Introduction
Hydrogen sulfide (H2S) has previously
been identified as a signaling molecule that exhibits numerous
physiological and pathological activities (1). Exogenous sodium hydrosulfide (NaHS)
releases H2S, in order to induce physiological
responses. Previous studies have demonstrated that H2S
may relax vascular and ileal smooth muscle in the cardiovascular
system (2), increase colonic
secretion and reduce gastric injury in the gastrointestinal system
(3), attenuate neuronal injury
(4), and prevent the development
of hypertension (5). The roles of
H2S also include the inhibition of oxidative stress
(6), the production of lipid
peroxidation and inflammatory factors (7), and the activation of ATP-sensitive
potassium channels (KATP) (8).
H2S regulates hepatic-related injury, and
is produced in the liver by both cystathionine-γ-lyase (CSE) and
cystathionine-β-synthase (CBS), which use L-cysteine as a substrate
to produce H2S. H2S exhibits
anti-inflammatory and cytoprotective activities against hepatic
ischemia reperfusion (I/R) injury (9). Carbon tetrachloride
(CCl4)-induced downregulation of H2S
production and CSE expression is associated with the development of
increased intrahepatic resistance, portal hypertension, and hepatic
fibrosis in a rat model of liver cirrhosis (10). A previous study suggested that
H2S and CSE exhibit anti-fibrotic effects in pulmonary
fibrosis (11). However, to the
best of our knowledge, there have been few attempts to investigate
the role of H2S in hepatic fibrosis.
Hepatic fibrosis is a common response to chronic
liver injury caused by various diseases. The mechanisms underlying
the development of hepatic fibrosis consist predominantly of the
activation of hepatic stellate cell (HSCs), and the accumulation of
extracellular matrix components within the liver (12). The renin angiotensin system (RAS)
is involved in the pathogenesis of fibrosis, both in the heart and
various other organs (13,14). Notably, activated human HSCs
express RAS components and synthesize angiotensin II. Furthermore,
angiotensin II type 1 receptors (AGTR1) are located in HSCs
(15). Previous studies have
demonstrated that the control of RAS activation by AGTR1
antagonists may have therapeutic potential in the treatment of
hepatic fibrosis. H2S and NaHS decrease AGTR1 binding,
as well as AGTR1 binding affinity in spontaneously hypertensive
rats (16). The present study
aimed to investigate whether H2S affects hepatic
fibrosis by regulating the expression of AGTR1.
Materials and methods
Materials and reagents
Pathogen-free male Wistar rats (weighing 200–300 g)
were purchased from XiPuer-Rubicam Experimental Animals, Ltd.
(Shanghai, China). Analytical grade CCl4 and
DL-propargylglycine (PAG) were purchased from Beijing Dingguo
Changsheng Biotechnology Co., Ltd. (Beijing, China). Analytical
grade zinc cetate, N,N-dimethy-p-phenylenediamine, HCl,
trichloroacetic acid, and NaHS were purchased from Sigma-Aldrich
(St. Louis, MO, USA). The rat ELISA kit was purchased from
Sigma-Aldrich, the reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) kit was obtained from Takara Bio, Inc.
(Tokyo, Japan), and the Western Blotting kit was from Abcam
(Cambridge, UK). The current study was approved by the ethics
committee of Shanghai Jiaotong University Affiliated Sixth People's
Hospital (Shanghai, China).
Experimental design
The rats were randomly divided into four groups
(n=14/group): Normal control group, model hepatic fibrosis group,
NaHS group, and PAG group. NaHS is a H2S donor, and PAG
is a CBS inhibitor. Each group was then randomly divided into two
subgroups each containing seven rats; one of the subgroups received
treatment for three weeks, whereas the other one received treatment
for four weeks. The rats were maintained in a sterile environment
with ad libitum access to drinking water, and underwent a 12
h light/dark cycle. Hepatic fibrosis was induced using 5 ml/kg 40%
CCl4 in corn oil tree time weekly for three or four
weeks in all groups, except for the normal control group. The rats
in the PAG group were intraperitoneally injected with 45
µmol/kg/day PAG, a CBS inhibitor. The rats in the NaHS group
were intraperitone-ally injected with 56 µmol/kg/day NaHS,
H2S donor. An equal volume of saline was
intraperitoneally injected into the control and model group rats.
The rats were then sacrificed with 2% pentobarbital (H. Lundbeck
Co., Copenhagen, Denmark) at week three or four following modeling,
depending on their subgroup.
Serum biochemical measurements
The serum expression levels of alanine
aminotransferase (ALT), aspartate aminotransferase (AST), and
albumin (ALB) were determined using commercially available kits
(Diatech Diagnostics, Hungary) using the Autobiochemical Analyzer
(Toshiba, Tokyo, Japan) following centrifugation at 3,000 × g at
4°C of the blood collected at 4,000 rpm for 15 min. The serum
expression levels of hyaluronidase (HA), laminin protein (LN),
procollagen III (PcIII), and collagen IV (cIV) were measured using
an ELISA kit.
Histopathological examination
The livers were removed and washed with saline to
remove excess blood. Liver tissue sections (5 µm) were fixed
in formalin and embedded in paraffin prior to examination. The
liver sections were then stained using hematoxylin and eosin (HE)
and Masson's trichrome (Wuhan Biotechnology Ltd., Co., Wuhan,
China), in order to determine the stage of hepatic fibrosis. The
fibrotic stages were scored according to Brunt (17): S0, no fibrosis; S1, portal fibrous
expansion; S2, thin fibrous septa emanating from portal triads; S3,
fibrous septa bridging portal triads and central veins; S4,
cirrhosis.
RT-qPCR
RT-qPCR was performed in order to analyze the mRNA
expression levels of CSE in hepatic tissue, as previously described
(18). Total RNA (2 µg) was
extracted from the hepatic samples and reverse transcribed using
the RevertAid First-Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's instructions. The PCR primer sequences from Takara
Bio, Inc. (Tokyo, Japan) were as follows: CSE, forward
5′-CCACCACAACGATTACCCA-3′, reverse 5′-TCAGCACCCAGAGCCAAAG-3′; and
β-actin, forward 5′-TCCTGACCCTGAAGTACCCCATTG-3′, and reverse
5′-GGAACCGCTCATTGCCGATAGT-3′. RT-qPCR was performed using the SYBR
Green qPCR Super Mixture (Takara Bio, Inc.) and an ABI Prism 7500
Sequence Detection System (Applied Biosystems Life Technologies,
Foster City, CA, USA). Amplification was performed with the
following cycles: 95°C for 2 min, followed by 40 cycles of
denaturing at 95°C for 15 sec, and annealing at 60°C for 32 sec.
All reactions were performed in triplicate. Data analysis was
performed using the 2−ΔΔCT method, as described by Livak
and Schmittgen (19), with β-actin
acting as a reference gene.
Western blot analysis
The protein expression levels of AGTR1 were detected
by western blotting. The membrane protein fractions were prepared
as previously described (20).
Briefly, 100 mg liver tissue samples were first homogenized
ultrasonically for 4 min using a Tissue Pulverizer (BioSpec
Products, Bartlesville, OK, USA). The samples were then lysed in
radioimmunoprecipitation buffer, separated by 10% SDS-PAGE and
electro-transferred to nitrocellulose membranes (EMD Millipore,
Billerica, MA, USA). The membranes were then blocked with 5%
non-fat dry milk in tris-buffered saline containing
Tween® 20 [TBST; 10 mmol/l Tris-hydrochloric acid (HCl)
pH 8.0, 150 mmol/l NaCl, and 1% Tween® 20]. The
membranes were probed with a rabbit polyclonal AGTR1 antibody (cat.
no. sc-1173; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at a
dilution of 1:1,000 in blocking solution (10 mM Tris-HCl, 5%
powdered milk, 2% bovine serum albumin, 0.1% Tween® 20,
pH 7.6; Sigma-Aldrich). Following extensive washing with TBST, the
membranes were incubated for 1 h with a secondary horseradish
peroxidase (HRP)-conjugated goat anti-rabbit antibody (cat. no.
A6154; Sigma-Aldrich) at a dilution of 1:2,000 in
phosphate-buffered saline (PBS). After a final washing step with
TBST, the blots were visualized using an enhanced chemiluminescence
kit (GE Healthcare Life Sciences, Chalfont, UK). In order to detect
β-actin, the membranes were stripped with 0.2% SDS, 0.1%
mercaptoethanol, and 1 M Tris pH 6.8 for 30 min at 70°C, prior to
being incubated with a mouse monoclonal β-actin antibody (cat. no.
A5441, Sigma-Aldrich) at a dilution of 1:4,000 in blocking solution
for 1 h. The membranes were then incubated with the HRP-conjugated
goat anti-rabbit antibody (cat. no. A6154, Sigma-Aldrich) at a
dilution of 1:2,000 in PBS for 1 h. The results of the enhanced
chemiluminescence analysis were digitized by conventional scanning,
and quantified using computerized image analysis software Alpha
Imager 2000, according to the manufacturer's instructions. The
densitometric results were expressed in relation to the ratio of
AGTR1 and β-actin.
Measurement of H2S serum
levels
The serum levels of H2S were measured as
described previously (13).
Briefly, 75 µl aliquots of sera were mixed with 100
µl distilled water, and 300 µl 10% trichloroacetic
acid. The reaction was terminated by the addition of 150 µl
1% zinc acetate. A total of 20 µM
N,N-dimethyl-p-phenylenediamine sulfate in 7.2 M HCl, and
FeCl3 (30 µM; 133 µl) in 1.2 M HCl was
then added to the solution. Following a 15 min incubation at room
temperature, the absorbance of the resulting solution was measured
using a UV-2550 spectrophotometer at 670 nm (Shimadzu Corporation,
Tokyo, Japan). All samples were assayed in triplicate, and the
levels of H2S were calculated against the NaHS
calibration curve (0.122–250 µM).
Statistical analysis
The results are expressed as the mean ± standard
deviation. The data were analyzed by a one-way analysis of variance
followed by Newman-Keuls comparisons using SPSS version 17.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of H2S on
CCl4-induced hepatic fibrosis
Treatment with NaHS significantly attenuated the
CCl4-induced serum expression levels of ALT and AST,
whereas PAG increased the serum expression levels of AST and ALT.
Conversely, the serum expression levels of ALB were decreased in
the PAG group, and increased in the NaHS group (Fig. 1). The observed
CCl4-induced impaired liver function and the protective
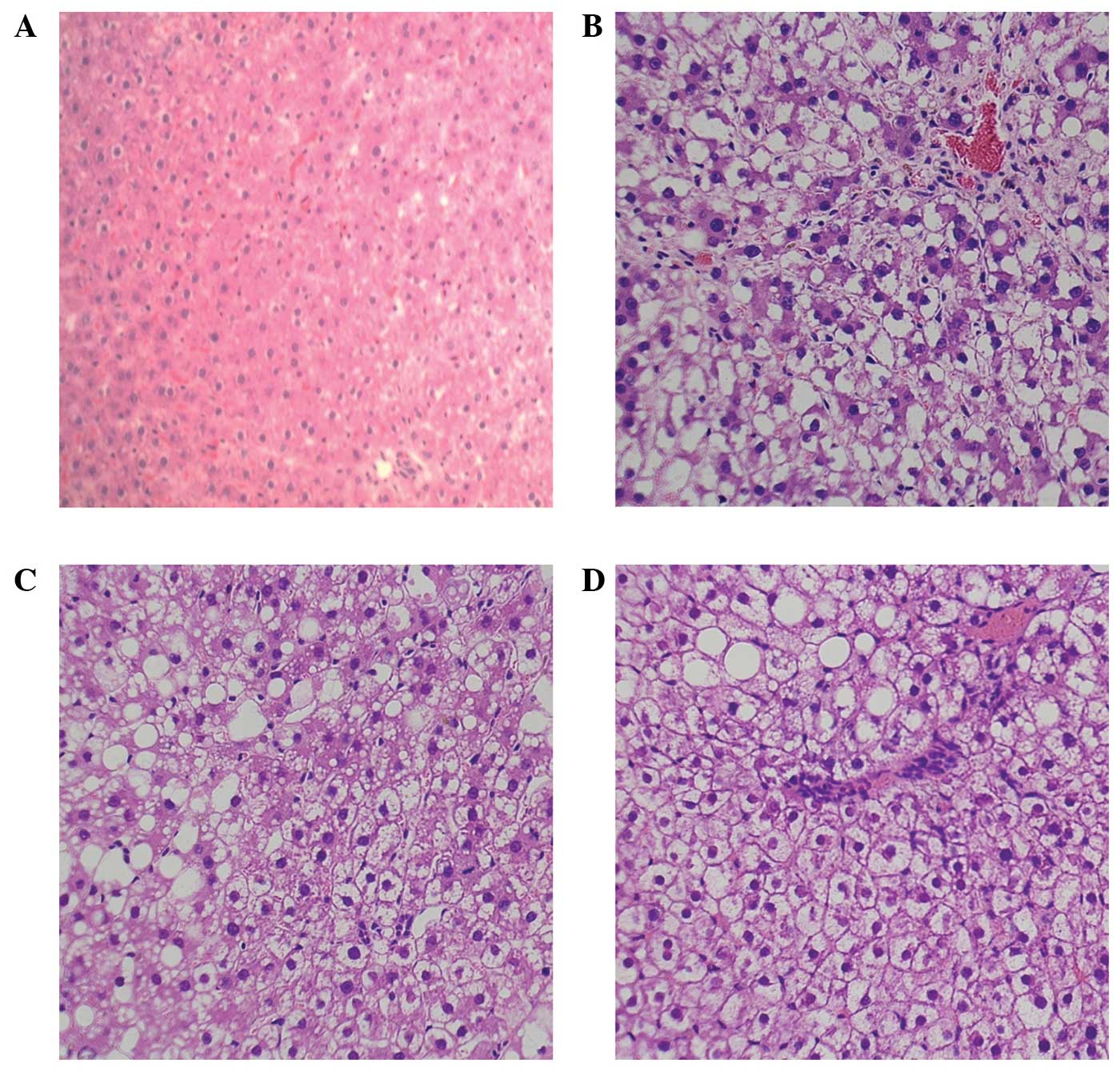
effects of H2S were supported by histological
alterations. The liver sections from the normal control group rats
exhibited normal histology (Table
I), whereas the liver sections from the CCl4-induced
model group rats exhibited focal necrosis and degeneration. NaHS
delayed both necrosis and vacuolization, whereas PAG aggravated
hepatic injury and caused increased inflammatory cell
infiltration.
 | Table INumber of rats present in each stage
of hepatic fibrosis for each group. |
Table I
Number of rats present in each stage
of hepatic fibrosis for each group.
| Groups | S0 | S1 | S2 | S3 | S4 |
|---|
| Normal control
group | 14 | | | | |
| Model group (3
w) | | | 3 | 4 | |
| NaHS group (3
w) | | 2 | 2 | 3 | |
| PAG group (3
w) | | | 2 | 5 | |
| Model group (4
w) | | | | 3 | 4 |
| NaHS group (4
w) | | 1 | 2 | 3 | 1 |
| PAG group (4
w) | | | | 2 | 5 |
Effects of NaHS and PAG on
CCl4-induced hepatic fibrosis
Evidence of liver injury in the model group, which
received an intraperitoneal injection of CCl4, was
indicated by significantly increased serum expression levels of HA,
LN, PcIII, and cIV (Fig. 2),
indicative of fibrosis. Pathological damage was evident when
compared with the control group. Treatment with PAG resulted in
increased serum expression levels of HA, LN, PcIII, and cIV, as
compared with the control group, whereas treatment with NaHS
resulted in significantly lower serum expression levels of HA, LN,
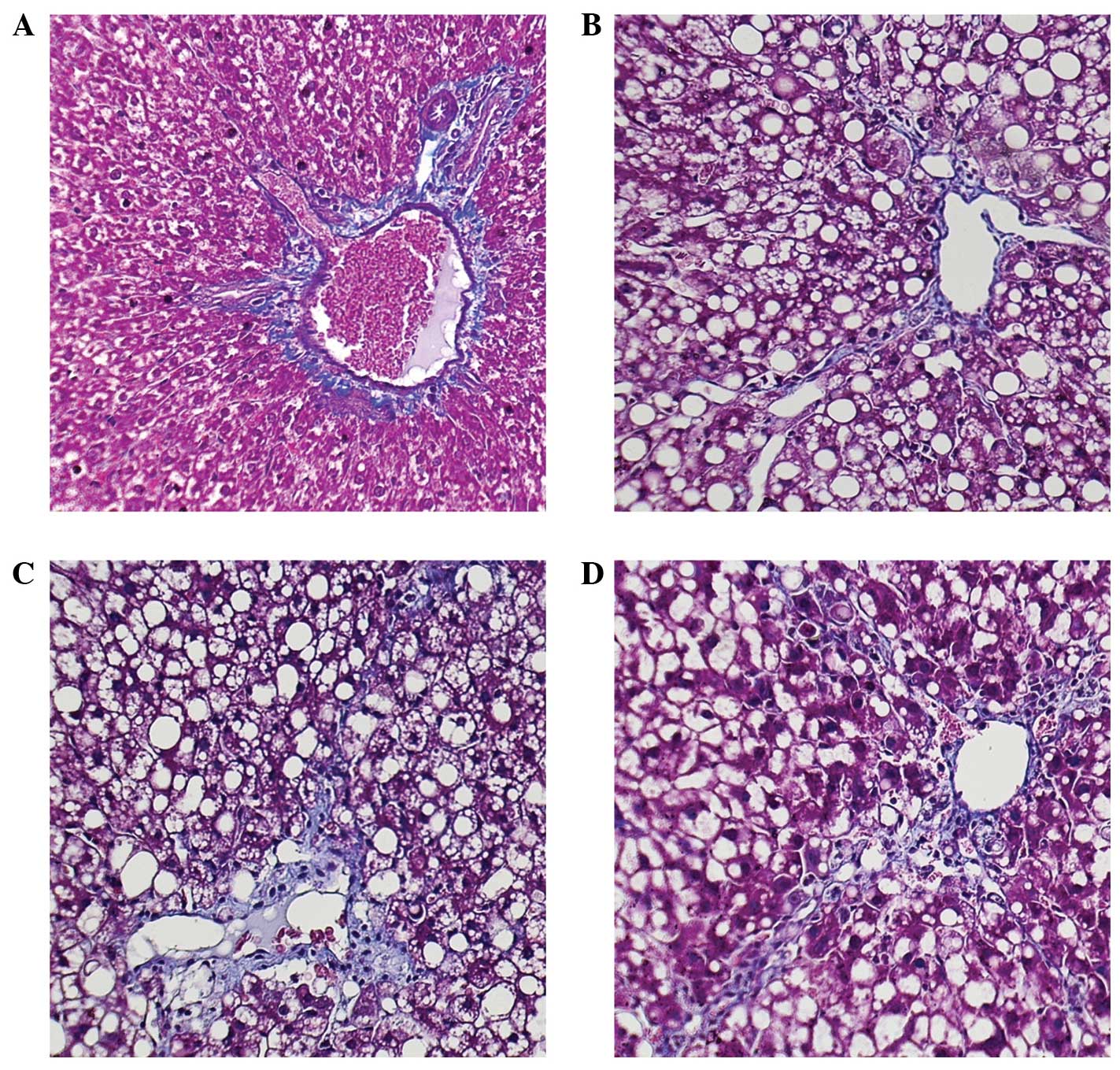
PcIII, and cIV compared with the model group (Fig. 2). The results of HE staining
demonstrated that the PAG group exhibited early-onset necrosis and
increased inflammatory cell infiltration, as compared with the
control group (Figs. 3 and
4). The subgroup treated for four
weeks with PAG exhibited severe liver necrosis. The number of blue
pixels in the Masson-stained liver sections was measured in order
to quantify the amount of collagen fibers. The results of the
Masson staining of the control group liver sections suggested a
near absence of collagen fibers, whereas treatment with
CCl4 significantly increased the number of blue pixels
in the liver sections, suggesting a large number of collagen fibers
were present. Treatment with NaHS resulted in a significantly lower
number of blue pixels, whereas treatment with PAG was associated
with a significantly higher number of blue pixels, as compared with
the control and model groups (Figs.
5 and 6).
Effects of NaHS and PAG on the mRNA
expression levels of CSE and on H2S synthesis in rat
liver
The gene encoding the H2S-forming enzyme
CSE was detected in the rat liver, by RT-qPCR of liver RNA. The
mRNA expression levels of CSE in the model group were markedly
lower, as compared with the normal control group, these results
were accentuated by PAG treatment. Conversely, in the NaHS group,
CSE content was significantly increased compared with the model
group (Fig. 7A). The serum
expression levels of H2S in the CCl4-treated
rats were markedly lower than in the normal control group,
suggesting that CCl4 significantly reduced hepatic
H2S-producing activity (Fig. 7B).
Effects of NaHS and PAG on the expression
levels of AGTR1 in CCl4-induced hepatic fibrosis
The hepatic protein expression levels of AGTR1 were
evaluated by immunoblot analysis. In the model group,
CCl4-induced hepatic fibrosis resulted in the
significant upregulation of AGTR1 expression, as compared with the
normal control group. Conversely, treatment with NaHS resulted in a
significant downregulation of AGTR1 expression, as compared with
the control group; however, this finding was not significant. PAG
treatment significantly increased the expression levels of AGTR1,
as compared with the control group (Fig. 8).
Discussion
CCl4 is widely used to induce a chemical
model of hepatic fibrosis. CCl4 is metabolized to a
trichloromethyl radical, leading to increased lipid peroxidation,
depletion of glutathione, impaired hepatic anti-oxidant activity,
and hepatocyte necrosis (21). Fan
et al (22) reported that
H2S administration attenuated hepatic fibrosis and
collagen I protein expression in rats exhibiting
CCl4-induced hepatic fibrosis, inhibited cellular
proliferation, and induced cell cycle arrest and apoptosis of
activated HSCs. Jha et al (23) demonstrated that H2S
significantly attenuated hepatic I/R injury via preservation of the
intracellular redox balance and inhibition of apoptosis during I/R
injury. These results suggested that H2S may serve as a
promising therapeutic agent in the treatment of hepatic I/R
injury.
HSCs have a crucial role in the onset of hepatic
fibrosis. HSCs express AGTR1 (15), and are activated by the binding of
angiotensin II to AGTR1, which in turn leads to the secretion of
extracellular matrix components resulting in the development of
hepatic fibrosis (24). Activated
HSCs also express numerous cytokines, which accelerate hepatic
inflammation (24).
Fibrogenesis in chronic liver disease is stimulated
by angiotensin II via AGTR1, and may be modulated by
angiotensin-converting enzyme inhibitors and AGTR1 antagonists
(25,26). In the present study, advanced liver
fibrosis was effectively induced by CCl4. The results of
the present study demonstrated that the protein expression levels
of AGTR1 were negatively correlated with the degree of liver
fibrosis. Töx et al (27)
showed that angiotensin II may influence transforming growth factor
(TGF)-β-mediated processes via AGTR1, by enhancing Smad2 gene
expression in the liver.
Tan et al (28) previously investigated the
protective role of H2S on CCl4-induced acute
hepatotoxicity, as well as the prophylactic and therapeutic effects
of H2S on long-term CCl4-induced cirrhosis
and portal hypertension, mediated by the multiple functions of
H2S, including antioxidation, anti-inflammation,
cytoprotection, and anti-fibrosis. The results of the study
indicated that the use of H2S may provide potent
therapeutic effects against liver cirrhosis and portal
hypertension.
The regulation of sinusoidal resistance depends on
the aggregation of HSCs around sinusoidal endothelial cells
(29). A previous study
demonstrated that H2S is an autocrine neurotransmitter
that is involved in the regulation of HSC contraction and the
maintenance of portal venous pressure via KATP channels
(29). H2S counteracts
impaired vasodilation and HSC contraction, thus reducing portal
hypertension in cirrhotic livers (29).
Angiotensin II has been shown to increase the
expression levels of hepatic TGF-β1 during the development of
hepatic fibrosis (30). Connective
tissue growth factor (CTGF) is a hepatic profibrotic mediator,
which is a downstream target of TGF-β1 in HSCs (31,32).
Tamaki et al (33)
demonstrated that telmisartan (an AGTR1 receptor blocker) inhibited
hepatic fibrosis, induced downregulation of tumour necrosis
factor-α, TGF-β1, and CTGF mRNA expression, and reduced the number
of α-smooth muscle actin-positive cells in the liver.
In conclusion, the results of the present study
demonstrated that H2S was able to inhibit liver
fibrosis, and hinder the formation of hepatic fibrosis.
Downregulation of AGTR1 was closely associated with the progression
of liver fibrosis, suggesting that H2S may inhibit the
expression of AGTR1. The results of the present study contribute to
the understanding of the protective effects of H2S in
liver fibrosis, but whether the mechanisms underlying
H2S protection, its correlation with angiotensin
receptor inhibitors, and its application in human hepatic fibrosis
has similar protective effects requires further study.
Acknowledgments
The present study was supported by grants from the
National Nature Science Foundation of China (grant nos. 81302093
and 81272752).
References
|
1
|
Łowicka E and Bełtowski J: Hydrogen
sulfide (H S) - the third gas of interest for pharmacologists.
Pharmacol Rep. 59:4–24. 2007.
|
|
2
|
Zhao W, Zhang J, Lu Y and Wang R: The
vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP)
channel opener. EMBO J. 20:6008–6016. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fiorucci S, Antonelli E, Distrutti E, et
al: Inhibition of hydrogen sulfide generation contributes to
gastric injury caused by anti-inflammatory nonsteroidal drugs.
Gastro-enterology. 129(1): 210–1224. 2005. View Article : Google Scholar
|
|
4
|
Zhang LM, Jiang CX and Liu DW: Hydrogen
sulfide attenuates neuronal injury induced by vascular dementia via
inhibiting apoptosis in rats. Neurochem Res. 34:1984–1992. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhong G, Chen F, Cheng Y, et al: The role
of hydrogen sulfide generation in the pathogenesis of hypertension
in rats induced by inhibition of nitric oxide synthase. J
Hypertens. 21:1879–1885. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kimura Y, Goto Y and Kimura H: Hydrogen
sulfide increases glutathione production and suppresses oxidative
stress in mitochondria. Antioxid Redox Signal. 12:1–13. 2010.
View Article : Google Scholar
|
|
7
|
Lou LX, Geng B, Du JB and Tang CS:
Hydrogen sulphide-induced hypothermia attenuates stress-related
ulceration in rats. Clin Exp Pharmacol Physiol. 35:223–228.
2008.
|
|
8
|
Zhong GZ, Li YB, Liu XL, et al: Hydrogen
sulfide opens the KATP channel on rat atrial and ventricular
myocytes. Cardiology. 115:120–126. 2010. View Article : Google Scholar
|
|
9
|
Kang K, Zhao M, Jiang H, et al: Role of
hydrogen sulfide in hepatic ischemia-reperfusion-induced injury in
rats. Liver Transpl. 15:1306–1314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fiorucci S, Antonelli E, Mencarelli A, et
al: The third gas: H S regulates perfusion pressure in both the
isolated and perfused 2 normal rat liver and in cirrhosis.
Hepatology. 42:539–548. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fang L, Li H, Tang C, et al: Hydrogen
sulfide attenuates the pathogenesis of pulmonary fibrosis induced
by bleomycin in rats. Can J Physiol Pharmacol. 87:531–538. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tacke F and Weiskirchen R: Update on
hepatic stellate cells: Pathogenic role in liver fibrosis and novel
isolation techniques. Expert Rev Gastroenterol Hepatol. 6:67–80.
2012. View Article : Google Scholar
|
|
13
|
Stock P, Liefeldt L, Paul M and Ganten M:
Local renin-angiotensin systems in cardiovascular tissues:
Localization and functional role. Cardiology. 86(Suppl 1): 2–8.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshiji H, Kuriyama S, Yoshii J, et al:
Angiotensin-II type 1 receptor interaction is a major regulator for
liver fibrosis development in rats. Hepatology. 34:745–750. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bataller R, Sancho-Bru P, Ginès P, et al:
Activated human hepatic stellate cells express the
renin-angiotensin system and synthesize angiotensin II.
Gastroenterology. 125:117–125. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao X, Zhang LK, Zhang CY, et al:
Regulatory effect of hydrogen sulfide on vascular collagen content
in spontaneously hypertensive rats. Hypertens Res. 31:1619–1630.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brunt EM: Nonalcoholic steatohepatitis:
Definition and pathology. Semin Liver Dis. 21:3–16. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen P, Sun B, Chen H, et al: Effects of
carbon monoxide releasing molecule-liberated CO on severe acute
pancreatitis in rats. Cytokine. 49:15–23. 2010. View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Nickenig G, Laufs U, Schnabel P, et al:
Down-regulation of aortic and cardiac AT1 receptor gene expression
in transgenic (mRen-2) 27 rats. Br J Pharmacol. 121:134–140. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan L and Kaplowitz N: Glutathione in
liver diseases and hepatotoxicity. Mol Aspects Med. 30:29–41. 2009.
View Article : Google Scholar
|
|
22
|
Fan HN, Wang HJ, Yang-Dan CR, et al:
Protective effects of hydrogen sulfide on oxidative stress and
fibrosis in hepatic stellate cells. Mol Med Rep. 7:247–53.
2013.
|
|
23
|
Jha S, Calvert JW and Duranski MR:
Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury:
Role of antioxidant and antiapoptotic signaling. Am J Physiol Heart
Circ Physiol. 295:H801–H806. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sakaida I, Matsumura Y, Kubota M, et al:
The prolyl 4-hydroxylase inhibitor HOE 077 prevents activation of
Ito cells, reducing procollagen gene expression in rat liver
fibrosis induced by choline-deficient L-amino acid-defined diet.
Hepatology. 23:755–63. 1996.PubMed/NCBI
|
|
25
|
Jonsson JR, Clouston AD, Ando Y, et al:
Angiotensin-converting enzyme inhibition attenuates the progression
of rat hepatic fibrosis. Gastroenterology. 121:148–155. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tuncer I, Ozbek H, Ugras S and Bayram I:
Anti-fibrogenic effects of captopril and candesartan cilexetil on
the hepatic fibrosis development in rat. The effect of AT1-R
blocker on the hepatic fibrosis. Exp Toxicol Pathol. 55:159–166.
2003.PubMed/NCBI
|
|
27
|
Töx U, Scheller I, Kociok N, et al:
Expression of angiotensin II receptor type 1 is reduced in advanced
rat liver fibrosis. Dig Dis Sci. 52:1995–2005. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tan G, Pan S, Li J, et al: Hydrogen
sulfide attenuates carbon tetrachloride-induced hepatotoxicity,
liver cirrhosis and portal hypertension in rats. Plos One.
6:e259432011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Distrutti E, Mencarelli A, Santucci L, et
al: The methionine connection: Homocysteine and hydrogen sulfide
exert opposite effects on hepatic microcirculation in rats.
Hepatology. 47:659–667. 2008. View Article : Google Scholar
|
|
30
|
Bataller R, Gabele E, Parsons CJ, et al:
Systemic infusion of angiotensin II exacerbates liver fibrosis in
bile duct-ligated rats. Hepatology. 41:1046–1055. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grotendorst GR: Connective tissue growth
factor: A mediator of TGF-beta action on fibroblasts. Cytokine
Growth Factor Rev. 8:171–179. 1997. View Article : Google Scholar
|
|
32
|
Paradis V, Dargere D, Bonvoust F, et al:
Effects and regulation of connective tissue growth factor on
hepatic stellate cells. Lab Invest. 82:767–774. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tamaki Y, Nakade Y, Yamauchi T, et al:
Angiotensin II type 1 receptor antagonist prevents hepatic
carcinoma in rats with nonalcoholic steatohepatitis. J
Gastroenterol. 48:491–503. 2013. View Article : Google Scholar
|