Introduction
Trans-9 octadecenoic acid (EA) is produced during
catalytic partial hydrogenation in the production of hardened fats
and during deodorization of commodity oils, and it is also present
in dairy fats (1). Trans-11
vaccenic acid (TVA) is a known positional and geometric isomer of
oleic acid and is the dietary precursor of cis-9, trans-11
conjugated linoleic acid (CLA) (2,3).
This fatty acid is an intermediate of ruminal biohydrogenation,
which has received significant attention due to its health
benefits, associated with and without CLA (3–5).
Cis-9, trans-11 CLA is one of a number of positional and geometric
isomers of linoleic acid (6). This
CLA isomer exerts several isomer-specific effects, including
anti-atherosclerotic, anti-inflammatory and anticarcinogenic
effects, and immune system modulation (3). These three fatty acids have received
attention due to their specific effects and their wide distribution
in human food.
In low-density lipoprotein (LDL) receptor-deficient
mice, a diet of EA-rich hydrogenated vegetable shortening for 14
weeks led to increased atherosclerosis, compared with butter rich
in TVA (7). When TVA-/CLA-enriched
butter was fed to rodents, the serum levels of cholesterol and
triacylglycerol, and the extent of atherosclerosis were reduced
compared with those fed with regular butter (8,9). In
rabbits, the ratio of atherogenic to antiatherogenic lipoproteins,
very-low-density lipoprotein (VLDL)+LDL to high-density lipoprotein
(HDL), was observed to be significantly lower in animals fed
TVA/CLA-enriched butter, compared with those fed
trans-10-C18:1-enriched group (9).
In addition, milk fat-rich cis-9, trans-11 CLA has been
demonstrated to reduce the atherogenic process in hyperlipidemic
hamsters (10), and bovine milk
fat, enriched with conjugated linoleic and vaccenic acids,
attenuates allergic airway disease in mice (11). The above findings were obtained
using butter, which was naturally enriched with EA, TVA or cis-9,
trans-11 CLA, or all three, however, the pure form of these fatty
acids was not used, and further investigations are required to
confirm these findings. Therefore, in the present study,
system-wide analyses were performed to assess the potential effects
of pure EA, TVA or cis-9, trans-11 CLA in mice.
Materials and methods
Subjects
All experiments involving animals were approved by
the Animal Care and Use Committee of Pusan National University
(Miryang, Korea; PNU-2012-0056). Male, 6-week-old, Institute of
Cancer Research (ICR) mice (Japan SLC Inc., Shizuoka, Japan) were
acclimated for 7 days prior to assessment of health status. The
study was approved by the ethics committee of Pusan National
University (Miryang, Korea). The mice were individually housed with
sawdust bedding and were maintained in environmentally controlled
rooms (ambient temperature, 22±2°C; relative humidity, 50±10%)
under a 12/12 h light-dark cycle (lights on 8:00 am–8:00 pm). Food
and water were available ad libitum. The repeated dose (4
weeks) investigation was performed in 32 mice, which were divided
into four groups of eight animals: Control (7% soybean oil), EA
(6.5% soybean oil + 0.5% EA), TVA (6.5% soybean oil + 0.5% TVA) and
CLA (6.5% soybean oil + 0.5% cis-9, trans-11 CLA), as shown in
Table I. The dosages of each fatty
acid were selected on the basis of the worldwide consumption report
(12). The fatty acids were
obtained from Matreya LLC (Pleasant Gap, PA, USA). The experimental
diets were manufactured by Feedlab (Kyung-ki, Seoul, Korea), and no
deterioration of the fatty acids in the diets was observed
following storage for 4 weeks (data not shown).
 | Table IComposition of the experimental
diets. |
Table I
Composition of the experimental
diets.
| Ingredient | Control | EA | TVA | CLA |
|---|
| Corn starch | 39.8 | 39.8 | 39.8 | 39.8 |
| Casein-vitamin
free | 20.0 | 20.0 | 20.0 | 20.0 |
| Maltodextrin | 13.2 | 13.2 | 13.2 | 13.2 |
| Sucrose | 10.0 | 10.0 | 10.0 | 10.0 |
| Soybean oil | 7.0 | 6.5 | 6.5 | 6.5 |
| Powdered
cellulose | 5.0 | 5.0 | 5.0 | 5.0 |
| AIN 93G mineral
mix | 3.5 | 3.5 | 3.5 | 3.5 |
| AIN 93G vitamin
mix | 1.0 | 1.0 | 1.0 | 1.0 |
| L-cystine | 0.3 | 0.3 | 0.3 | 0.3 |
| Choline
bitartrate | 0.25 | 0.25 | 0.25 | 0.25 |
|
t-Butyl-hydroquinone | 0.0014 | 0.0014 | 0.0014 | 0.0014 |
| Pure fatty acid | 0.0 | 0.5 | 0.5 | 0.5 |
| Total | 100 | 100 | 100 | 100 |
| Proximate
composition | | | | |
| Crude fat (%) | 6.4±0.0 | 6.9±0.0 | 6.9±0.0 | 7.1±0.0 |
| Crude protein
(%) | 15.9±0.0 | 15.8±0.1 | 16.2±0.1 | 14.4±0.1 |
| Calorie
(kcal/g) | 4.319 | 4.396 | 4.379 | 4.355 |
| Fatty acid
composition (%) | | | | |
| EA | ND | 2.7±0.02a | ND | ND |
| TVA | ND | ND | 2.8±0.27 | ND |
| CLA | ND | ND | ND | 2.4±0.03 |
| Oleic acid | 5.3±0.05 | 4.5±0.14 | 4.8±0.28 | 4.7±0.07 |
| Linoleic acid | 12.3±0.18 | 10.4±0.32 | 11.1±0.51 | 10.2±0.15 |
| Linolenic
acid | 1.6±0.02 | 1.4±0.04 | 1.5±0.07 | 1.4±0.02 |
The total fatty acid compositions of the diets were
analyzed and are described in Table
I. Briefly, the total lipids were extracted from 4 g of feed
using 20 ml chloroform/methanol (2:1; v/v; Burdick & Jackson,
Muskegon, MI, USA). The extracted lipids were subsequently
converted into fatty acid methyl esters using 14% (w/v) boron
trifluoride-methanol (cat. no. B1252; Sigma-Aldrich, St. Louis, MO,
USA), according to a previously published method (13). The fatty acid methyl esters were
then subjected to gas chromatography (Agilent 7890A GC system;
Agilent Technologies, Inc., Santa Clara, CA, USA) using a system
equipped with a 7863 series auto-sampler, 7683B series injector and
flame ionization detector. An SP™-2560 fused silica capillary
column (L × I.D. 100 m × 0.25 mm, df 0.20 μm) was used
(Supelco Inc., Bellefonte, PA, USA) to analyze the fatty acid
compositions of the samples. The peaks were routinely identified by
comparing the duration of retention with those of the standards,
including Supelco 37 Component Fatty acid methyl esters mix
(47885-U), trans-11-octadecenoicmethyl ester (46905-U;
Sigma-Aldrich) and cis-9, trans-11 CLA (1255; Matreya LLC, Pleasant
Gap, PA, USA). The percentages of the individual fatty acids were
calculated as the ratio of each area compared with that of the
total identified fatty acids, as described previously (14). The four experimental diets
contained a similar concentration of fatty acids. The
concentrations of the three assessed fatty acids decreased to 0.25%
from an initial concentration of 0.5%, which may have been due to
losses acquired during production (Table I). The body weights and food intake
of each mice were measured every 2 days. In addition, the general
appearance, behavior and signs of morbidity and mortality were
observed every 2 days throughout the experiment.
Blood sampling and blood metabolite
analysis
Following 4 weeks of dietary intervention, the mice
were sacrificed by CO2 asphyxiation and blood samples
were collected (5 ml) from the external jugular veins. The blood
was centrifuged at 400 × g at 4°C for 15 min and the supernatant
was collected and stored at −80°C until subsequent analysis.
The blood metabolites were analyzed using a Toshiba
Accute Biochemical Analyzer-TBA-40FR (Toshiba Medical Instruments,
Tochigi-ken, Japan), according to the manufacturer’s instructions.
A total of 10 metabolites, including blood urea nitrogen, calcium,
magnesium, triglyceride, glucose, non-esterified fatty acid,
albumin, total cholesterol, LDL cholesterol and HDL cholesterol
were analyzed. All the reagents required for this procedure were
purchased from Wako Pure Chemical Industries, Ltd. (Chuo-ku, Osaka,
Japan).
Anatomical pathology
Samples of the small intestine and liver
(n=80/group) were collected and fixed in formaldehyde solution
(Sigma-Aldrich). The samples for histopathological examination were
processed, embedded in paraffin (Sigma-Aldrich), cut to a thickness
of ~2–4 μm and stained using hematoxylin and eosin
(Sigma-Aldrich). The length of the villi were then quantified
(n=80/group) by measuring the distance between the crypt necks.
Western blotting
The relative protein quantities of cholesterol
7α-hydroxylase (CYP7α1) in the liver were determined using western
blotting. Briefly, the proteins (15 μg protein/lane) were
separated using 10% sodium dodecyl sulfate polyacryl-amide gel
electrophoresis (SDS-PAGE; Bio-Rad Laboratories, Inc., Hercules,
CA, USA) and were subsequently transferred onto a
polyvinylidenedifluoride membrane (GE Healthcare Life Sciences.,
Piscataway, NJ, USA). The membrane was blocked with
phosphate-buffered saline (PBS)-T, containing 10% 10X PBS (pH 7.6),
0.2 M Tris base, 1.37 M NaCl and 10% Tween-20, with 5% skim milk
(BD Biosciences, San Jose, CA, USA), for 1 h at room temperature.
The membrane was incubated overnight with specific primary
antibodies against rabbit monoclonal β-actin (1:1,000; cat. no.
ab32575; Abcam, Cambridge, MA, USA) and mouse monoclonal CYP7α1
(1:1,000; cat. no. ab77157; Abcam) at 4°C. Following washing three
times with TBS-T, the membrane was incubated with horseradish
peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin G
(1:1,000; cat. no. ab6721; Abcam) for 30 min at room temperature.
The membrane was then washed with TBS-T and antibody binding was
visualized using an enhanced chemiluminescence system and detection
kit (GE Healthcare Life Sciences, Pittsburgh, PA, USA), according
to the manufacturer’s instructions. The membrane was then exposed
to X-ray film (Fujifilm Corporation, Minato-ku, Tokyo, Japan) for 1
min (β-actin) or 3 min (CYP7α1). The film was scanned and bands
were quantified using the Image J® 1.43 software (NIH,
Bethesda, MD, USA). The protein levels were normalized against that
of β-actin on the same membrane.
Cytokine production
The concentrations of tumor necrosis factor-α
(TNF-α) in the plasma were determined using an ELISA kit (cat. no.
88-7324; eBioscience, Inc., San Diego, CA, USA) at the end of the
experimental period, according to the manufacturer’s instructions.
The absorbance was then measured at 450 nm using a Multiskan EX
Microplate Reader (Thermo Fisher Scientific Inc., Waltham, MA,
USA).
Statistical analysis
All data are expressed as the mean ± standard error
of the mean and were analyzed using one-way analysis of variance
with SPSS 11.5 software (SPSS Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results and Discussion
Changes in daily food intake, body weight
and organ parameters
Changes in the general condition and external
appearance of the rodents were observed in mice fed with EA or TVA.
The animals of these two groups were small and weak in appearance,
and their fur, eyes and oral cavity conditions were poor (data not
shown). Although all the mice had a similar daily food intake
(Fig. 1A), the body weights of the
animals in the EA, TVA and CLA groups were all gradually decreased
following 10 days administration of the assessed fatty acids
(Fig. 1B). However, no significant
change was observed in the lengths of the intestine or colon
(Fig. 1C). In addition, the
weights of the mediastinal adipose tissue (MAT) of the animals in
these three groups were decreased significantly, compared with the
control group (P<0.05; Fig.
1C).
A previous study reported that TVA exerted no effect
on either the body weight or food intake in obese or lean rats
during experimental periods (15).
In another previous study, no significant differences in body
weight or body weight gain were observed between groups treated
with enriched dairy fat compared with the control throughout the
experimental period (16).
However, in the present study, the body weight decreased gradually
following treatment with TVA, the reason for this difference
remains to be elucidated and requires further investigation.
Ratio of organ/body weight (%)
Significant effects of the three assessed fatty
acids on organ weights was observed (Table II). The weights of the liver and
testis of mice in the EA, TVA and CLA groups were all lower
compared with those in the control groups (P<0.05). The weights
of the spleen, kidney and heart of mice in the EA and TVA groups
were also decreased (P<0.05; Table
II). However, a previous study reported no gross pathological
changes or differences in organ weight following 4 weeks of
treatment with TVA (16).
 | Table IIRatio of organ/body weight (%) in
mice following repeated oral administration of EA, TVA or CLA for 4
weeks. |
Table II
Ratio of organ/body weight (%) in
mice following repeated oral administration of EA, TVA or CLA for 4
weeks.
| Organ | Control | EA | TVA | CLA |
|---|
| Brain | 1.6±0.0a | 1.7±0.1 | 1.7±0.0 |
| Heart | 0.7±0.0b,a | 0.6±0.0b | 0.6±0.0b | 0.6±0.0a,b |
| Kidney | 2.0±0.1a | 1.6±0.0c | 1.7±0.1b,c | 1.8±0.0a,b |
| Large
intestine | 2.4±0.2 | 1.9±0.1 | 2.5±0.2 | 1.9±0.2 |
| Liver | 5.8±0.2a | 4.3±1.0c | 4.9±0.3b | 5.1±0.2b |
| Lung | 1.2±0.1a | 1.0±0.1a,b | 0.9±0.1b,c | 1.1±0.2a,b |
| Small
intestine | 5.5±0.1 | 5.2±0.3 | 5.4±0.4 | 5.3±0.2 |
| Spleen | 0.5±0.0a | 0.2±0.0b,c | 0.3±0.0b,c | 0.7±0.3a |
| Stomach | 4.5±0.5 | 3.6±0.3 | 3.4±0.3 | 3.4±0.4 |
| Testis | 0.9±0.0a | 0.6±0.1c | 0.8±0.0b | 0.8±0.0b |
Analysis of blood metabolites
A metabolic profile test (MPT) was initially
designed as a pre-symptomatic diagnostic aid, based on the
statistical analyses of blood metabolites, to provide an early
warning of certain types of metabolic imbalances (17). Previously, it was used to measure
physiological characteristics for selection programs designed to
improve animal production traits (18). In the present study, 10 blood
metabolites were analyzed to determine if any of these factors were
correlated with the consumption of pure EA, TVA or CLA. No
significant changes were observed in the TVA or CLA group, compared
with the control. The EA group exhibited lower levels of Mg and
triglycerides compared with the other groups (P<0.05; Table III). The levels of Mg,
triglycerides and glucose were significantly decreased in the EA
group compared with those in the control group (P<0.05).
 | Table IIIAnalysis of blood metabolites in mice
following repeated oral administration of EA, TVA or CLA for 4
weeks. |
Table III
Analysis of blood metabolites in mice
following repeated oral administration of EA, TVA or CLA for 4
weeks.
| Metabolite | Control | EA | TVA | CLA |
|---|
| BUN (mg/dl) | 13.9±3.66a | 15.5±2.92 | 21.1±3.70 | 14.6±3.06 |
| Ca (mg/dl) | 6.6±1.12 | 5.2±0.72 | 6.9±0.37 | 7.2±0.38 |
| Mg (mg/dl) | 2.7±0.11a,b | 2.1±0.18b | 2.8±0.25a | 2.6±0.18a |
| Triglyceride
(mg/dl) | 49.6±2.73a | 18.4±3.61b | 49.6±17.13a | 61.4±19.37a |
| Glucose
(mg/dl) | 159.4±8.66a | 126.0±9.97b | 147.3±18.99a,b | 149.9±18.97a,b |
| NEFA (ueq/l) | 448.0±66.78 | 335.6±48.04 | 388.3±95.66 | 442.6±94.62 |
| Albumin (g/dl) | 2.1±0.08 | 2.0±0.15 | 2.1±0.10 | 2.3±0.09 |
Circulating levels of cholesterol and the
expression of cholesterol 7-hydroxylase
Neither the total cholesterol concentrations or the
HDL:total cholesterol ratio in the plasma were significantly
different between the groups (Fig.
2A–C). However, the presence of TVA in the diet reduced the
levels of plasma LDL cholesterol, compared with the other groups
(P<0.05; Fig. 2A–C). In
addition, although no significant difference was observed, the
expression of CYP7α1 was increased in the TVA and CLA groups
(Fig. 2D). EA is the predominant
trans fat in hydrogenated vegetable oils and it has been confirmed
that the consumption of EA exerts negative effects on health
(5,19,20).
In particular, EA increases the activity of cholesterylester
transfer protein, which increases the level of VLDL cholesterol and
reduces the level of HDL cholesterol (21). CYP7α1 is a rate-limiting enzyme in
the synthesis of bile acid from cholesterol via the classical
pathway (22). A previous study
reported primarily neutral effects of TVA/CLA-enriched butter on
atherogenic risk factors and reduced aorta fatty streak development
(5,9). However, the ratios of atherogenic to
anti-atherogenic lipoproteins, VLDL+LDL to HDL, are significantly
lower in TVA/CLA-enriched butter groups compared with those of
trans-10-C18:1-enriched groups (9).
Histochemical analysis of the structure
of the mouse small intestine
No significant liver injury was observed in any of
the groups following 4 weeks of dietary intervention with EA, TVA
or CLA (Fig. 3A). However,
morphological analyses demonstrated that EA and TVA intervention
structurally shortened the length of villi, whereas CLA increased
the villi length compared with that of the control (Fig. 3B and C). Villi are finger-like
projections in the small intestine, which are important for the
digestion and absorption of food (23). These results suggested that EA and
TVA had negative effects (P<0.05) on villi morphogenesis in the
small intestines of the mice, where CLA exerted a positive effect
(P<0.05; Fig. 3D).
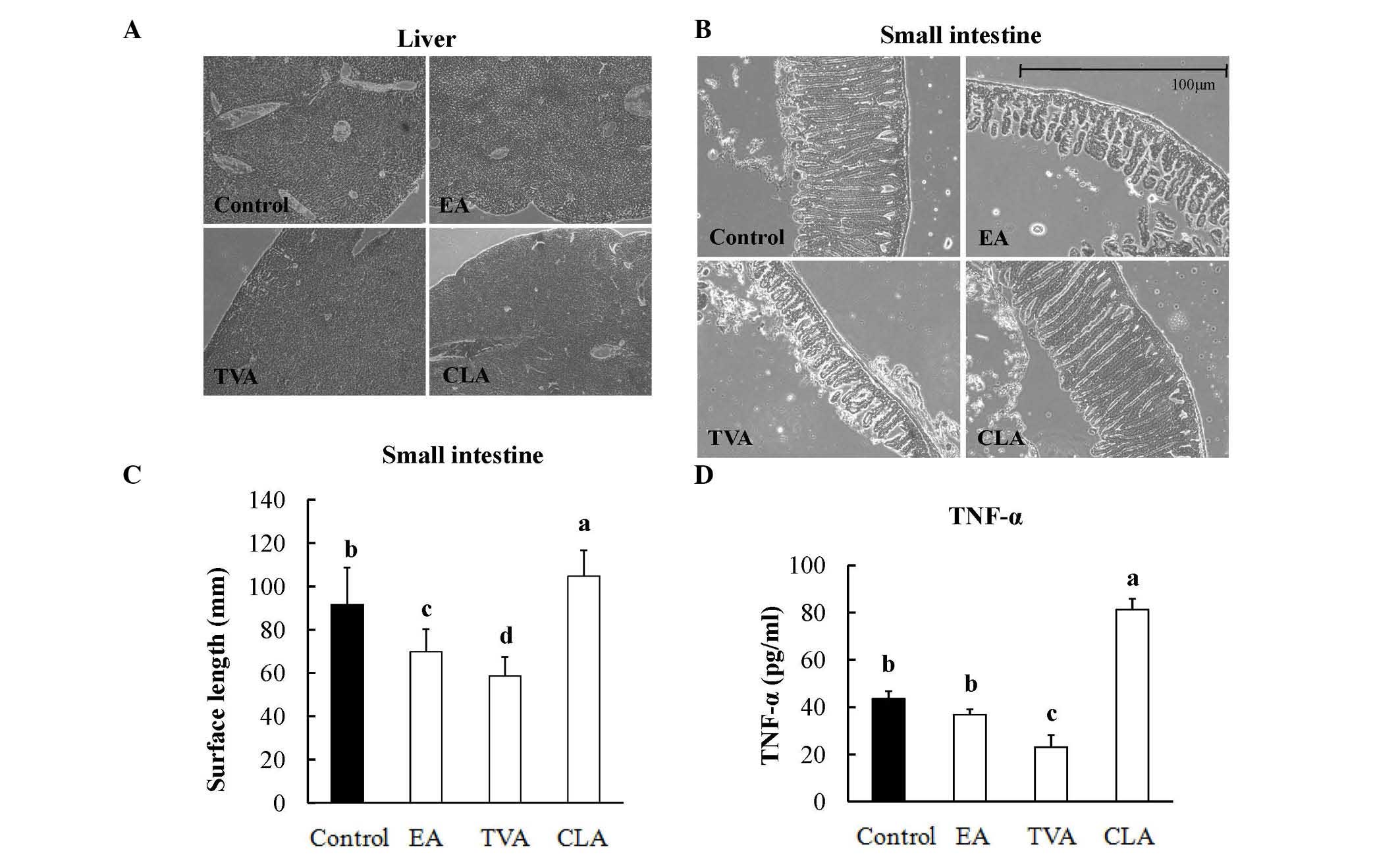 | Figure 3Histochemical analysis of the small
intestines and analysis of cytokine production in the plasma of
mice fed EA, TVA or CLA for 4 weeks. Representative images of the
(A) liver and (B) small intestine (n=80/group; magnification, x100;
Olympus CX22). (C) Quantification of the villus length in mice fed
EA, TVA or CLA for 4 weeks. (D) Expression levels of TNF-α (n=8)
were determined following treatment with EA, TVA or CLA for 4 weeks
(abc and dP<0.05, compared with the control group).
EA, octadecenoic acid; TVA, trans-11 vaccenic acid; CLA, cis-9,
trans-11 conjugated linoleic acid; TNF, tumor necrosis factor. |
Cytokine production in the plasma of
mice
Cytokines are fundamental in modulating
inflammation, phagocytosis, tissue injury and cell death.
Accordingly, the present study measured the plasma levels of
circulating TNF-α. The CLA group exhibited higher levels of TNF-α
(P<0.05), whereas the TVA group exhibited lower levels, compared
with the control group (Fig. 3D).
It has been confirmed that the plasma levels of TNF-α are
significantly lower in rats fed CLA for 6 weeks compared with
control-fed rats 2 h following LPS challenge, whereas the
production of TNF-α in PBS-injection control mice remains unchanged
(24). Additionally, a previous
report indicated that dietary CLA inhibits the production of TNF-α
in weaned pigs challenged with LPS at the protein and mRNA
expression levels (25). Previous
studies have also indicated that CLA inhibits LPS-stimulated
production of TNF-α and expression, partially due to inhibition of
the binding activity of nuclear factor-κB. However, the present
study did investigate the effects of LPS challenge, therefore, the
increased expression of TNF-α by CLA may offer potential in
immune-modulation. A pro-inflammatory response can be beneficial
for bacterial clearance, however, overactivation of the
inflammatory response can lead to cell injury and shock. Therefore,
consideration of the appropriate dietary concentrations of CLA is
required.
In conclusion, the administration of EA, TVA or CLA
in the present study reduced the body weights of the mice,
specifically the weights of the liver, testis and MAT. Treatment
with EA decreased the levels of magnesium and triglycerides, and
the length of the villi, whereas treatment with CLA increased the
length of the villi and the expression of TNF-α. TVA also reduced
the length of the villi, and the levels of LDL and TNF-α. Overall,
the mice exhibited different responses to EA, TVA and CLA, however,
the underlying mechanism remains to be elucidated and requires
further investigation.
Acknowledgments
This study was supported by a grant from the
concentrated research professor program for Konkuk University,
Seoul, Republic of Korea.
References
|
1
|
Low JN, Scrimgeour C and Horton P: Elaidic
acid (trans-9-octa-decenoic acid). Acta Cryst. E61:o3730–o3732.
2005.
|
|
2
|
Bauman DE, Baumgard LH, Corl BA and
Griinari JM: Biosynthesis of conjugated linoleic acid in ruminants.
J Anim Sci. E77:1–15. 2000.
|
|
3
|
Wang T and Lee HG: Advances in Research on
cis-9, trans-11 Conjugated Linoleic Acid: A Major Functional
Conjugated Linoleic Acid Isomer. Crit Rev Food Sci Nutr.
55:720–731. 2015. View Article : Google Scholar
|
|
4
|
Wang Y, Lu J, Ruth MR, Goruk SD, Reaney
MJ, Glimm DR, Vine DF, Field CJ and Proctor SD: Trans-11 vaccenic
acid dietary supplementation induces hypolipidemic effects in JCR:
LA-cp rats. J Nutr. 138:2117–2122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bassett C, Edel AL, Patenaude AF,
McCullough RS, Blackwood DP, Chouinard PY, Paquin P, Lamarche B and
Pierce GN: Dietary vaccenic acid has antiatherogenic effects in
LDLr-/-Mice. J Nutr. 140:18–24. 2010. View Article : Google Scholar
|
|
6
|
Dilzer A and Park YH: Implication of
conjugated linoleic acid (CLA) in human health. Crit Rev Food Sci
Nutr. 52:488–513. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dupasquier CMC, Patenaude AF, Blackwood
DP, Chouinard Y, Lamarche B and Pierce GN: Elaidic and vaccenic
TFAs have different effects on atherosclerotic development in low
density lipoprotein receptor deficient (LDLr-/-) mice. Ann Nutr
Metab. 1:2662007.
|
|
8
|
Lock AL, Horne CA, Bauman DE and Salter
AM: Butter naturally enriched in conjugated linoleic acid and
vaccenic acid alters tissue fatty acids and improves the plasma
lipoprotein profile in cholesterol-fed hamsters. J Nutr.
135:1934–1939. 2005.PubMed/NCBI
|
|
9
|
Roy A, Chardigny JM, Bauchart D, Ferlay A,
Lorenz S, Durand D, Gruffat D, Faulconnier Y, Sébédio JL and
Chilliard Y: Butters rich either in trans-10-C18:1 or in
trans-11-C18:1 plus cis-9, trans-11 CLA differentially affect
plasma lipids and aortic fatty streak in experimental
atherosclerosis in rabbits. Animal. 1:467–476. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Valeille K, Ferezou J, Amsler G,
Quignard-Boulange A, Parquet M, Gripois D, Dorovska-Taran V and
Martin JCA: cis-9, trans-11-conjugated linoleic acid-rich oil
reduces the outcome of atherogenic process in hyperlipidemic
hamster. Am J Physiol Renal Physiol. 289:H652–H659. 2005.
View Article : Google Scholar
|
|
11
|
Kanwar RK, Macgibbon AK, Black PN, Kanwar
JR, Rowan A, Vale M and Krissansen GW: Bovine milk fat enriched in
conjugated linoleic and vaccenic acids attenuates allergic airway
disease in mice. Clin Exp Allergy. 38:208–218. 2008.
|
|
12
|
Craig-Schmidt MC: World-wide consumption
of trans fatty acids. Atheroscler Suppl. 7:1–4. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Folch J, Lees M and Sloane Stanley GH: A
simple method for the isolation and purification of total lipids
from animal tissues. J Biol Chem. 226:497–509. 1957.PubMed/NCBI
|
|
14
|
Wang T, Oh JJ, Lim JN, Hong JE, Kim JH,
Kim JH, Kang HS, Choi YJ and Lee HG: Effects of lactation stage and
individual performance on milk cis-9, trans-11 conjugated linoleic
acids content in dairy cows. Asian-Aust J Anim Sci. 26:189–194.
2013. View Article : Google Scholar
|
|
15
|
Blewett HJ, Gerdung CA, Ruth MR, Proctor
SD and Field CJ: Vaccenic acid favourably alters immune function in
obese JCR: LA-cp rats. Br J Nutr. 102:526–536. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Anadon A, Martinez-Larranaga MR, Martinez
MA, Ares I, Ramos E, Gomez-Cortes P, Juarez M and de la Fuente MA:
4-Week repeated oral dose toxicity study of dairy fat naturally
enriched in vaccenic, rumenic and α-linolenic acids in rats. J
Agric Food Chem. 59:8036–8046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Payne JM: The Compton metabolic profile
test. Proc R Soc Med. 65:181–183. 1972.PubMed/NCBI
|
|
18
|
Kato Y, Ito M and Hirooka H: Genetic
parameters of serum vitamin A and total cholesterol concentrations
and the genetic relationships with carcass traits in an F1 cross
between Japanese Black sires and Holstein dams. J Anim Sci.
89:951–958. 2011. View Article : Google Scholar
|
|
19
|
Meijer GW, Tol AV, Berkel TV and
Weststrate JA: Effect of dietary elaidic versus vaccenic acid on
blood and liver lipids in the hamster. Atherosclerosis. 157:31–40.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fournier N, Attia N, Rousseau-Ralliard D,
Vedie B, Destaillats F, Grynberg A and Paul JL: Deleterious impact
of elaidic fatty acid on ABCA1-mediated cholesterol efflux from
mouse and human macrophages. Biochim Biophys Acta. 182:303–312.
2012. View Article : Google Scholar
|
|
21
|
Abbey M and Nestel PJ: Plasma cholesteryl
ester transfer protein activity is increased when trans-elaidic
acid is substituted for cis-oleic acid in the diet.
Atherosclerosis. 106:99–107. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jelinek DF, Andersson S, Slaughter CA and
Russell DW: Cloning and regulation of cholesterol 7
alpha-hydroxylase, the rate-limiting enzyme in bile acid
biosynthesis. J Biol Chem. 265:8190–8197. 1990.PubMed/NCBI
|
|
23
|
Ryan G; Tissue Engineering the Small
Intestine. Clin Gastroenterol Hepatol. 11:354–358. 2013. View Article : Google Scholar
|
|
24
|
Yang M and Cook ME: Dietary conjugated
linoleic acid decreased cachexia, macrophage tumor necrosis
factor-α production and modifies splenocyte cytokines production.
Exp Biol Med (Maywood). 228:51–58. 2003.
|
|
25
|
Zhao L, Yin J, Li D, Lai C, Chen X and Ma
D: Conjugated linoleic acid can prevent tumor necrosis factor gene
expression by inhibiting nuclear factor binding activity in
peripheral blood mononuclear cells from weaned pigs challenged with
lipopolysaccharide. Arch Anim Nutr. 59:429–438. 2005. View Article : Google Scholar
|

















