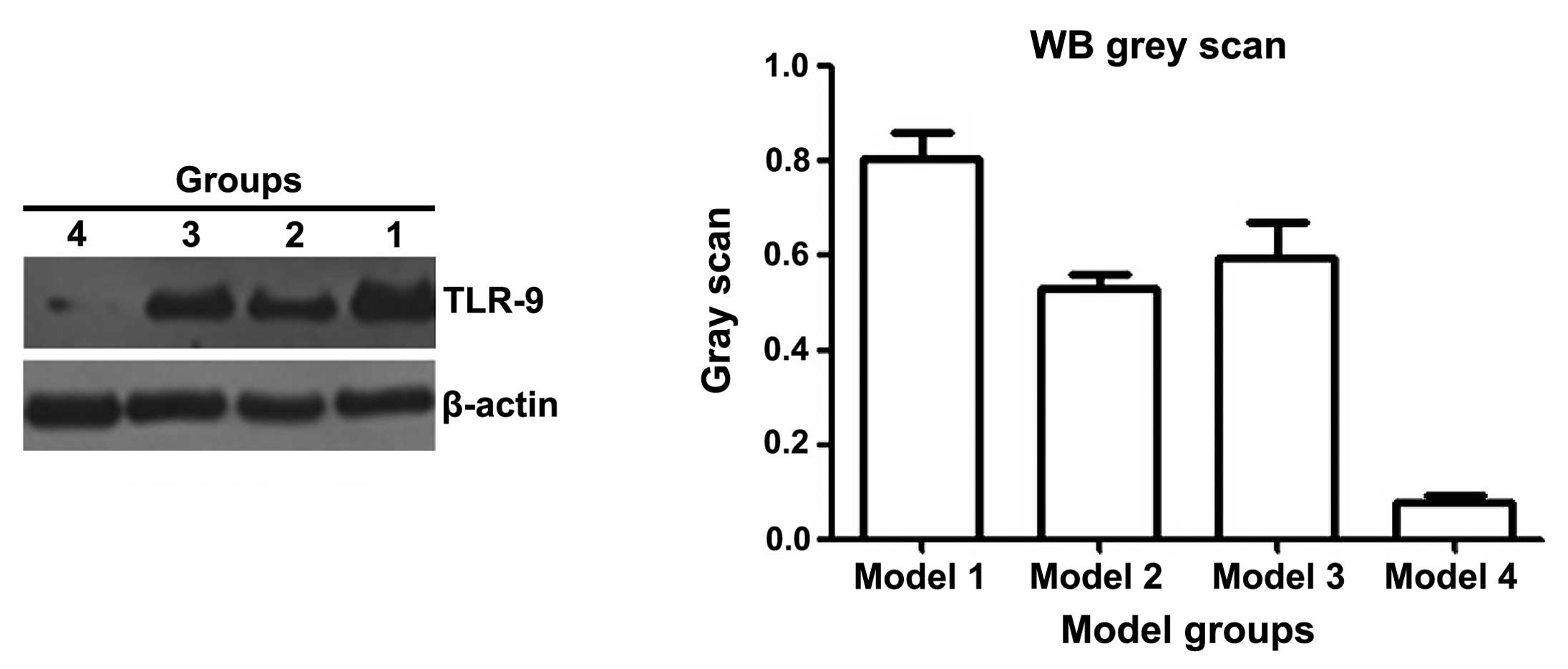
The expression of TLR9 can be used to predict
glucocorticoid response in patients with SLE (17). Therefore, the expression of TLR9 in
the kidneys of the BABL/C-lpr mice were examined. The
immunohistochemical (Fig. 3) and
western bolt analyses (Fig. 4)
suggested that the expression of TLR9 in kidneys of the mice
treated with DAPT and glucocorticoid was markedly decreased,
compared with the other treatment groups. These results suggested
that DAPT may improve the response to glucocorticoids in lupus
nephritis by reducing the expression of TLR9. There may be an
association between DAPT and TLR9.
Discussion
TLRs are pattern recognition receptors in the innate
immune system, which recognize specific pathogen-associated
molecular patterns, conserved among micro-organisms (23). A total of 12 TLRs have been
identified in a number of autoimmune diseases, including rheumatoid
arthritis and SLE, exhibit increased expression levels in patients
with SLE (24,25). The expression of TLR9 is increased
in patients with SLE (26), and
the SLE disease activity index (SLEDAI), and expression levels of
anti-dsDNA, anatoxin-a synthetase and TLR9 are significantly higher
in patients with active SLE, with the expression of TLR9
significantly higher in steroid-resistant, compared with
steroid-sensitive blood samples prior to treatment with
corticosteroid. Positive correlations have also been observed
between the expression of TLR9, the SLEDAI score and anti-dsDNA,
and negative correlations have been observed between the expression
of TLR9 and the expression levels of C3 and C4 (27). In the present study, the
development of lupus nephritis was observed in mice treated with
DAPT. Specifically, the proteinuria and renal histopathology of the
mice treated with DAPT and glucocorticoid were improved, compared
with those treated with either drug alone. Therefore, DAPT may have
enhanced the anti-lupus effects of glucocorticoid treatment in the
BABL/C-lpr mice. The expression of TLR9 has been observed to
correlate with glomerular injury (28). TLR7 and TLR9-induced PDC
stimulation can account for the reduced activity of glucocorticoids
in inhibiting the interferon pathway in patients with SLE in
vivo and in two lupus-affected mouse strains in vitro
(29). Corticosteroids have no
effect on the expression of TLR9, which explains to t lack of
corticoid response in certain patients with SLE. The expression of
TLR9 can be used to predict glucocorticoid response in patients
with SLE (28). Therefore, the
expression of TLR9 in BABL/C-lpr mouse kidneys was analyzed using
immunohistochemistry. The results demonstrated that the expression
of TLR9 in the mice treated with DAPT and glucocorticoid was lower
than those treated with either drug alone and those, which received
no treatment. Notch1 inhibition may reduce the expression of TLR9
in glomerular tissues and improve the response to corticosteroid
treatment in BABL/C-lpr mice.
TLR9 is localized to the cell surface or endosomes
of several types of cell, notably of APCs, including dendritic
cells and B cells (30–32). TLR9 deficiency in certain lupus
models may lead to reductions or alterations in anti chromatin
antibodies (33). Genome-wide
association investigations have revealed TLR9 genes located in
susceptibility regions for SLE (34). The expression of TLR9 may
contribute to renal and immunological disorders and to the presence
of anti-dsDNA antibodies (35). In
the present study, a positive correlation was observed between the
expression of TLR9 and clinical and laboratory indices (SLEDAI,
anti-dsDNA, IL10, C3 and C4) in patients with SLE, which suggested
that TLR9 may represent a potential biomarker for SLE. Although the
mechanisms underlying the involvement of TLR9 in SLE remains to be
fully elucidated, a previous study demonstrated that the expression
of TLR9 is associated with disease active indexes, including the
systemic SLEDAI (36). The failure
of T cells to upregulate Notch1 upon activation may be a key
feature of active SLE and represents a potential therapeutic target
(37). The level of Notch1 may be
negatively associated with SLE activity. In the present study, DAPT
was used to inhibit the Notch-1 pathway in order to investigate the
effects on lupus nephritis in BABL/C-lpr mice. Lower expression
levels of TLR9 were observed in the Model 4 mice compared with the
mice in Models 1–3. Corticosteroids in combination with
cyclophosphamide has been used previously to suppress proliferative
lupus nephritis (38). The results
of the present study suggested that DAPT reduced the expression of
TLR9 and relieve corticosteroid resistance in BABL/C-lpr mouse
kidneys. These results suggested a novel method for reducing the
dosage of corticosteroids in the clinical treatment for patients
with SLE.
Acknowledgments
This study was supported by the Graduate Project for
Freedom to Explore in Central South University (grant no.
2012zzts124; Hunan, China). The authors would like to thank Dr Keda
Yang, Dr Baihua Luo and Dr Wenmei Wang for their descriptions of
the pathological sections.
References
|
1
|
D’Cruz DP, Khamashta MA and Hughes GR:
Systemic lupus erythematosus. Lancet. 369:587–596. 2007. View Article : Google Scholar
|
|
2
|
Borchers AT, Leibushor N, Naguwa SM, et
al: Lupus nephritis: A critical review. Autoimmun Rev. 12:174–194.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mak A and Kow NY: The pathology of T cells
in systemic lupus erythematosus. J Immunol Res. 2014:4190292014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mackern-Oberti JP, Llanos C, Vega F, et
al: Role of dendritic cells in the initiation, progress and
modulation of systemic autoimmune diseases. Autoimmun Rev.
14:127–139. 2015. View Article : Google Scholar
|
|
5
|
Al Gadban MM, Alwan MM, Smith KJ and
Hammad SM: Accelerated vascular disease in systemic lupus
erythematosus: Role of macrophage. Clin Immunol. 157:133–144. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Laky K, Evans S, Perez-Diez A and Fowlkes
BJ: Notch signaling regulates antigen sensitiviy of naive CD4+ T
cells by tuning co-stimulation. Immunity. 42:80–94. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohishi K, Varnum-Finney B, Serda RE, et
al: The Notch ligand, Delta-1, inhibits the differentiation of
monocytes into macrophages but permits their differentiation into
dendritic cells. Blood. 98:1402–1407. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng P, Zhou J and Gabrilovich D:
Regulation of dendritic cell differentiation and function by Notch
and Wnt pathways. Immunol Rev. 234:105–119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Backer RA, Helbig C, Gentek R, et al: A
central role for Notch in effector CD8(+) T cell differentiation.
Nat Immunol. 15:1143–1151. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng J, Jiang HY, Li J, et al:
Microrna-23b promotes tolerogenic properties of dendritic cells in
vitro through inhibiting notch1/nf-kappab signalling pathways.
Allergy. 67:362–370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dervovic DD, Liang HC, Cannons JL, et al:
Cellular and molecular requirements for the selection of in
vitro-generated cd8 t cells reveal a role for notch. J Immunol.
191:1704–1715. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Riella LV, Ueno T, Batal I, et al:
Blockade of notch ligand delta1 promotes allograft survival by
inhibiting alloreactive th1 cells and cytotoxic t cell generation.
J Immunol. 187:4629–4638. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Amsen D, Blander JM, Lee GR, et al:
Instruction of distinct cd4 t helper cell fates by different notch
ligands on antigen-presenting cells. Cell. 117:515–526. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dongre A, Surampudi L, Lawlor RG, et al:
Non-canonical Notch signaling drives activation and differentiation
of peripheral CD4(+) T cells. Front Immunol. 5:542014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Talora C, Campese AF, Bellavia D, et al:
Notch signaling and diseases: An evolutionary journey from a simple
beginning to complex outcomes. Biochim Biophys Acta. 1782:489–497.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang W, Xu W and Xiong S: Blockade of
notch1 signaling alleviates murine lupus via blunting macrophage
activation and m2b polarization. J Immunol. 184:6465–6478. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teachey DT, Seif AE, Brown VI, et al:
Targeting notch signaling in autoimmune and lymphoproliferative
disease. Blood. 111:705–714. 2008. View Article : Google Scholar
|
|
19
|
Rauen T, Grammatikos AP, Hedrich CM, et
al: Camp-responsive element modulator alpha (cremalpha) contributes
to decreased notch-1 expression in t cells from patients with
active systemic lupus erythematosus (sle). J Biol Chem.
287:42525–42532. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barrat FJ and Coffman RL: Development of
tlr inhibitors for the treatment of autoimmune diseases. Immunol
Rev. 223:271–283. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Comery TA, Martone RL, Aschmies S, et al:
Acute gamma-secretase inhibition improves contextual fear
conditioning in the tg2576 mouse model of alzheimer’s disease. J
Neurosci. 25:8898–8902. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Watson ML, Rao JK, Gilkeson GS, et al:
Genetic analysis of mrl-lpr mice: Relationship of the fas apoptosis
gene to disease manifestations and renal disease-modifying loci. J
Exp Med. 176:1645–1656. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takeda K and Akira S: Toll-like receptors
in innate immunity. Int Immunol. 17:1–14. 2005. View Article : Google Scholar
|
|
24
|
Roelofs MF, Wenink MH, Brentano F, et al:
Type i interferons might form the link between toll-like receptor
(tlr) 3/7 and tlr4-mediated synovial inflammation in rheumatoid
arthritis (ra). Ann Rheum Dis. 68:1486–1493. 2009. View Article : Google Scholar
|
|
25
|
Marshak-Rothstein A: Toll-like receptors
in systemic autoimmune disease. Nat Rev Immunol. 6:823–835. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lyn-Cook BD, Xie C, Oates J, et al:
Increased expression of toll-like receptors (tlrs) 7 and 9 and
other cytokines in systemic lupus erythematosus (sle) patients:
ethnic differences and potential new targets for therapeutic drugs.
Mol Immunol. 61:38–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ghaly NR, Kotb NA, Nagy HM and Ragehel SM:
Toll-like receptor 9 in systemic lupus erythematosus, impact on
glucocorticoid treatment. J Dermatolog Treat. 24:411–417. 2013.
View Article : Google Scholar
|
|
28
|
Papadimitraki ED, Tzardi M, Bertsias G, et
al: Glomerular expression of toll-like receptor-9 in lupus
nephritis but not in normal kidneys: Implications for the
amplification of the inflammatory response. Lupus. 18:831–835.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guiducci C, Gong M, Xu Z, et al: Tlr
recognition of self nucleic acids hampers glucocorticoid activity
in lupus. Nature. 465:937–941. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jarrossay D, Napolitani G, Colonna M, et
al: Specialization and complementarity in microbial molecule
recognition by human myeloid and plasmacytoid dendritic cells. Eur
J Immunol. 31:3388–3393. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kadowaki N, Ho S, Antonenko S, et al:
Subsets of human dendritic cell precursors express different
toll-like receptors and respond to different microbial antigens. J
Exp Med. 194:863–869. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dorner M, Brandt S, Tinguely M, et al:
Plasma cell toll-like receptor (tlr) expression differs from that
of b cells and plasma cell tlr triggering enhances immunoglobulin
production. Immunology. 128:573–579. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Horton CG, Pan ZJ and Farris AD: Targeting
toll-like receptors for treatment of SLE. Mediators Inflamm.
2010:4989802010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Harley IT, Kaufman KM, Langefeld CD, et
al: Genetic susceptibility to sle: new insights from fine mapping
and genome-wide association studies. Nat Rev Genet. 10:285–290.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Piotrowski P, Lianeri M, Wudarski M, et
al: Contribution of toll-like receptor 9 gene single-nucleotide
polymorphism to systemic lupus erythematosus. Rheumatol Int.
33:1121–1125. 2013. View Article : Google Scholar :
|
|
36
|
Mu R, Sun XY, Lim LT, et al: Toll-like
receptor 9 is correlated to disease activity in Chinese systemic
lupus erythematosus population. Chin Med J (Engl). 125:2873–2877.
2012.
|
|
37
|
Sodsai P, Hirankarn N, Avihingsanon Y, et
al: Defects in notch1 upregulation upon activation of t cells from
patients with systemic lupus erythematosus are related to lupus
disease activity. Lupus. 17:645–653. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Henderson L, Masson P, Craig JC, et al:
Treatment for lupus nephritis. Cochrane Database Syst Rev.
12:CD0029222012.PubMed/NCBI
|