Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune
disease characterized by the hyperplasia of synovial tissue and
progressive destruction of articular cartilage, bone and ligaments
(1–2). The pathogenesis of RA is a complex
process mediated by an interdependent network of cytokines,
proteolytic enzymes and prostanoids (3). There thus remains a tremendous unmet
clinical requirement for more effective therapeutic strategies,
with a goal of sustained remission for a greater number of patients
with RA.
Current therapeutic strategies pursued by the
biopharmaceutical industry include those that target the key
kinases involved in the pathogenesis of RA. One such enzyme is the
spleen tyrosine kinase (SYK), which is a master regulator in
coupling activated immunoreceptors to the mobilization of
downstream signal transduction cascades that affect diverse
biological functions (4). Given
the central role of SYK in transmission of antigen receptor signals
that are critical for autoantibody production and the various
innate immune effector functions, pharmacological inhibition of the
catalytic function of SYK is expected to have pleiotropic
anti-inflammatory effects in the pathogenesis of autoimmune
disorders (5).
The present study explored whether the selective SYK
inhibitor P505-15 impacted the development of collagen-induced
arthritis (CIA) disease in vivo in mice. The results of the
present study indicated that P505-15 may serve as a lead candidate
for further development of selective SYK inhibitors for the
potential treatment of RA.
Materials and methods
Animals
A total of 24 male DBA1/J mice (28–30 g; 8 weeks
old) were obtained from Charles River Breeding Laboratories
(Wilmington, MA, USA). All animal experiments were approved by the
Institutional Animal Use Committee of Shandong University (Jinan,
China). The study was approved by the ethics committee of Shandong
Provincial Hospital, Shandong University (no. G2015698). The mice
were housed with a 12-h light/dark cycle. They had ad
libitum access to food and water in cages (n=6) under
controlled temperature and humidity.
CIA induction
To induce CIA in DBA1/J mice as previously described
(6), type II collagen (CII;
Chondrex, Redmond, WA, USA) was dissolved overnight in 0.1 N acetic
acid (4 mg/ml; (Chondrex) with gentle rotation at 4°C. The mice
were injected intradermally at the base of the tail with 100
μg CII emulsified 1:1 in complete Freund’s adjuvant
(Chondrex) and then received a second injection with 100 μg
CII emulsified 1:1 in incomplete Freund’s adjuvant 21 days
later.
Assessment of arthritis
Clinical arthritis scores were evaluated using a
scale of 0–4 for each limb as previously described (7). Hind paw thickness was measured with
an electric caliper placed across the ankle joint at the widest
point. The paw thickness index was defined as the percent increase
in the diameter of the arthritic ankle at specific time-points as
compared with the diameter determined on day 21. On day 38, the
mice were sacrificed, and joint tissue samples from each animal
were harvested for end-point histological analysis.
Administration of P505-15
On day 24 after the first immunization, CIA mice
(n=12 per group) received oral doses of vehicle (0.5%
methylcellulose in water) or P505-15 (15 mg/kg) twice daily for 14
days as previously reported (8).
On day 38, mice were anesthetized by subcutaneous injection of
ketamine (Fort Dodge Animal Health, Webster County, IN, USA) and
exsanguinated via cardiac puncture. Joints were stained with
hematoxylin and eosin (H&E) for histology.
Histological assessment
Mouse joint tissue specimens from mice with CIA were
fixed with 10% formalin, decalcified for 15 days in 10% EDTA,
dehydrated and embedded in paraffin. Sections (6 μm) were
stained with H&E and Safranin O-fast green for light microscopy
(Labophot-2; Nikon, Tokyo, Japan). The mouse joint sections were
scored for changes in synovial inflammation and bone erosion on a
scale of 0–4 as described in the previous literature (7). Rabbit anti-mouse monoclonal anti-CD68
antibody (1:2,000 dilution; cat. no. 76308; Cell Signaling
Technology, Beverly, MA, USA) incubated for 2 h at 37°C was used to
identify the synovial macrophages. Safranin O-fast green staining
was scored 0–3 (7). For
immunohistochemical staining, expression of CD68 in the synovial
tissue of all ankle joints present was scored semiquantitatively on
a five-point scale (9). A score of
0 represented minimal expression, while a score of 4 represented
abundant expression of a marker.
Assessment of anti-CII antibodies and
cytokines
Sera were obtained from anesthetized animals by
retroorbital puncture at the end of the study. Serum levels of
anti-CII immunoglobulin (Ig)G1 and −IgG2a were measured by ELISA
(Chondrex, Redmond, WA, USA). The levels of tumor necrosis factor
(TNF)-α, interleukin (IL)-6 and IL-17 were measured by TNF-α, IL-6
and IL-17 ELISA kits (R&D Systems, Minneapolis, MN, USA)
according to the manufacturer’s instructions.
Human RA synovial cells
Synovial tissue from 10 RA patients was digested
with 0.5 mg/ml collagenase A in RPMI medium for 2 h at 37°C. The
cells were washed twice with 10% phosphate-buffered
saline/Dulbecco’s modified Eagle’s medium and filtered using a
0.70-μm cell strainer (Falcon, BD Biosciences, Franklin
Lakes, NJ, USA). The cells were washed and 3×106 cells
were plated in six-well plates. After overnight incubation, the
cells were treated with the selective SYK inhibitor P505-15 (100
μmol/l) for 48 h. The cells were then treated with 20 ng/ml
IL-1β (R&D Systems) for 4 h. The cytokines in the cell
supernatants were quantified by ELISA. Results are expressed as the
average percent of the values in untreated controls. Written
consent was obtained from all patients and all experiments were
approved by the Institutional Ethics Committee of Shandong
University (Jinan, China).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Statistical analyses were performed using SPSS 16.0
(SPSS, Inc., Chicago, IL, USA). Statistical comparisons were
performed using one-way analysis of variance. The significance of
differences between groups was determined using Student’s unpaired
t-test. P<0.05 was considered to indicate a significant
difference between values.
Results
P505-15 treatment attenuates arthritis
score and joint damage in CIA mice
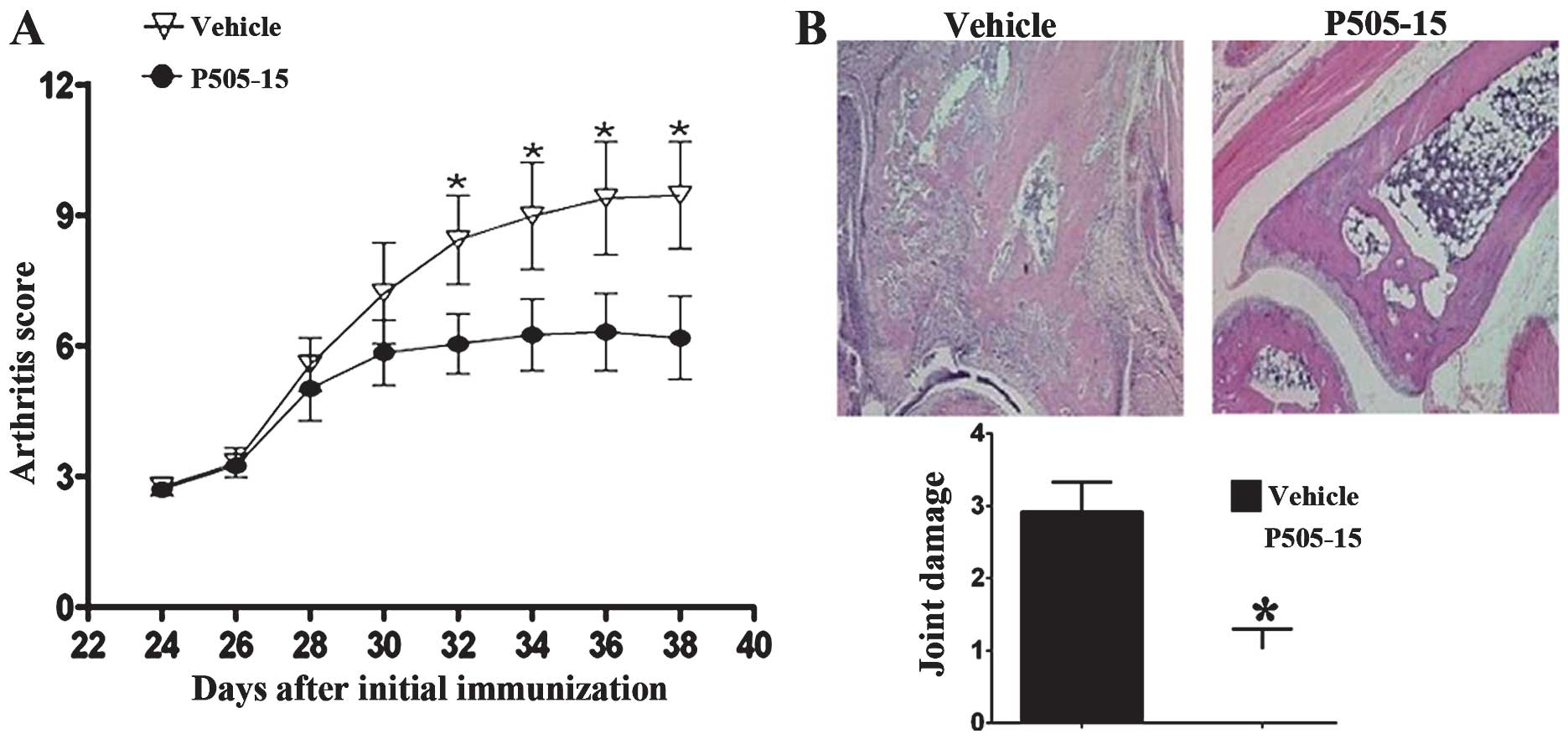
The mice started developing arthritis 24 days after
the first immunization. Clinical scores of CIA in DBA1/J mice were
recorded every 2 days from 24 days after the first immunization.
P505-15 treatment significantly attenuated the clinical symptoms of
arthritis from day 32 onwards (Fig.
1A). H&E staining (Fig.
1B) showed that the joints of vehicle-treated CIA mice showed
marked infiltration of leukocytes and bone damage. In the joints of
P505-15-treated CIA mice, the infiltration of inflammatory cells
and bone erosion were significantly inhibited (Fig. 1B).
P505-15 reduces cartilage destruction and
macrophage infiltration in CIA mice
In addition, the present study investigated the
efficacy of P505-15 treatment in CIA bone erosion using safranin-O
staining. The results indicated that the joints of CIA mice showed
evident cartilage destruction, which was significantly reduced in
the CIA mice treated with P505-15 (Fig. 2A and B).
The synovial tissue sections were also assessed for
the expression of CD68 using immunohistochemistry. A significant
reduction in CD68 expression was observed in the CIA mice treated
with P505-15 compared with that in the control group (P<0.01;
Fig. 2C and D).
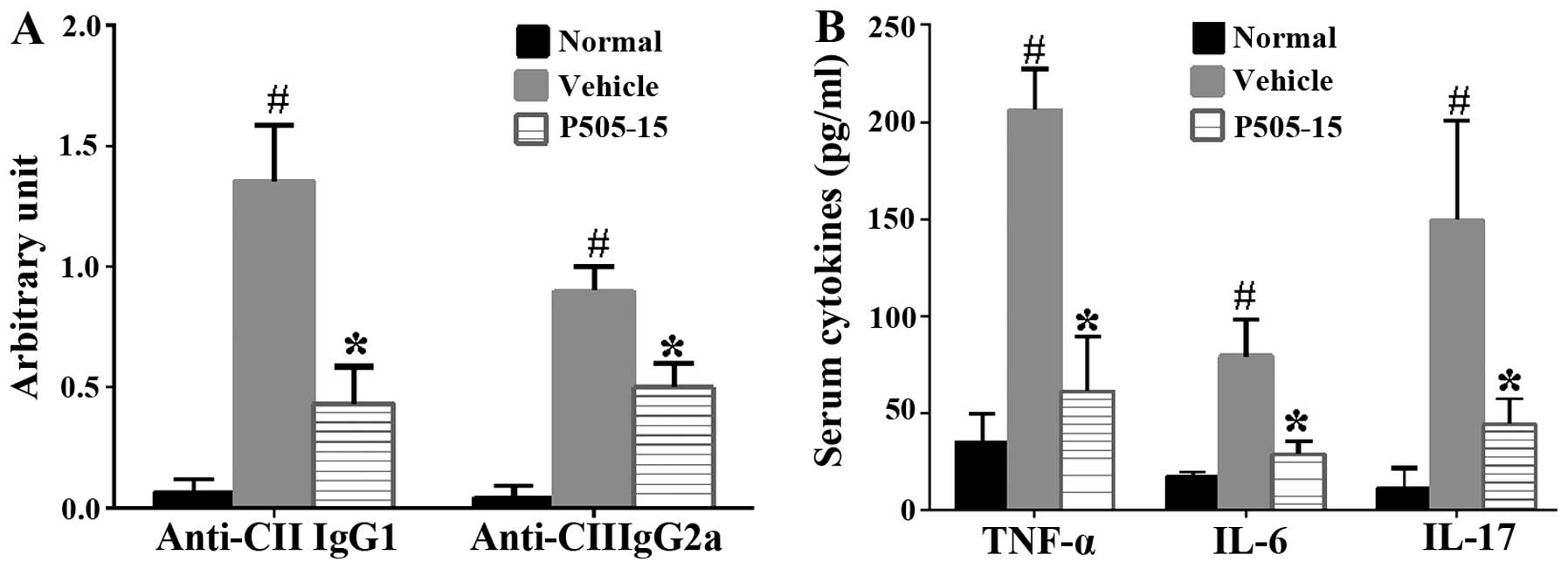
P505-15 treatment inhibits anti-CII IgG1,
-IgG2a and pro-inflammatory cytokines in CIA mice
The effects of P505-15 administration on the serum
anti-CII IgG1 and −IgG2a were assessed. The results indicated that
P505-15 treatment significantly reduced the number of anti-CII
antibodies relative to that in vehicle-treated CIA controls
(P<0.01; Fig. 3A). CIA is
characterized by marked expression of pro-inflammatory cytokines
(10). To ascertain whether
P505-15 inhibits this characteristic, P505-15-treated and
-untreated mice with CIA were bled on day 38. Serum TNF-α, IL-6 and
IL-17 levels were measured using an ELISA (Fig. 3B). The levels of these
pro-inflammatory cytokines were markedly increased in the
vehicle-treated group compared with those in the normal group,
which was significantly attenuated by treatment with P505-15
(P<0.01). The results therefore suggested that P505-15 may have
a therapeutic effect to reduce the severity of CIA by inhibiting
the production of inflammatory cytokines.
P505-15 treatment significantly decreases
inflammation in human RA synovial cells
To assess whether a similar phenomenon to that in
the mouse model of CIA can be observed in human RA, RA synovial
cells were cultured in the presence or absence of the SYK inhibitor
P505-15 (100 μmol/l) for 48 h. The cells were then treated
with 20 ng/ml IL-1β (R&D Systems) for 4 h. Supernatants were
then assayed for TNF-α, IL-6 and IL-17. The results indicated that
P505-15 treatment significantly reduced the levels of TNF-α, IL-6
and IL-17 (Fig. 4). The results
therefore suggested that SYK inhibition attenuates experimental
arthritis via inhibiting the generation of a pro-inflammatory
response.
Discussion
The results of the present study suggested that SYK
warrants clinical investigation as a therapeutic target in
autoimmune arthritis. In addition to the known anti-inflammatory
effects of SYK (11), the present
study was the first, to the best of our knowledge, to show that SYK
inhibition has a significant impact on CIA using a highly accurate
pre-clinical autoimmune arthritis model. More importantly, the
present study reported the effects of SYK inhibition in human RA
synovial cells.
Pharmacological inhibition of SYK using fostamatinib
or other small molecule inhibitors as potential immunomodulatory
agents is being actively pursued for the treatment of autoimmune
and inflammatory disorders in a number of in vivo models of
immune-mediated injury (5,11,12).
However, to the best of our knowledge, P505-15, a highly specific
and potent inhibitor of purified SYK, has not been tested in
rheumatoid arthritis models. SYK is a protein tyrosine kinase that
couples B-cell receptor (BCR) activation with downstream signaling
pathways, affecting cell survival and proliferation (13). P505-15 has been shown to inhibit
BCR-dependent secretion of the chemokines CCL3 and CCL4 by CLL
cells, and leukemia cell migration toward the tissue homing
chemokines CXCL12, CXCL13, and beneath stromal cells, which
demonstrates that the selective SYK inhibitor P505-15 is highly
effective in the inhibition of CLL survival and tissue homing
circuits, and supports the therapeutic development of these agents
in patients with CLL, other B-cell malignancies and autoimmune
disorders (13). The present study
implicated SYK in the pathogenesis of RA and demonstrated that
P505-15 is highly effective for ameliorating CIA disease. These
results are also supported by a study showing that genetic
deficiency of SYK in the hematopoietic compartment completely
blocked the development of arthritis and also prevented the
appearance of periarticular bone erosions (14). In further support of the results of
the present study, it was reported that SYK inhibition suppresses
the development of lupus disease and ameliorates established
disease in lupus-prone mice, and may therefore represent a valuable
treatment for patients with systemic lupus erythematosus (15,16).
In addition to preventing the production of
pathogenic autoantibodies, including serum anti-CII IgG1 and
−IgG2a, the present study observed that the pro-inflammatory
cytokine production in CIA was inhibited by P505-15. In addition,
SYK has been reported to have a crucial role in macrophage
activation (17,18). Consistent with this observation,
the present study showed that blockade of intracellular SYK
inhibited macrophage activation. Strengths of the present study,
however, include the use of a genuine autoimmune model of RA using
a well characterized selective SYK inhibitor. The striking findings
of the present study using this experimental model suggested that
clinical studies on SYK as a therapeutic target in RA are
desirable.
References
|
1
|
Lee HS, Woo SJ, Koh HW, Ka SO, Zhou L,
Jang KY, Lim HS, Kim HO, Lee SI and Park BH: Regulation of
apoptosis and inflammatory responses by insulin-like growth factor
binding protein 3 in fibroblast-like synoviocytes and experimental
animal models of rheumatoid arthritis. Arthritis Rheumatol.
66:863–873. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Firestein GS: Evolving concepts of
rheumatoid arthritis. Nature. 423:356–361. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meier FM, Frerix M, Hermann W and
Müller-Ladner U: Current immunotherapy in rheumatoid arthritis.
Immunotherapy. 5:955–974. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Riccaboni M, Bianchi I and Petrillo P:
Spleen tyrosine kinases: biology, therapeutic targets and drugs.
Drug Discov Today. 15:517–530. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tan S, Liao C, Lucas C, Stevenson C and
DeMartino JA: Targeting the SYK-BTK axis for the treatment of
immunological and hematological disorders: recent progress and
therapeutic perspectives. Pharmacol Ther. 138:294–309. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moon SJ, Park JS, Woo YJ, Lim MA, Kim SM,
Lee SY, Kim EK, Lee HJ, Lee WS, Park SH, Jeong JH, et al:
Rebamipide suppresses collagen-induced arthritis through reciprocal
regulation of th17/treg cell differentiation and heme oxygenase 1
induction. Arthritis Rheumatol. 66:874–885. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Criado G, Risco A, Alsina-Beauchamp D,
Pérez-Lorenzo MJ, Escós A and Cuenda A: Alternative p38 MAPKs are
essential for collagen-induced arthritis. Arthritis Rheumatol.
66:1208–1217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Spurgeon SE, Coffey G, Fletcher LB, Burke
R, Tyner JW, Druker BJ, Betz A, DeGuzman F, Pak Y, Baker D, Pandey
A, et al: The selective SYK inhibitor P505-15 (PRT062607) inhibits
B cell signaling and function in vitro and in vivo and augments the
activity of fludarabine in chronic lymphocytic leukemia. J
Pharmacol Exp Ther. 344:378–387. 2013. View Article : Google Scholar :
|
|
9
|
Tak PP, Smeets TJ, Daha MR, Kluin PM,
Meijers KA, Brand R, Meinders AE and Breedveld FC: Analysis of the
synovial cell infiltrate in early rheumatoid synovial tissue in
relation to local disease activity. Arthritis Rheum. 40:217–225.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Souza PP and Lerner UH: The role of
cytokines in inflammatory bone loss. Immunol Invest. 42:555–622.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kaur M, Singh M and Silakari O: Inhibitors
of switch kinase ‘spleen tyrosine kinase’ in inflammation and
immune-mediated disorders: a review. Eur J Med Chem. 67:434–446.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ghosh D and Tsokos GC: Spleen tyrosine
kinase: an Src family of non-receptor kinase has multiple functions
and represents a valuable therapeutic target in the treatment of
autoimmune and inflammatory diseases. Autoimmunity. 43:48–55. 2010.
View Article : Google Scholar
|
|
13
|
Hoellenriegel J, Coffey GP, Sinha U,
Pandey A, Sivina M, Ferrajoli A, Ravandi F, Wierda WG, O’Brien S,
Keating MJ and Burger JA: Selective, novel spleen tyrosine kinase
(Syk) inhibitors suppress chronic lymphocytic leukemia B-cell
activation and migration. Leukemia. 26:1576–1583. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jakus Z, Simon E, Balázs B and Mócsai A:
Genetic deficiency of Syk protects mice from autoantibody-induced
arthritis. Arthritis Rheum. 62:1899–1910. 2010.PubMed/NCBI
|
|
15
|
Chauhan AK and Moore TL: Immune complexes
and late complement proteins trigger activation of Syk tyrosine
kinase in human CD4(+) T cells. Clin Exp Immunol. 167:235–245.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng GM, Liu L, Bahjat FR, Pine PR and
Tsokos GC: Suppression of skin and kidney disease by inhibition of
spleen tyrosine kinase in lupus-prone mice. Arthritis Rheum.
62:2086–2092. 2010.PubMed/NCBI
|
|
17
|
Ulanova M, Asfaha S, Stenton G, Lint A,
Gilbertson D, Schreiber A and Befus D: Involvement of Syk protein
tyrosine kinase in LPS-induced responses in macrophages. J
Endotoxin Res. 13:117–125. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stenton GR, Ulanova M, Déry RE, Merani S,
Kim MK, Gilchrist M, Puttagunta L, Musat-Marcu S, James D,
Schreiber AD and Befus AD: Inhibition of allergic inflammation in
the airways using aerosolized antisense to Syk kinase. J Immunol.
169:1028–1036. 2002. View Article : Google Scholar : PubMed/NCBI
|