1. Introduction
Gab2 belongs to the Grb-associated binder (Gab)
family of docking proteins, which also includes mammalian Gab1,
Gab3 and Gab4, Drosophila daughter of sevenless, and
Caenorhabditis elegans suppressor of Clr-1 (1). Gab2 is reported to contain a
pleckstrin homology (PH) domain at the N-terminus, several
proline-rich motifs (PXXP) and multiple tyrosine residues, which
couple with SH2-containing molecules in a phosphorylation-dependent
manner (1,2). The Gab2 PH domain preferentially
combines phosphatidylinositol 3,4,5-P3 (PIP3) (3). In Gab2, two of the proline-rich
motifs are Grb2-Src homology (SH3) domain binding sites (4), which are vital for binding Gab2 to
upstream receptors through the Shc-Grb2 complex (5). Gab2 was initially identified as a
major binding site of SHP2 phosphatase, which is one of SH2
domain-containing tyrosine phosphatases, in interleukin
(IL)-3-stimulated hematopoietic cells (6). Subsequently, this adapter protein was
found to be widely involved in a variety of other signaling
processes, including the erythropoietin, thrombopoietin, stem cell
factor receptor (SCFR), Flt-3 ligand, and the T-cell and B-cell
antigen receptor (TCR and BCR, respectively) signaling pathways
(7–9).
Gab2 is not only important in signaling systems, but
it is important in other physiological activities. Overexprssion of
Gab2 enhances the activation of cytokine-dependent extracellular
signal-regulated kinase (ERK) mitogen-activated protein kinase
(MAPK) and gene expression (9,10).
Gab2−/− mice are viable and generally healthy; however, the
response of Gab2-knockout mast cells to stimulation via the high
affinity lgE receptor (FcεRI) is defective (11). It has been demonstrated that Gab2
adaptor function is intrinsically required for the response of
hematopoietic cells to early-acting cytokines, resulting in
defective hematopoiesis in Gab2-deficient mice (12). In addition to a role in abnormal
development, Gab2 is increasingly being described as associated
with mammary tumorigenesis and hematological malignancies. Gab2 is
essential for epidermal growth factor (EGF) signaling and breast
cancer cell proliferation (13,14).
Gab2 has also been considered as a key intracellular intermediate
for leukemic transformation, mediated by BCR-ABL (15), and Gab2 is pivotal in the expansion
of Friend virus-infected erythroid progenitor cells (16). In the present review, the role of
Gab2 protein in signal transduction and its emerging role in cancer
are discussed.
2. Structure, recruitment and function of
Gab2
The Gab2 gene is located on chromosome 11q13.4-q13.5
in humans, and the molecular weight of Gab2 protein is 97–100 kD.
Gab2 is expressed ubiquitously at high levels, particularly in the
brain, kidney, lung, heart, testis and ovary (2). Gab2 contains an N-terminal Pleckstrin
homology (PH) domain, a central praline-rich domain (PRD) and
multiple tyrosines within potential binding motifs, which are
favored by various SH-2 and 3 domain-containing proteins (Fig. 1) (2,17).
All three domains, particularly the PH domain, are highly conserved
in the process of organic evolution.
The N-terminal PH domain is the most conserved, and
its binding to PIP3 is involved in membrane recruitment of Gab2.
Previous reports have indicated that PH domain may also be involved
in regulating intracellular signaling (Fas-signaling pathway), not
just a localization module (1,18).
The PRD contains numerous PXXP motifs, mediating the interaction
with SH3 domain-containing proteins, including Grb2. As shown in
Fig. 1, there are multiple sites
of tyrosine phosphorylation, which may interact with SH2
domain-containing proteins, including SHP2 and p85. This
interaction is important for the function of Gab2 in mediating
intracellular signaling pathways, which are crucial for normal cell
growth, differentiation, development and apoptosis (19).
3. Gab2 in signal transduction
Gab proteins integrate and amplify signals from
cytokines, growth factors and antigen receptors, as well as from
cell adhesion molecules. They also diversify signals by channeling
the input information from activated receptors into signal pathways
with distinct biological functions (1). The interactions of Gab2 with other
signaling molecules are dependent on its phosphorylation status. In
unstimulated cells, Gab2 is located in the cytoplasm, while upon
activation by growth hormone (GH), EGF, IL-2/3/15,
granuloctye-stimulating factor, interferon (IFN), or T/B cell
receptors (20,21), Gab2 can be recruited to the cell
membrane by combining with PIP3 via the PH domain. Subsequently,
the PRD of Gab2 can interact with Grb2 to from a Gab2-Grb2-Shc
complex, which mediates rapid tyrosine phosphorylation. The
activated Gab2 contains several docking sites for certain key SH2
domain-containing molecules, including the tyrosine phosphatase,
SHP2, and the p85 of subunit of phosphatidylinositol 3-kinase
(PI3K), the recruitment and activation of which are induced. At
present, the SHP2/rat sarcoma viral oncogene (RAS) and PI3K/AKT
pathways are considered to be the two major effector arms of the
Gab2 protein.
In the Gab2-SHP2 mediated RAS/ERK pathway, the
tyrosine phosphatase, SHP2, is an important binding effector of
Gab2 downstream, which contains tandem SH2 domains, the most
N-terminal of which confers auto-inhibition of the C-terminal
phosphatase domain (22). Gab2
protein contains two SHP2 binding sites, which, if phosphorylated,
act as a bisphosphoryl tyrosine activation motif (BTAM) and confers
simultaneous binding of the two SH2 domains, thereby relieving
auto-inhibition and activating the RAS/ERK signaling pathway
(22,23). Thus, SHP2 interaction partners,
including Gab2 protein may act, not only as a recruitment platform,
but also as an allosteric activator. Gab2 tyrosine phosphorylation
site coupling with SH2 domains to activate SHP2 and Gab2 protein
regulates diverse biological endpoints, including cell adhesion and
the migration of Ba/F3 haematopoietic cells (6), epithelial morphogenesis in MDCK cells
(24) and acinar growth of MCF-10A
mammary epithelial cells (14). In
addition, in certain cellular contexts, the Gab2-SHP2 complex
positively regulates other downstream pathways, including
c-Kit-induced RAC activation (25)
and β1-integrin- and growth factor-induced PI3K activation
(6,14).
For the PI3K/AKT signaling pathway, Gab2 has three
important tyrosine residues, Y452, Y476 and Y584 sites, for the p85
regulatory subunit of PI3K, which induces the activation of PI3K
(18). Activated PI3K leads to the
production of phosphatidylinositol-phosphates (PIPs), which bind to
the PH domain of Gab2, enhancing the recruitment of Gab2 and
promoting the activation of PI3K (26). Thus, a positive feed-back loop is
formed to amplify the PI3K/AKT signaling pathway, and the mechanism
to produce specific physiological effects is important. It has been
demonstrated that Gab1/Gab2 regulates cell survival via the
SHP2/ERK and PI3K/AKT pathways in B-cells, and a low level of PI3K
activity inhibits Gab2-SHP2 interaction (18), suggesting that PI3K activity is
essential for the Gab2/SHP2/ERK signaling pathway.
In addition to the binding sites for SHP2 and p85,
Gab2 also contains numerous YXXP motifs, the potential binding
sites for Crk family proteins, which are responsible for c-Jun
N-terminal kinase (JNK) activation (27). Yu M et al demonstrated that
Gab2, via its association with SHP2, is required for SCF-evoked
activation of the RAC/JNK pathway and mast cell proliferation
(25). Biochemical analyses and
genetic investigations, as well as yeast-two-hybrid (Y2H) screens
have also identified additional Gab effector proteins (Fig. 2), including PLcγ (28), Crk families (29,30),
adaptor proteins of the Shc (10),
SHIP lipid phosphatase (31),
Ras-GTPase activating protein (RasGAP) (32), GC-GAP (31) and the transcriptional activators,
signal transducer and activator of transcription (STAT)3 and STAT5
(33,34). However, the detailed mechanism of
these effectors interact with Gab2 remain to be fully
elucidated.
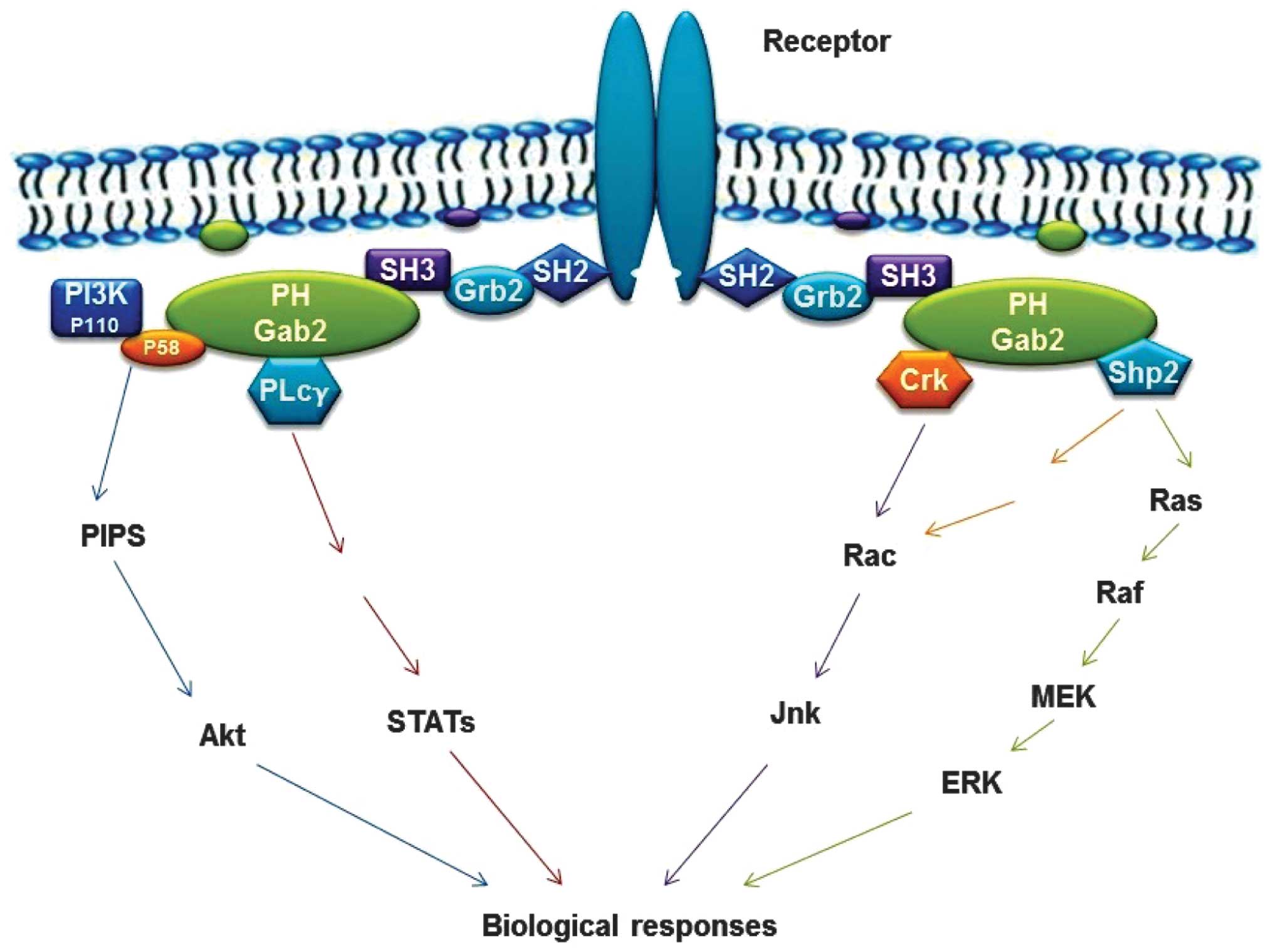 | Figure 2Schematic diagram of the roles of
Gab2 protein in signal transduction (1). Characteristics of the mechanism are
that the phosphotyrosine residues within the cytoplasmic tails of
the activated surface receptors act as binding sites for the
SH2-domain of Grb2, which then connects Gab2 via its C-terminal
SH3-domain. Activated receptors lead to tyrosine phosphorylation of
Gab2 protein and subsequent recruitment of SH2-domain-containing
effectors, including SHP2, P85, PLCγ, Crk and STATs. PH-domain
confers recruitment of Gab2 to plasma membrane patches enriched in
phosphatidyl-inositol-phosphates. Gab2, Grb-associated binder-2;
SH2 Src homology 2; SHP, SH2-containing protein tyrosine
phosphatase; PLC γ, phospholipase C-γ; RAS, rat sarcoma viral
oncogene; Erk, extracellular signal-regulated kinase; MEK,
mitogen-activated protein kinase kinase; Jnk, Janus kinas; STAT,
signal transducer and activator of transcription; PI3K,
phosphatidylinositol 3-kinase. |
4. Gab2 in cancer
Breast cancer
It has been reported that the expression of Gab2 is
reduced in invasive cancer and lymph node metastases, compared with
ductal carcinoma in situ (DCIS), although it remains higher
than in normal breast tissue (35). Overexpression of Gab2 in MCF-10A
cells, an immortalized, non-transformed human mammary epithelial
cell line, contributes to increased proliferation and alterations
in dependency on EGF and other growth factors (14). By contrast, ablation of Gab2 in
several breast cancer cell lines, inhibiting genomic
amplifications, leads to a decrease in proliferation, due to a
reduction in cell-cycle progression and increased apoptosis, and a
reduction in their invasive potential (36). Although the mechanisms by which
Gab2 contribute to breast cancer remain to be fully elucidated, the
recruitment of SHP2 and subsequent activation of the RAS/MAPK
pathway are reported to be required (13). Overexpression of Gab2 in MCF-10A
cells promotes enhanced cell migration by modulating the activation
of Rashomolog gene family, member A, which is dependent on the
SHP2-binding sites (37). Previous
investigation has suggested that the RAS/MAPK pathway modulates
SHP2 recruitment in a p90 ribosomal S6 kinase (RSK)-dependent
manner, and RSK-mediated Gab2 phosphorylation inhibits mammary
epithelial cell migration (38).
Gab2 is required for efficient ErbB2-driven mammary
tumorigenesis and metastatic spread (13,39).
Gab2 acts downstream of Neu, also termed ErbB2 and HER2, and is
tyrosyl-phosphorylated upon activation of signal transduction
(13). Gab2 and ErbB2 are
co-amplified in a subset of breast carcinoma, and co-expression of
Gab2 with ErbB2 results in an invasive phenotype, and increases
proliferation of MCF-10A mammary cells in a three-dimensional
culture. This effect is mediated through downstream SHP2/ERK
signaling and is independent of PI3K/AKT activation (13). Agents that interact with the Gab2
or Gab2-mediated pathways may be useful for treating breast tumors
overexpressing Gab2 and/or HER2. Several studies using the MCF-10A
model system and transgenic mouse models have indicated that, in
addition to the above-mentioned HER2, Gab2 also cooperates with
other oncogenes linked to the development of breast cancer,
including the SRC family.
The small interfering (si)RNA-mediated silencing of
Gab2 in breast cancer lines exhibiting Gab2 amplification has
suggested a dependency on Gab2 for cell proliferation, cell-cycle
progression, survival and invasion, which is likely mediated
through altered PI3K and MAPK signaling (36). Qian P et al also observed
that the p44/42 MAPK-matrix metalloproteinase (MMP)-2/MMP-9 pathway
can be used to enhance mammary carcinoma cell migration and
invasion consequent to let-7 g depletion by increasing the
expression of Gab2 and fibronectin1 (40). In addition, the inhibition or
knockdown of the expression of JNK2 in mammary cancer cells reduces
tumor cell invasion, and JNK2 conveys these effects in response to
a variety of receptor tyrosine kinases, expressed by breast cancer
cells by regulating the expression of Gab2 and its downstream
signaling (41). These findings
highlight a novel role for the Gab2 protein and its role in
signaling regulation as a primary genetic diver of breast
tumorigenesis.
Melanoma
As mentioned above, Gab2 is a scaffolding protein
that mediates interactions with various signaling pathways,
including RAS/ERK and PI3K/AKT signaling. The development of
melanoma is inextricably associated with oncogenic activation of
these signaling pathways (42,43).
Metastatic melanomas express significantly higher levels of Gab2,
compared with primary melanomas and melanocytic nevi, identifying
Gab2 as a molecular marker for neoplastic progression (44). Furthermore, Gab2 promotes tumor
cell migration and invasion by activating PI3K/AKT signaling, and
enhances tumor growth and metastasis in vivo, suggesting a
role for Gab2-mediated signaling in promoting metastatic capability
in melanoma (45).
Neuroblastoma v-ras oncogene homolog (NRAS) and
v-raf murine sarcoma viral oncogene homolog B1 (BRAF) are oncogenes
in melanoma, which are critical for tumor initiation (46). Oncogenic mutations in NRAS can
activate the MAPK and PI3K/AKT pathways, whereas mutant BRAF
activates the MAPK pathway (47).
Gab2 amplification is associated with melanoma arising from
sun-protected sites and often occurs independently from oncogenic
NRAS or BRAF mutations or amplifications of the KIT gene (44). However, Gab2 is co-expressed with
NRAS in melanoma cell lines and tumor samples, and its expression
correlates with metastatic potential. Overexpression of Gab2 leads
to increased metastatic potential with anchorage independence in
soft agar, and a previous report revealed that the cooperative
activity of Gab2 in NRAS-driven melanoma increases anchorage
independent growth by improving survival of Gab2-expressing cells,
enhancing tumorigenesis in vivo and facilitating an
angiogenic switch through the upregulation of HIF-1a and VEGF by
MAPK signaling, but not PI3K signaling, in Gab2/NRAS-driven
tumorigenesis (47).
Ovarian cancer
Compared with its role in breast cancer and
melanoma, the function of Gab2 in ovarian carcinoma is less well
understood. Genomic amplifications of Gab2 have been described in
~16% of ovarian carcinoma cases (48). The expression of Gab2 predominantly
regulates the migratory behaviors of ovarian cancer cells, and
overexpression of Gab2 enhances migration and invasion, and
downregulates the expression of E-cadherin in ovarian cancer cells
with low baseline expression levels of Gab2. Conversely, silencing
of Gab2 inhibits the migration and invasion, and positively
regulates E-cadherin expression in ovarian cancer cells with
high-Gab2 expression (49). The
neuregulin/ErbB3 signaling module is important for activation of
the PI3K pathway, and promotes cell growth in a subset of ovarian
cancer (50). In addition, a
previous study has reported that the overexpression of Gab2
activates the epithelial-mesenchymal transition program through
activation of the PI3K/Zeb1 pathway, and inhibits the expression of
E-cadherin in a subset of ovarian cancer (49). Notably, the OVCAR5 cell lines used
in this study exhibit no ErbB3 signaling, and the TOV21G and
lgrov-1 cells express low levels of ErbB3 protein, suggesting that
the expression of erbB3 and Gab2 contribute to activation of the
PI3K pathway in different subsets of ovarian cancer (50). Therefore, the Gab2 acted as ErbB3,
which is important in ovarian cancer.
Dunn GP et al also identified that Gab2 as an
ovarian cancer oncogene, which potently transforms immortalized
ovarian and fallopian tube secretory epithelial cells through the
activation of PI3K signaling (51). The novel Gab2/PI3K/Zeb1 pathway can
be targeted by PI3K and mammalian target of rapamycin (mTOR)
inhibitors and can be potentially used to treat Gab2-driven ovarian
cancer in combination with standard chemotherapy. In addition, a
previous clinical study indicated that novel candidate genes,
including UR11, Gab2 and PAK4, may be specifically targeted for the
treatment of high-grade serous and endometrioid types of ovarian
tumor (52).
Leukemia
The first evidence for the critical contribution of
Gab2 to leukemogenesis was an investigation, which demonstrated
that myeloid progenitors from Gab2-deficient mice are resistant to
transformation by the BCR-ABL oncoprotein, which arises from a
chromosomal translocation found in >90% of patients with chronic
myeloid leukaemia (CML). The oncogenic protein tyrosine kinase,
BCR-ABL, the product of the Philadelphia chromosome, interacts with
Grb2 and Gab2 signaling, and triggers hematopoietic cell
proliferation (53). In
BCR/ABL-positive CML bone marrow, Gab2-positive myeloid cells are
significantly more frequent, compared with normal bone marrow
(53). These findings indicate
that Gab2 is part of a protein complex that is important, if not
essential, in BCR/ABL-driven CML. In addition, BCR-ABL1 is not only
present in CML patients, but also occurs in 20–30% of patients with
acute lymphoblastic leukemia (ALL) (54,55).
Gab2 is an important signal transducer of BCR-ABL1, which combines
growth factor and cytokine receptors with downstream effectors,
including the PI3K/AKT/mTOR, SHP2/RAS/ERK and JAK/STAT pathways
(54). Gab2 does not possess any
intrinsic catalytic activity; however, by coupling to effector
molecules with distinct enzymatic properties, it leads to the
amplification, integration and diversification of the
BCR/ABL-derived signaling (1,15).
Following recruitment, Gab2 becomes tyrosine phosphorylated, binds
to effector proteins, includng PI3K and SHP2 and activates the AKT
and ERK signaling pathways (1,56,57).
In a similar manner, Gab2 is also involved in multiple nonreceptor
tyrosine kinase-linked signaling networks, mediated by
erythropoietin or granulocyte colony-stimulating factors receptors
(9,58). The pivotal role of Gab2 in BCR-ABL
signaling is further demonstrated by observations that short
hairpin (sh) RNA-mediated silencing of endogenous Gab2 inhibits the
proliferation and colony formation of CD34+ cells from
patients with CML, but not in cells isolated from healthy donors
(59). These findings suggest that
human CML may depend on BCR/ABL-driven Gab2 signaling and identify
Gab2 as a potential therapeutic target. In addition, a study by
Zatkova A et al indicated that, in addition to the mixed
lineage leukemia gene, Gab2 is a novel candidate target gene of
chromosome arm 11q amplification in acute myeloid leukemia
(AML)/myelodysplastic syndrome (60).
Despite the significant clinical success of BCR-ABL
tyrosine kinase inhibitors (TKIs) in the treatment of CML,
mechanisms of TKI-resistance have evolved resulting in CML
remaining one of the most difficult types of cancer to treat
(61). SHP2 and Gab2 have been
demonstrated to be required for BCR/ABL-induced myeloid
transformation and leukemia cell proliferation, suggesting that the
Gab2-SHP2 axis is an important signaling event in leukemia
(15,59). Enhanced sensitivity to the
inhibition of Gab2, SHP2 and STAT5 has been observed in
BCR/ABL-transformed cell lines. Phosphorylated Gab2 Y452, a PI3K
recruitment site, confers Gab2-mediated TKI resistance, while Gab2
knockdown or haploinsufficiency increases TKI sensitivity (55). Ding J et al also indicated
that SUP-B15, a Ph+ cell line, expresses unusually high levels of
Gab2, potentially causing TKI resistance (54). Constitutive phosphorylation of SHP2
is associated with the binding of SHP2 with the p85 PI3K regulatory
subunit and Gab2, which is sufficient for KITD814V-induced
myeloproliferative disease (MPD). By contrast, the SHP2 inhibitor
enhances the efficacy of the PI3K inhibitor in suppressing
KITD814V-induced ligand-independent growth in vitro and MPD
in vivo (62). Furthermore,
targeting of the N-SH2 domain of SHP2 with monobodies markedly
reduces its interaction with Gab2 and has significant effects on
downstream signaling in BCR/ABL-drived CML (63). These findings suggest that the
Gab2-SHP2 axis may be exploited as a novel modulator of TKI
sensitivity in CML and as a potential therapeutic target in
TKI-resistant disease. Notably, a previous study demsontrated that,
at equimolar concentrations, dasatinib is more effective in
preventing Gab2 tyrosine and serine/threonine phosphorylation,
compared with imatinib, suggesting that dasatinib may be an
alterative in the clinical therapy of CML (64).
BCR-ABL stability and oncogenic signaling in CML
cells are under the control of Janus kinase-2 (JAK2) (65). The inhibition of JAK2 reduces the
levels of tyrosine phosphorylation of Shc and Gab2, and reduces
activation of the RAS, PI3K and STAT5 pathways, thereby inducing
apoptosis in CD34+ cell from patients with CML in blast
crisis (65). STAT5 is a critical
transcription factor for normal hematopoiesis, and its sustained
activation is connected with hematological malignancy. A
persistently active mutant of STAT5 (STAT5as711F)
associates with Gab2 in myeloid leukemias and promotes growth in
vitro via the activation of AKT activation (66). Nagao T et al found that
apoptosis may be suppressed in PVTL-1 cells, an AML cell line,
through inactivation of GSK3 by Lyn, and of JAK2-V617F and is also
suppressed by the activation of STAT5 by JAK2-V617F (67). Tyrosine-phosphorylated-STAT5 can be
tracked using flow cytometry or immunostaining and is a biomarker
associated with poor prognosis in patients with juvenile
myelomonocytic leukemia (JMML) (66) and AML (68).
JMML, an MPD of young children characterized by
cytokine hypersensitivity of myeloid progenitors, is associated
with a mutation in the RAS pathway (69,70).
PTPN11 is the most common target of genetic mutations in JMML
(71,72), and 35% of patients with JMML have
activating mutations in tyrosine phosphatase PTPN11 (SHP2), a known
positive regulation of the RAS pathway. These mutations, including
the congenital mutation D61 G and somatic mutation E76K, disrupt
the inhibitory intramolecular interaction between the N-terminal
SH2 (N-SH2) and catalytic domains, leading to hyperactivation of
SHP2 (71,73). Furthermore, interactions of mutant
SHP2 with tyrosine-phosphorylated signaling partners, including
Gab1 and Gab2, are enhanced by mutations in the N-SH2 domain
(74,75). Gab2 as an important regulatory
protein and is vital in the mutant SHP2-mediated RAS pathway. In
addition, SHP2 mutations and the SHP2 binding protein, Gab2, are
associated with hyperactivation of the ERK, AKT and STAT5 pathways
in JMML, suggesting novel approaches to JMML therapy (76).
Gab2 in other types of malignancy
Gab2 is overexpressed in malignant lung tissues,
compared with normal lung tissues, suggesting Gab2 has a novel role
in the development of lung cancer (77). The association between c-Met, and
PI3K and Gab2 in small cell lung cancer enhances cell motility and
invasion as an important consequence of c-Met signaling (78). In addition, a previous study
reported that Gab2 positively regulates mucin synthesis and goblet
cell hyperplasia through an IL-13-mediated TYK2/STAT6 pathway in
lung cancer and chronic obstructive pulmonary disease (79). In addition, Gab2-mediated signaling
may result in the activation of AKT and promote invasion in glioma
cell via the AKT/mTOR pathway (80). Lee SH et al demonstrated
that Gab2 is over-expressed in malignant gastric cells, compared
with normal epithelial cells, suggesting that the expression of
Gab2 may be involved in the development of gastric cancer (81). The aforementioned evidence suggests
that the overexpression of Gab2 and its signaling are important in
human malignancies, however, additional functional investigations
are required to identify more key proteins, which combine with
Gab2, and are involved in the proliferation, differentiation and
migration of tumor cells. This is vital for understanding
carcinogenesis and devising novel therapeutic approaches.
5. Conclusion and perspective
As summarized in the present review, it has been
demonstrated over several years that numerous kinases and
phosphorylation events regulate Gab2 signal transduction, the
signaling of which is summarized in Fig. 2. The pathophysiology of the
expression of Gab2 and/or Gab2 signal transduction is complex and
dependent upon oncogenic processes and host cell biological
responses. Although a body of evidence has demonstrated that
aberrant Gab2 and/or Gab2 signaling is closely associated with
malignant biological properties of tumors, particularly in breast
and ovarian cancer, melanoma and leukemia, the detailed mechanism
remains to be fully elucidated. Therefore, further investigations
are warranted to improve understanding.
Notably, the EMT and cancer stem cells (CSCs) are
also critical in cancer pathogenesis (82). Activation of the EMT triggers tumor
cell invasion and metastasis to distant organs via the
downregulation of intercellular adhesion molecules, including
E-cadherin and occludin, and the upregulation of mesenchymal
markers, including vimentin and N-cadherin (83). The EMT is also involved in the
acquisition of CSC properties, and EMT-inducing CSCs have been
considered an important origin of CSCs (82). In addition to their capacities in
tumor initiation, CSCs have also been implicated in tumor invasion
and metastasis (83). Improving
understanding of the mechanism underlying the activation of
different signaling pathways by Gab2 in promoting tumor cell
metastasis, migration and recurrence, combined with current
understanding of EMT and CSCs, may provide novel insight for
designing effective therapies to treat different types of
cancer.
Acknowledgments
This study was supported by grants from the Science
and Technology Foundation of Guizhou Province (grant no.
2013J2312).
References
|
1
|
Wöhrle FU, Daly RJ and Brummer T:
Function, regulation and pathological roles of the Gab/DOS docking
proteins. Cell Commun Signal. 7:222009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gu H and Neel BG: The 'Gab' in signal
transduction. Trends Cell Biol. 13:122–130. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu M, Lowell CA, Neel BG and Gu H:
Scaffolding adapter Grb2-associated binder 2 requires Syk to
transmit signals from FcepsilonRI. J Immunol. 176:2421–2429. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lock LS, Royal I, Naujokas MA and Park M:
Identification of an atypical Grb2 carboxyl-terminal SH3 domain
binding site in Gab docking proteins reveals Grb2-dependent and
-independent recruitment of Gab1 to receptor tyrosine kinases. J
Biol Chem. 275:31536–31545. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gu H, Maeda H, Moon JJ, Lord JD, Yoakim M,
Nelson BH and Neel BG: New role for Shc in activation of the
phosphatidylinositol 3-kinase/Akt pathway. Mol Cell Biol.
20:7109–7120. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu WM, Hawley TS, Hawley RG and Qu CK:
Role of the docking protein Gab2 in beta (1)-integrin signaling
pathway-mediated hematopoietic cell adhesion and migration. Blood.
99:2351–2359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hibi M and Hirano T: Gab-family adapter
molecules in signal transduction of cytokine and growth factor
receptors, and T and B cell antigen receptors. Leuk Lymphoma.
37:299–307. 2000.PubMed/NCBI
|
|
8
|
Wickrema A, Uddin S, Sharma A, Chen F,
Alsayed Y, Ahmad S, Sawyer ST, Krystal G, Yi T, Nishada K, et al:
Engagement of Gab1 and Gab2 in erythropoietin signaling. J Biol
Chem. 274:24469–24474. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishida K, Yoshida Y, Itoh M, Fukada T,
Ohtani T, Shirogane T, Atsumi T, Takahashi-Tezuka M, Ishihara K,
Hibi M, et al: Gab-family adapter proteins act downstream of
cytokine and growth factor receptors and T- and B-cell antigen
receptors. Blood. 93:1809–1816. 1999.PubMed/NCBI
|
|
10
|
Nishida K, Wang L, Morii E, Park SJ,
Narimatsu M, Itoh S, Yamasaki S, Fujishima M, Ishihara K, Hibi M,
et al: Requirement of Gab2 for mast cell development and KitL/c-Kit
signaling. Blood. 99:1866–1869. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gu H, Saito K, Klaman LD, Shen J, Fleming
T, Wang Y, Pratt JC, Lin G, Lim B, Kinet JP, et al: Essential role
for Gab2 in the allergic response. Nature. 412:186–190. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Diaz-Flores E, Li G, Wang Z, Kang
Z, Haviernikova E, Rowe S, Qu CK, Tse W, Shannon KM, et al:
Abnormal hemato-poiesis in Gab2 mutant mice. Blood. 110:116–124.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bentires-Alj M, Gil SG, Chan R, Wang ZC,
Wang Y, Imanaka N, Harris LN, Richardson A, Neel BG and Gu H: A
role for the scaffolding adapter GAB2 in breast cancer. Nat Med.
12:114–121. 2006. View
Article : Google Scholar
|
|
14
|
Brummer T, Schramek D, Hayes VM, Bennett
HL, Caldon CE, Musgrove EA and Daly RJ: Increased proliferation and
altered growth factor dependence of human mammary epithelial cells
overexpressing the Gab2 docking protein. J Biol Chem. 281:626–637.
2006. View Article : Google Scholar
|
|
15
|
Sattler M, Mohi MG, Pride YB, Quinnan LR,
Malouf NA, Podar K, Gesbert F, Iwasaki H, Li S, Van Etten RA, et
al: Critical role for Gab2 in transformation by BCR/ABL. Cancer
Cell. 1:479–492. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Teal HE, Ni S, Xu J, Finkelstein LD, Cheng
AM, Paulson RF, Feng GS and Correll PH: GRB2-mediated recruitment
of GAB2, but not GAB1, to SF-STK supports the expansion of Friend
virus-infected erythroid progenitor cells. Oncogene. 25:2433–2443.
2006. View Article : Google Scholar
|
|
17
|
Pan XL, Ren RJ, Wang G, Tang HD and Chen
SD: The Gab2 in signal transduction and its potential role in the
pathogenesis of Alzheimer's disease. Neurosci Bull. 26:241–246.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maus M, Medgyesi D, Kövesdi D, Csuka D,
Koncz G and Sármay G: Grb2 associated binder 2 couples B-cell
receptor to cell survival. Cell Signal. 21:220–227. 2009.
View Article : Google Scholar
|
|
19
|
Sármay G, Angyal A, Kertész A, Maus M and
Medgyesi D: The multiple function of Grb2 associated binder (Gab)
adaptor/scaffolding protein in immune cell signaling. Immunol Lett.
104:76–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pyarajan S, Matejovic G, Pratt JC, Baksh S
and Burakoff SJ: Interleukin-3 (IL-3)-induced c-fos activation is
modulated by Gab2-calcineurin interaction. J Biol Chem.
283:23505–23509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tripathi A and Sodhi A: Growth
hormone-induced production of cytokines in murine peritoneal
macrophages in vitro: Role of JAK/STAT, PI3K, PKC and MAP kinases.
Immunobiology. 214:430–440. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Neel BG, Gu H and Pao L: The 'Shp'ing
news: SH2 domain-containing tyrosine phosphatases in cell
signaling. Trends Biochem Sci. 28:284–293. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gu H, Pratt JC, Burakoff SJ and Neel BG:
Cloning of p97/Gab2, the major SHP2-binding protein in
hematopoietic cells, reveals a novel pathway for cytokine-induced
gene activation. Mol Cell. 2:729–740. 1998. View Article : Google Scholar
|
|
24
|
Maroun CR, Naujokas MA, Holgado-Madruga M,
Wong AJ and Park M: The tyrosine phosphatase SHP-2 is required for
sustained activation of extracellular signal-regulated kinase and
epithelial morphogenesis downstream from the met receptor tyrosine
kinase. Mol Cell Biol. 20:8513–8525. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu M, Luo J, Yang W, Wang Y, Mizuki M,
Kanakura Y, Besmer P, Neel BG and Gu H: The scaffolding adapter
Gab2, via Shp-2, regulates kit-evoked mast cell proliferation by
activating the Rac/JNK pathway. J Biol Chem. 281:28615–28626. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang TT, Li H, Cheung SM, Costantini JL,
Hou S, Al-Alwan M and Marshall AJ: Phosphoinositide
3-kinase-regulated adapters in lymphocyte activation. Immunol Rev.
232:255–272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Montagner A, Yart A, Dance M, Perret B,
Salles JP and Raynal P: A novel role for Gab1 and SHP2 in epidermal
growth factor-induced Ras activation. J Biol Chem. 280:5350–5360.
2005. View Article : Google Scholar
|
|
28
|
Holgado-Madruga M, Emlet DR, Moscatello
DK, Godwin AK and Wong AJ: A Grb2-associated docking protein in
EGF- and insulin-receptor signalling. Nature. 379:560–564. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gual P, Shigematsu S, Kanzaki M, Grémeaux
T, Gonzalez T, Pessin JE, Le Marchand-Brustel Y and Tanti JF: A
Crk-II/TC10 signaling pathway is required for osmotic
shock-stimulated glucose transport. J Biol Chem. 277:43980–43986.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Crouin C, Arnaud M, Gesbert F, Camonis J
and Bertoglio J: A yeast two-hybrid study of human p97/Gab2
interactions with its SH2 domain-containing binding partners. FEBS
lett. 495:148–153. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao C, Ma H, Bossy-Wetzel E, Lipton SA,
Zhang Z and Feng GS: GC-GAP, a Rho family GTPase-activating protein
that interacts with signaling adapters Gab1 and Gab2. J Biol Chem.
278:34641–34653. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simister PC and Feller SM: Order and
disorder in large multi-site docking proteins of the Gab
family-implications for signalling complex formation and inhibitor
design strategies. Mol Biosyst. 8:33–46. 2012. View Article : Google Scholar
|
|
33
|
Nyga R, Pecquet C, Harir N, Gu H,
Dhennin-Duthille I, Régnier A, Gouilleux-Gruart V, Lassoued K and
Gouilleux F: Activated STAT5 proteins induce activation of the PI
3-kinase/Akt and Ras/MAPK pathways via the Gab2 scaffolding
adapter. Biochem J. 390:359–366. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ni S, Zhao C, Feng GS, Paulson RF and
Correll PH: A novel Stat3 binding motif in Gab2 mediates
transformation of primary hematopoietic cells by the Stk/Ron
receptor tyrosine kinase in response to Friend virus infection. Mol
Cell Biol. 27:3708–3715. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fleuren ED, O'Toole S, Millar EK, McNeil
C, Lopez-Knowles E, Boulghourjian A, Croucher DR, Schramek D,
Brummer T, Penninger JM, et al: Overexpression of the oncogenic
signal transducer Gab2 occurs early in breast cancer development.
Int J Cancer. 127:1486–1492. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bocanegra M, Bergamaschi A, Kim YH, Miller
MA, Rajput AB, Kao J, Langerød A, Han W, Noh DY, Jeffrey SS, et al:
Focal amplification and oncogene dependency of GAB2 in breast
cancer. Oncogene. 29:774–779. 2010. View Article : Google Scholar
|
|
37
|
Herrera Abreu MT, Hughes WE, Mele K, Lyons
RJ, Rickwood D, Browne BC, Bennett HL, Vallotton P, Brummer T and
Daly RJ: Gab2 regulates cytoskeletal organization and migration of
mammary epithelial cells by modulating RhoA activation. Mol Biol
Cell. 22:105–116. 2011. View Article : Google Scholar
|
|
38
|
Zhang X, Lavoie G, Fort L, Huttlin EL,
Tcherkezian J, Galan JA, Gu H, Gygi SP, Carreno S and Roux PP: Gab2
phosphorylation by RSK inhibits Shp2 recruitment and cell motility.
Mol Cell Biol. 33:1657–1670. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ke Y, Wu D, Princen F, Nguyen T, Pang Y,
Lesperance J, Muller WJ, Oshima RG and Feng GS: Role of Gab2 in
mammary tumorigenesis and metastasis. Oncogene. 26:4951–4960. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qian P, Zuo Z, Wu Z, Meng X, Li G, Wu Z,
Zhang W, Tan S, Pandey V, Yao Y, et al: Pivotal role of reduced
let-7g expression in breast cancer invasion and metastasis. Cancer
Res. 71:6463–6474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nasrazadani A and Van Den Berg CL: c-Jun
N-terminal Kinase 2 regulates multiple receptor tyrosine kinase
pathways in mouse mammary tumor growth and metastasis. Genes
Cancer. 2:31–45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yajima I, Kumasaka MY, Thang ND, Goto Y,
Takeda K, Yamanoshita O, Iida M, Ohgami N, Tamura H, Kawamoto Y, et
al: RAS/RAF/MEK/ERK and PI3K/PTEN/AKT signaling in malignant
melanoma progression and therapy. Dermatol Res Pract.
2012:3541912012.
|
|
43
|
McCubrey JA, Steelman LS, Abrams SL, Lee
JT, Chang F, Bertrand FE, Navolanic PM, Terrian DM, Franklin RA,
D'Assoro AB, et al: Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT
pathways in malignant transformation and drug resistance. Adv
Enzyme Regul. 46:249–279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chernoff KA, Bordone L, Horst B, Simon K,
Twadell W, Lee K, Cohen JA, Wang S, Silvers DN, Brunner G, et al:
GAB2 amplifi-cations refine molecular classification of melanoma.
Clin Cancer Res. 15:4288–4291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Horst B, Gruvberger-Saal SK, Hopkins BD,
Bordone L, Yang Y, Chernoff KA, Uzoma I, Schwipper V, Liebau J,
Nowak NJ, et al: Gab2-mediated signaling promotes melanoma
metastasis. Am J Pathol. 174:1524–1533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Davies H, Bignell GR, Cox C, Stephens P,
Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W,
et al: Mutations of the BRAF gene in human cancer. Nature.
417:949–954. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang Y, Wu J, Demir A, Castillo-Martin M,
Melamed RD, Zhang G, Fukunaga-Kanabis M, Perez-Lorenzo R, Zheng B,
Silvers DN, et al: GAB2 induces tumor angiogenesis in NRAS-driven
melanoma. Oncogene. 32:3627–3637. 2013. View Article : Google Scholar
|
|
48
|
Brown LA, Kalloger SE, Miller MA, Shih
IeM, McKinney SE, Santos JL, Swenerton K, Spellman PT, Gray J,
Gilks CB, et al: Amplification of 11q13 in ovarian carcinoma. Genes
Chromosomes Cancer. 47:481–489. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang Y, Sheng Q, Spillman MA, Behbakht K
and Gu H: Gab2 regulates the migratory behaviors and E-cadherin
expression via activation of the PI3K pathway in ovarian cancer
cells. Oncogene. 31:2512–2520. 2012. View Article : Google Scholar :
|
|
50
|
Sheng Q, Liu X, Fleming E, Yuan K, Piao H,
Chen J, Moustafa Z, Thomas RK, Greulich H, Schinzel A, et al: An
activated ErbB3/NRG1 autocrine loop supports in vivo proliferation
in ovarian cancer cells. Cancer Cell. 17:298–310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dunn GP, Cheung HW, Agarwalla PK, Thomas
S, Zektser Y, Karst AM, Boehm JS, Weir BA, Berlin AM, Zou L, et al:
In vivo multiplexed interrogation of amplified genes identifies
GAB2 as an ovarian cancer oncogene. Proc Natl Acad Sci USA.
111:1102–1107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Davis SJ, Sheppard KE, Pearson RB,
Campbell IG, Gorringe KL and Simpson KJ: Functional analysis of
genes in regions commonly amplified in high-grade serous and
endometrioid ovarian cancer. Clin Cancer Res. 19:1411–1421. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Aumann K, Lassmann S, Schöpflin A, May AM,
Wöhrle FU, Zeiser R, Waller CF, Hauschke D, Werner M and Brummer T:
The immunohistochemical staining pattern of Gab2 correlates with
distinct stages of chronic myeloid leukemia. Hum Pathol.
42:719–726. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ding J, Romani J, Zaborski M, MacLeod RA,
Nagel S, Drexler HG and Quentmeier H: Inhibition of PI3K/mTOR
overcomes nilotinib resistance in BCR-ABL1 positive leukemia cells
through translational down-regulation of MDM2. PLoS One.
8:e835102013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wohrle FU, Halbach S, Aumann K, Schwemmers
S, Braun S, Auberger P, Schramek D, Penninger JM, Laßmann S, Werner
M, et al: Gab2 signaling in chronic myeloid leukemia cells confers
resistance to multiple Bcr-Abl inhibitors. Leukemia. 27:118–129.
2013. View Article : Google Scholar
|
|
56
|
Brummer T, Larance M, Herrera Abreu MT,
Lyons RJ, Timpson P, Emmerich CH, Fleuren ED, Lehrbach GM, Schramek
D, Guilhaus M, et al: Phosphorylation-dependent binding of 14–3 –3
terminates signalling by the Gab2 docking protein. EMBO J.
27:2305–2316. 2008. View Article : Google Scholar
|
|
57
|
Wöhrle FU, Daly RJ and Brummer T: How to
Grb2 a Gab. Structure. 17:779–781. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Carlberg K and Rohrschneider LR:
Characterization of a novel tyrosine phosphorylated 100-kDa protein
that binds to SHP-2 and phosphatidylinositol 3′-kinase in myeloid
cells. J Biol Chem. 272:15943–15950. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Scherr M, Chaturvedi A, Battmer K,
Dallmann I, Schultheis B, Ganser A and Eder M: Enhanced sensitivity
to inhibition of SHP2, STAT5 and Gab2 expression in chronic myeloid
leukemia (CML). Blood. 107:3279–3287. 2006. View Article : Google Scholar
|
|
60
|
Zatkova A, Schoch C, Speleman F, Poppe B,
Mannhalter C, Fonatsch C and Wimmer K: GAB2 is a novel target of
11q amplification in AML/MDS. Genes Chromosomes Cancer. 45:798–807.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Adams SJ, Aydin IT and Celebi JT: GAB2 - a
scaffolding protein in cancer. Mol Cancer Res. 10:1265–1270. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mali RS, Ma P, Zeng LF, Martin H, Ramdas
B, He Y, Sims E, Nabinger S, Ghosh J, Sharma N, et al: Role of SHP2
phosphatase in KIT-induced transformation: Identification of SHP2
as a druggable target in diseases involving oncogenic KIT. Blood.
120:2669–2678. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sha F, Gencer EB, Georgeon S, Koide A,
Yasui N, Koide S and Hantschel O: Dissection of the BCR-ABL
signaling network using highly specific monobody inhibitors to the
SHP2 SH2 domains. Proc Natl Acad Sci USA. 110:14924–14929. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Halbach S, Rigbolt KT, Wöhrle FU, Diedrich
B, Gretzmeier C, Brummer T and Dengjel J: Alterations of Gab2
signalling complexes in imatinib and dasatinib treated chronic
myeloid leukaemia cells. Cell Commun Signal. 11:302013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Samanta A, Perazzona B, Chakraborty S, Sun
X, Modi H, Bhatia R, Priebe W and Arlinghaus R: Janus kinase 2
regulates Bcr-Abl signaling in chronic myeloid leukemia. Leukemia.
25:463–472. 2011. View Article : Google Scholar :
|
|
66
|
Kotecha N, Flores NJ, Irish JM, Simonds
EF, Sakai DS, Archambeault S, Diaz-Flores E, Coram M, Shannon KM,
Nolan GP, et al: Single-cell profiling identifies aberrant STAT5
activation in myeloid malignancies with specific clinical and
biologic correlates. Cancer Cell. 14:335–343. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Nagao T, Kurosu T, Umezawa Y, Nogami A,
Oshikawa G, Tohda S, Yamamoto M and Miura O: Proliferation and
survival signaling from both Jak2-V617F and Lyn involving GSK3 and
mTOR/p70S6K/4EBP1 in PVTL-1 cell line newly established from acute
myeloid leukemia transformed from polycythemia vera. PLoS One.
9:e847462014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Heuser M, Sly LM, Argiropoulos B,
Kuchenbauer F, Lai C, Weng A, Leung M, Lin G, Brookes C, Fung S, et
al: Modeling the functional heterogeneity of leukemia stem cells:
Role of STAT5 in leukemia stem cell self-renewal. Blood.
114:3983–3993. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lauchle JO, Braun BS, Loh ML and Shannon
K: Inherited predispositions and hyperactive Ras in myeloid
leukemogenesis. Pediatr Blood Cancer. 46:579–585. 2006. View Article : Google Scholar
|
|
70
|
Emanuel PD: Juvenile myelomonocytic
leukemia and chronic myelomonocytic leukemia. Leukemia.
22:1335–1342. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tartaglia M, Niemeyer CM, Fragale A, Song
X, Buechner J, Jung A, Hählen K, Hasle H, Licht JD and Gelb BD:
Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia,
myelodysplastic syndromes and acute myeloid leukemia. Nat Genet.
34:148–150. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
72
|
Loh ML, Vattikuti S, Schubbert S, Reynolds
MG, Carlson E, Lieuw KH, Cheng JW, Lee CM, Stokoe D, Bonifas JM, et
al: Mutations in PTPN11 implicate the SHP-2 phosphatase in
leuke-mogenesis. Blood. 103:2325–2331. 2004. View Article : Google Scholar
|
|
73
|
Keilhack H, David FS, McGregor M, Cantley
LC and Neel BG: Diverse biochemical properties of Shp2 mutants.
Implications for disease phenotypes. J Biol Chem. 280:30984–30993.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yu WM, Daino H, Chen J, Bunting KD and Qu
CK: Effects of a leukemia-associated gain-of-function mutation of
SHP-2 phosphatase on interleukin-3 signaling. J Biol Chem.
281:5426–5434. 2006. View Article : Google Scholar
|
|
75
|
Kontaridis MI, Swanson KD, David FS,
Barford D and Neel BG: PTPN11 (Shp2) mutations in LEOPARD syndrome
have dominant negative, not activating, effects. J Biol Chem.
281:6785–6792. 2006. View Article : Google Scholar
|
|
76
|
Mohi MG, Williams IR, Dearolf CR, Chan G,
Kutok JL, Cohen S, Morgan K, Boulton C, Shigematsu H, Keilhack H,
et al: Prognostic, therapeutic, and mechanistic implications of a
mouse model of leukemia evoked by Shp2 (PTPN11) mutations. Cancer
cell. 7:179–191. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Xu XL, Wang X, Chen ZL, Jin M, Yang W,
Zhao GF and Li JW: Overexpression of Grb2-associated binder 2 in
human lung cancer. Int J Biol Sci. 7:496–504. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Maulik G, Madhiwala P, Brooks S, Ma PC,
Kijima T, Tibaldi EV, Schaefer E, Parmar K and Salgia R: Activated
c-Met signals through PI3K with dramatic effects on cytoskeletal
functions in small cell lung cancer. J Cell Mol Med. 6:539–553.
2002. View Article : Google Scholar
|
|
79
|
Zhang X, Zhang Y, Tao B, Wang D, Cheng H,
Wang K, Zhou R, Xie Q and Ke Y: Docking protein Gab2 regulates
mucin expression and goblet cell hyperplasia through TYK2/STAT6
pathway. FASEB J. 26:4603–4613. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Shi L, Sun X, Zhang J, Zhao C, Li H, Liu
Z, Fang C, Wang X, Zhao C, Zhang X, et al: Gab2 expression in
glioma and its implications for tumor invasion. Acta Oncol.
52:1739–1750. 2013. View Article : Google Scholar
|
|
81
|
Lee SH, Jeong EG, Nam SW, Lee JY, Yoo NJ
and Lee SH: Increased expression of Gab2, a scaffolding adaptor of
the tyrosine kinase signalling, in gastric carcinomas. Pathology.
39:326–329. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Cheng Q, Yi B, Wang A and Jiang X:
Exploring and exploiting the fundamental role of microRNAs in tumor
pathogenesis. Onco Targets Ther. 6:1675–1684. 2013.PubMed/NCBI
|
|
83
|
Fan YL, Zheng M, Tang YL and Liang XH: A
new perspective of vasculogenic mimicry: EMT and cancer stem cells
(Review). Oncol Lett. 6:1174–1180. 2013.PubMed/NCBI
|
















