Introduction
The temporomandibular joint (TMJ), a highly
specialized synovial joint, permits movement and function of the
mammalian jaw, which consists of a glenoid fossa, a condylar head
of the mandible and a fibrocartilaginous disc (1,2). The
glenoid fossa as well as the condyle develop from two distinct
mesenchymal condensations, the temporal and condylar blastemas, and
ossify through specific mechanisms: The glenoid fossa undergoes
intramembranous ossification, while the condyle is endochondral in
origin (3,4). Although TMJ development during
embryonic and post-natal life involves complex processes that are
well documented, little information is available regarding the
cellular and molecular mechanisms involved in TMJ
morphogenesis.
The condyle is a crucial growth site in the mandible
and displays similarities with the growth plate of the long bones.
It has four distinct zones, including a zone of hypertrophic
chondrocytes, a zone of flattened chondrocytes, a progenitor cell
layer and a fibrous cell layer (5). One key gene which was previously
reported to be expressed in the growth plate of the condylar
cartilage is Indian hedgehog (Ihh), which belongs to a family of
potent secreted signaling proteins (6). Ihh is critical for maintaining the
growth of adjacent proliferating chondrocytes and has an indirect
role in controlling the rate of chondrocyte differentiation by
acting in a negative feedback loop with parathyroid-hormone-related
protein (PTHrP) in the periarticular perichondrium (7,8).
Ihh, in conjunction with PTHrP, is essential for organizing the
growth plate (9). Ihh acts on
target cells through the cell-surface receptors Smoothened (Smo)
and Patched (Ptc) and in cooperation with primary cilia, and
signaling is mediated by the Gli family of transcription factors
(10–12). Gli1, a transcriptional activator of
Ihh targets, is transcriptionally upregulated by Ihh signaling. In
mice, cartilage development begins in the center of the condyle,
and Ihh begins to have a significant effect in the condylar
cartilage at E15.5 (6,13). In Ihh-/- mutant mice, the
organization of the growth-plate-like zone in the condyle is
disrupted, and the articular disc does not form (6). In summary, Ihh is crucial for TMJ
formation and development, where it appears to regulate the
condylar cartilage phenotype, chondroprogenitor cell function, as
well as growth and elongation events.
A previous study by our group showed that
downregulation of the Ihh signaling pathway is one of the causes of
congenital dysplasia of the TMJ in mice with short stature homeobox
2 (shox2) overexpression (14).
Shox2, a member of shox gene family, is found only in vertebrates,
suggesting its role in the development of the internal skeleton and
its associated structures (4,13,14).
The present study was conducted to determine whether overexpression
of Ihh may rescue the shox2 overexpression-associated effects on
the TMJ phenotype using a mouse model of Ihh and shox2
overexpression (Wnt1-Cre; pMes-stop shox2;
pMes-stop Ihh mice).
Materials and methods
Mouse embryo collection
The animal procedure used in the present study was
approved by the Institutional Animal Care and Use Committee (IACUC)
of Fujian University of Traditional Chinese Medicine (Fuzhou,
China). A total of 72 Wnt1-Cre; pMes-stop shox2;
pMes-stop Ihh mice, generated by crossing Wnt1-Cre;
pMes-stop shox2 mice (14)
with pMes-stop Ihh mice, and a total of 72 wild-type mice
were provided by the laboratory of Dr Yiping Chen (Department of
Cell and Molecular Biology, Tulane University, New Orleans, LA,
USA). The mice were exposed to a 12 h light/dark cycle in a
temperature (22±1°C) and humidity (56±1%) controlled environment.
They were maintained on a 0.3% sodium diet and were housed
five/cage. The mice were sacrificed with CO2 and the
embryos were extracted in phosphate-buffered saline (PBS; pH 7.4;
GE Healthcare Life Sciences; Logan, UT, USA) at 4°C. When the
presence of a vaginal plug was found in the morning, the age of the
embryo was defined as embryonic day 0.5 (E0.5). Embryonic heads
were fixed in 4% paraformaldehyde (PFA) (Sigma-Aldrich, St Louis,
MO, USA)/PBS at 4°C overnight. The heads of post-natal day 0 (P0),
P7, P14 and P21 mice were fixed and decalcified in Surgipath
Decalcifier I (Leica Biosystems Richmond Inc., Richmond, CA, USA)
for different times depending on the age of the mouse according to
the manufacturer's instructions (4).
Histological analyses
Paraffin-embedded heads were sectioned at a
thickness of 10 µm with a microtome. For histological
analysis of the TMJ, the serial sections were stained with azon
red/anilin blue staining (Sigma-Aldrich) according to standard
procedures. Briefly, paraffin sections were deparaffinizated in
xylene (Sigma-Aldrich) and rehydrated through a gradient series of
ethanol concentrations (Merck & Co., Inc., Kenilworth, NJ,
USA). They were then stained with 0.5% azon red (Sigma-Aldrich) for
2 h at 56°C, rinsed in 5% phospho-tungstic acid (Sigma-Aldrich) for
3 h at room temperature, stained with 0.5% anilin blue
(Sigma-Aldrich) for 2 h, then were dehydrated and mounted on
slides. The morphology of TMJ was observed under an Olympus BH-2
light microscope (Olympus, Tokyo, Japan).
Immunohistochemistry
Paraffin-embedded heads were sectioned at a
thickness of 8 µm for immunohistochemistry. Subsequent to
blocking with goat serum (1:10; Invitrogen Life Technologies,
Carlsbad, CA, USA) and incubation for 15 min at room temperature,
the sections were incubated with polyclonal antibodies against
runt-related transcription factor 2 (Runx2; 1:1,000; ab76956), sex
determining region Y-box 9 (Sox9; 1:500; ab26414), collagen type I
(col I) (1:500; ab34710), collagen type II (col II) (1:200;
ab53047), aggrecan (1:500; ab36861), matrix metalloproteinase 9
(MMP9) (1:300; ab38898), MMP13 (1:50; ab75606) and Ihh (1:200;
ab39634) from Abcam (Cambridge, MA, USA) overnight at 4°C. The
slides were then washed three times with PBS and were incubated
with a biotinylated horseradish peroxidase goat anti-rabbit
secondary antibody (1:1,000 dilution; Invitrogen Life Technologies)
for 20 min at 37°C. The slides were then washed three times with
the secondary antibody using PBS. Immunolabelling was visualized
with 0.05% diaminobenzidine (Invitrogen Life Technologies) in PBS
for 5 min at room temperature, and the slides were then rinsed for
10 min under running tap water. The TMJ immunohistochemical
staining was analyzed under the Olympus BH-2 light microscope.
In situ zymography
Heads of P0 mice were fixed in zinc-based fixative
(ZBF; 36.7 mM ZnCl, 27.3 mM ZnAc2 × 2H2O and
0.63 mM CaAc in 0.1 mM Tris pH 7.4; all from Sigma-Aldrich) and
frozen in optimum cutting temperature compound (Tissue-Tek; Sakura
Finetek USA, Inc., Torrance, CA, USA) compound using liquid
nitrogen. According to the manufacturer's instructions,
10-µm sections were incubated at 37°C for 2 h in a dark
humidified chamber with 1 mg/ml dye-quenched gelatin (100
µl; E12055; Molecular Probes Europe BV, Leiden, The
Netherlands), which was applied on top of the sections and was
covered with a cover-slip (14–17).
The gelatinolytic activity was observed as green fluorescence under
a fluorescence microscope (Axioskop 50; CarlZeiss, Oberkochen,
Germany).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay and bromodeoxyuridine (BrdU)
labeling assay
Apoptosis was tested using the In Situ Cell
Death Detection kit, alkaline phosphatase (Roche, Basel,
Switzerland). Paraffin-embedded heads were sectioned at 5 µm
and subjected to immunodetection according to the manufacturer's
instructions (4,14). At E14.5 or E16.5, pregnant mice
were injected with 1.5 ml labeling reagent/100 g using the BrdU
Labeling and Detection Kit II (Roche) for 2 h. The heads of
embryonic mice were fixed in Carnoy's fixative [6:3:1 ratio of
ethanol (Merck & Co., Inc.), chloroform (Sigma-Aldrich) and
glacial acetic acid (Merck & Co., Inc.)], ethanol-dehydrated,
paraffin-embedded and sectioned at 5 µm. The sections were
subjected to immunodetection for analysis of cell proliferation
according to the manufacturer's instructions (13).
Statistical analysis
All experiments were repeated at least three times,
and values are expressed as the mean ± standard deviation.
Statistical analyses of the data were performed using the Student's
t-test with SPSS software, version 13.0 (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Wnt1-Cre; pMes-stop shox2; pMes-stop Ihh
mice exhibit TMJ dysplasia
Histological analyses of TMJs did not indicate any
differences between wild-type mice and Wnt1-Cre;
pMes-stop shox2; pMes-stop Ihh mice at P0 and P7,
while TMJ dysplasia was observed at P14. To investigate the
phenotype of the TMJs, a time-course analysis of alternation in the
width of the glenoid fossa and condyle of the TMJ was performed.
The observation was focused on the widest part of the glenoid fossa
and condyle where the defect was most significant. The average
width (obtained from three measurements of different animals) of
the glenoid fossa and condyle in the wild-type mice at each
time-point was defined as 100%. That the width of the glenoid fossa
and condyle appeared to be similar between wild-type mice and
Wnt1-Cre; pMes-stop shox2; pMes-stop Ihh mice
at P0 and P7 (Fig. 1a–d). However,
at P14 and P21, the width of the condyle and glenoid fossa in the
Wnt1-Cre; pMes-stop shox2; pMes-stop Ihh mice
was decreased compared with that in the wild-type mice (P<0.05)
(Fig. 1e–j). These results
suggested that the TMJ dysplasia occurred primarily in the second
week following birth.
To assess the effect of the TMJ dysplasia on the
body weight in the Wnt1-Cre; pMes-stop Shox2;
pMes-stop Ihh mice, the body weight was determined at
different developmental stages (P0, P7, P14 and P21) (Fig. 1k). Mice stopped suckling and
started eating solid food at P21. The results demonstrated that the
body weight between wild-type mice and Wnt1-Cre;
pMes-stop shox2; pMes-stop Ihh mice was similar at P0
and P7; however, it was significantly different at P14 and P21
(P<0.05), indicating that the observed changes of the TMJ in
Wnt1-Cre; pMes-stop shox2; pMes-stop Ihh mice
may have affected the function of the TMJ, leading to a reduction
in body weight and the development of a wasting syndrome.
Wnt1-Cre; pMes-stop shox2; pMes-stop Ihh
mice display normal expression of Runx2 and Sox9 in the developing
TMJ
To determine whether the protein levels of Runx2 and
Sox9 were altered in the developing TMJ of Wnt1-Cre;
pMes-stop shox2; pMes-stop Ihh mice,
immunohistochemical analysis was performed. There were no
differences in Runx2 and Sox9 protein levels in the TMJ between
wild-type mice and Wnt1-Cre; pMes-stop shox2;
pMes-stop Ihh mice at E14.5 and E16.5 (Fig. 2a–h), suggesting that overexpression
of Ihh did not affect the formation and development of the TMJ
during the embryonic stage.
Wnt1-Cre; pMes-stop shox2; pMes-stop Ihh
mice show no abnormalities in the extracellular matrix (ECM)
composition
The ECM, including collagens, proteoglycans and
glycosaminoglycans, is the main component of articular cartilage
(4). It is essential for the
resistance to compressive forces and maintaining the tensile
properties of the cartilage. A previous study by our group showed
that overexpression of shox2 led to a reduction of col I and col II
expression (14). To account for
the dysplasia of TMJ in the Wnt1-Cre; pMes-stop
shox2; pMes-stop Ihh mice, the expression of several key
components of the ECM, including col I, col II and aggrecan was
assessed at P0 and P7 (Fig. 3a–l).
Immunohistochemcal analysis showed that the protein levels of col I
and col II were similar between wild-type and Wnt1-Cre;
pMes-stop shox2; pMes-stop Ihh mice at P0 and P7.
However, the protein levels of aggrecan were increased in the
condyle in the TMJ of Wnt1-Cre; pMes-stop shox2;
pMes-stop Ihh mice. These results suggested that
overexpression of Ihh rescued the ECM composition in the
Wnt1-Cre; pMes-stop shox2 mice.
Ihh is overexpressed in the TMJ of
Wnt1-Cre; pMes-stop shox2; pMes-stop Ihh mice
To determine whether the expression of Ihh was
significantly upregulated in the developing TMJ of Wnt1-Cre;
pMes-stop shox2; pMes-stop Ihh mice, the protein
levels of Ihh were assessed using immunohistochemistry at P0 and P7
(Fig. 3m–p). The protein levels of
Ihh were increased in the condyle of Wnt1-Cre; pMes-stop
shox2; pMes-stop Ihh mice compared with those in the
wild-type mice, supporting that Ihh was overexpressed in the
TMJ.
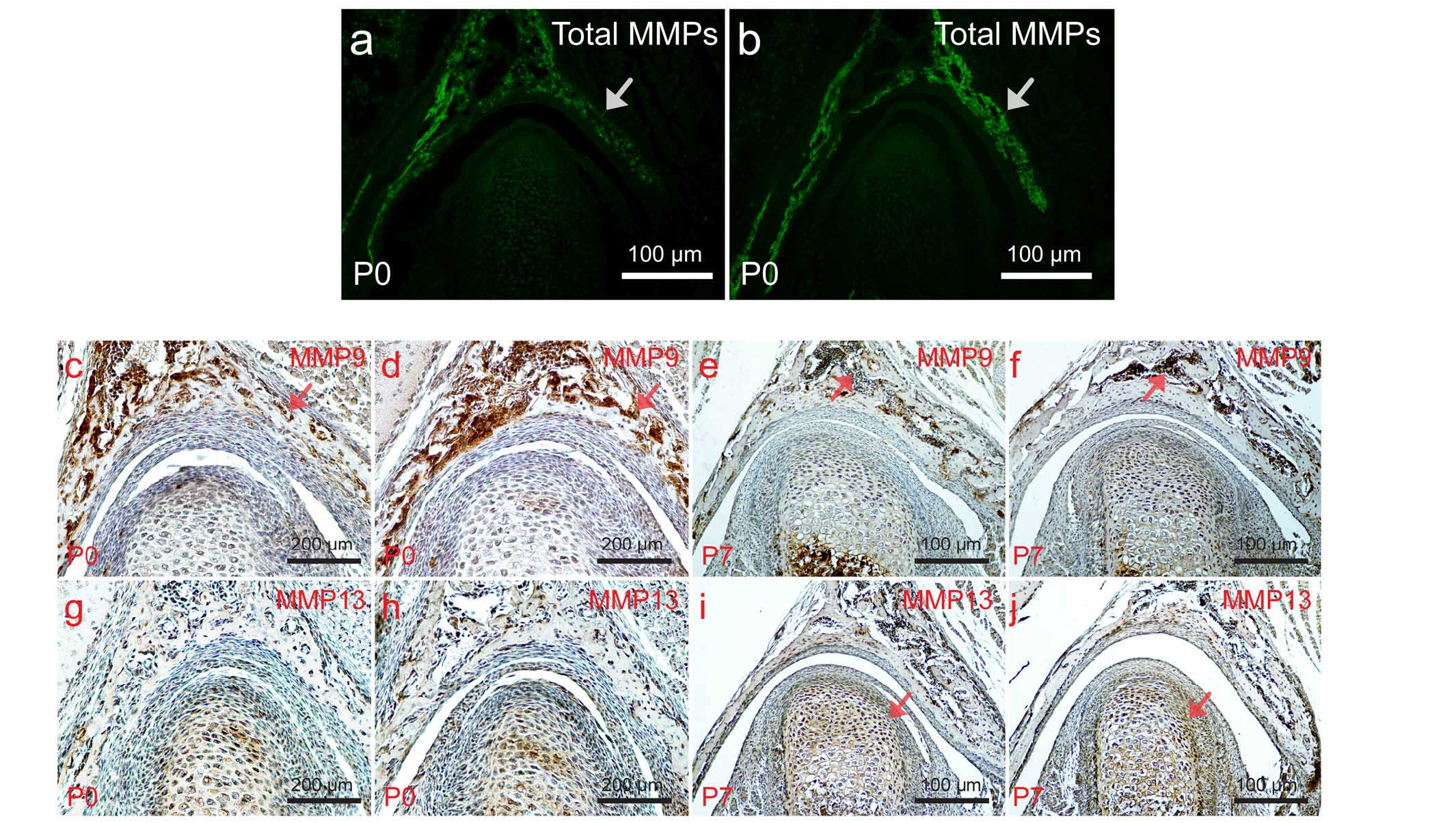
MMPs activity is upregulated in the TMJ
of Wnt1-Cre; pMes-stop shox2; pMes-stop Ihh mice
The previous study by our group showed that
overexpression of shox2 results in the upregulation of MMP activity
(14). In the present study, it
was therefore assessed whether overexpression of Ihh is able to
inhibit the enhancement of MMP activity. First, in situ
zymography was performed to determine total MMP activity in the
TMJs of wild-type mice and Wnt1-Cre; pMes-stop shox2;
pMes-stop Ihh mice at P0. As shown in Fig. 4a and b, enhanced MMP activity was
identified in the glenoid fossa; however, above-background levels
of MMP activity were also observed in the condyle. Consistent with
this enhanced total MMP activity, the expression of MMP9 and MMP13
in the TMJs of Wnt1-Cre; pMes-stop shox2;
pMes-stop Ihh mice was found to be enhanced compared with
that in the wild-type mice at P0 and P7 (Fig. 4c–j). These results indicated that
enhanced MMP activity is associated with dysplasia of the TMJ, and
that the shox2 overexpression-associated increases in MMP activity
was not sufficiently rescued by overexpression of Ihh.
Apoptosis is enhanced in the glenoid
fossa of the Wnt1-Cre; pMes-stop shox2; pMes-stop Ihh mice
The present study further examined whether the
overexpression of Ihh was able to decrease the apoptosis that
potentially contributed to the TMJ phenotype of the
Wnt1-Cre; pMes-stop shox2 mice. The results of the
TUNEL assay showed a significantly enhanced number of apoptotic
cells in the glenoid fossa of Wnt1-Cre; pMes-stop
shox2; pMes-stop Ihh mice compared to that in the
wild-type mice at P0 (P<0.05) (Fig.
5a–c), indicating that overexpression of Ihh did not inhibit
shox2 overexpression-associated apoptosis of glenoid fossa. In
addition, a BrdU labeling assay revealed there was no alteration in
the cell proliferation rate in the condyle of the wild-type mice
and Wnt1-Cre; pMes-stop shox2; pMes-stop Ihh
mice at E14.5 and E16.5 (Fig.
6a–f).
Discussion
The results of the present study provided solid
evidence that Ihh is required for the formation and development of
the TMJ. Compared with the results of a previous study by our group
(14), the results of the present
study demonstrated that overexpres-sion of Ihh rescued the shox2
overexpression-associated reduction of ECM components; however,
shox2-associated increases of MMP activity, MMP9 and MMP13
levels as well as apoptosis in the TMJ were not sufficiently
attenuated, suggesting that overexpression of Ihh partially rescued
shox2-associated congenital dysplasia of the TMJ in mice.
In the present study, detailed histological analysis
of the TMJ indicated changes in the width of glenoid fossa and
condyle between post-natal wild-type mice and Wnt1-Cre;
pMes-stop shox2; pMes-stop Ihh mice at P14 and
thereafter. The previous study by our group reported that
Wnt1-Cre; pMes-stop shox2 mice developed a congenital
dysplasia of TMJ, mostly likely attributed to the wasting syndrome,
since TMJ is essential for the movement and function of the jaw in
mammals (14). To investigate the
alterations of the TMJ that may have contributed to the decreases
in body weight, a time-course analysis of the body weight was
performed in the present study. The body weight was significantly
different between wild-type and Wnt1-Cre; pMes-stop
shox2; pMes-stop Ihh mice from P14, supporting that
alterations of the TMJ may have affected the observed body weight
changes.
Ihh is essential for TMJ formation and development,
and also for the maintenance of the proper structure and function
of the TMJ after it is formed. The expression of Smo, Pct, Gli1,
Gli2 and Gli3 as Hh receptors and effector genes accompanies Ihh
expression in the process of TMJ development (6,7). It
is currently elusive whether the reduction of Ihh expression is a
causative factor in the reduction of the ECM and the elevated MMP
activity in the TMJ of post-natal Wnt1-Cre; pMes-stop
shox2 mice; however, it is consistent with a key role for the
Ihh signaling pathway, namely the maintenance of the proper
structure and tissue homeostasis of the post-natal TMJ (14). The hypothesis of the present study
was that the overexpres-sion of Ihh rescues the TMJ phenotype
following upregulation of shox2. To test this hypothesis,
Wnt1-Cre; pMes-stop shox2; pMes-stop Ihh mice
were bred, in which the overexpression of Ihh in the TMJ was
confirmed using immunohistochemical analysis.
To investigate the cellular and molecular
alterations contributing to the dysplasia of the TMJ, changes in
osteogenic genes, the composition of the ECM, MMPs, apoptosis and
proliferation were assessed in a series of developmental stages of
Wnt1-Cre; pMes-stop shox2; pMes-stop Ihh mice.
During the progression of TMJ development, the transcription
factors Runx2 and Sox9 have been implicated in the development of
primary cartilage and endochondral ossification (2,18,19).
The present study identified that the cell proliferation and the
expression of Runx2 and Sox9 were at normal levels in the
developing TMJ, indicating upregulation of shox2 and Ihh did not
lead to any abnormalities in the progression of TMJ development. To
reveal the underlying mechanisms of the dysplasia of the TMJ, the
present study then examined changes of ECM composition, MMPs and
apoptosis in the TMJ of Wnt1-Cre; pMes-stop shox2;
pMes-stop Ihh mice.
Cartilage degradation results from the imbalance
between anabolism and catabolism due to increased matrix-degrading
proteases and decreased synthesis of matrix, which are largely
mediated by the imbalance between anabolic and catabolic cytokine
signaling molecules (20,21). These changes are char-acterized by
the significant upregulation of MMPs, which are able to cleave
aggrecan and collagen, the two most abundant ECM components of
articular cartilage (20,22). MMP9 has a central role in
connective tissue remodeling and in the turnover of basement
membrane (23), while MMP13 has a
crucial role in bone formation and remodeling, and is expressed in
terminal hypertrophic chondrocytes in the growth plate and in
osteoblasts (24,25). MMP9 degrades the aggrecan core
protein and denatured type II collagen resulting from initial
cleavage by activated MMP1 (26,27).
MMP13 degrades fibrillar collagens, including collagen II, and also
has the capacity to degrade aggrecan, the hydrodynamically large
aggregating proteoglycan of cartilage (28,29).
The results of the present study showed that overexpression of Ihh
rescued the ECM composition; however, the shox2-associated
increases in MMP activity and apoptosis were not attenuated by Ihh
overexpres-sion. Overall, these results indicated that
overexpression of Ihh was able to partially rescue the
shox-associated dysplasia of the TMJ.
In conclusion, the present study showed that
overexpression of Ihh partially rescued the shox2
overexpression-associated congenital dysplasia of the TMJ. Further
study is required to investigate the association between Ihh and
other signals involved in the formation and development of the TMJ.
An enhanced understanding of the underlying molecular mechanisms of
TMJ organogenesis will aid the improvement of diagnoses and the
development of novel therapeutic targets for TMJ disorders.
Acknowledgments
This work was supported by the National Natural
Science Foundation of China (grant no. 81373818), the Program for
New Century Excellent Talents in Fujian Province University (grant
no. JA14150), and the Developmental Fund of Chen Keji Integrative
Medicine (grant nos. CKJ2014014 and CKJ2015009). The authors
appreciate the help of Dr Yiping Chen at the Department of Cell and
Molecular Biology, Tulane University (New Orleans, LA, USA).
References
|
1
|
Hinton RJ: Genes that regulate
morphogenesis and growth of the temporomandibular joint: A review.
Dev Dyn. 243:864–874. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Y, Liu C, Rohr J, Liu H, He F, Yu J,
Sun C, Li L, Gu S and Chen Y: Tissue interaction is required for
glenoid fossa development during temporomandibular joint formation.
Dev Dyn. 240:2466–2473. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gu S, Wu W, Liu C, Yang L, Sun C, Ye W, Li
X, Chen J, Long F and Chen Y: BMPRIA mediated signaling is
essential for temporomandibular joint development in mice. PLoS
One. 9:e1010002014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li X, Liu H, Gu S, Liu C, Sun C, Zheng Y
and Chen Y: Replacing Shox2 with human SHOX leads to congenital
disc degeneration of the temporomandibular joint in mice. Cell
Tissue Res. 355:345–354. 2014. View Article : Google Scholar :
|
|
5
|
Ochiai T, Shibukawa Y, Nagayama M, Mundy
C, Yasuda T, Okabe T, Shimono K, Kanyama M, Hasegawa H, Maeda Y, et
al: Indian hedgehog roles in post-natal TMJ development and
orga-nization. J Dent Res. 89:349–354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shibukawa Y, Young B, Wu C, Yamada S, Long
F, Pacifici M and Koyama E: Temporomandibular joint formation and
condyle growth require Indian hedgehog signaling. Dev Dyn.
236:426–434. 2007. View Article : Google Scholar
|
|
7
|
Purcell P, Joo BW, Hu JK, Tran PV,
Calicchio ML, O'Connell DJ, Maas RL and Tabin CJ: Temporomandibular
joint formation requires two distinct hedgehog-dependent steps.
Proc Natl Acad Sci USA. 106:18297–18302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vortkamp A, Lee K, Lanske B, Segre GV,
Kronenberg HM and Tabin CJ: Regulation of rate of cartilage
differentiation by Indian hedgehog and PTH-related protein.
Science. 273:613–622. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lanske B, Karaplis AC, Lee K, Luz A,
Vortkamp A, Pirro A, Karperien M, Defize LH, Ho C, Mulligan RC, et
al: PTH/PTHrP receptor in early development and Indian
hedgehog-regulated bone growth. Science. 273:663–666. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Corbit KC, Aanstad P, Singla V, Norman AR,
Stainier DY and Reiter JF: Vertebrate Smoothened functions at the
primary cilium. Nature. 437:1018–1021. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koyama E, Young B, Nagayama M, Shibukawa
Y, Enomoto-Iwamoto M, Iwamoto M, Maeda Y, Lanske B, Song B, Serra
R, et al: Conditional Kif3a ablation causes abnormal hedgehog
signaling topography, growth plate dysfunction, and excessive bone
and cartilage formation during mouse skeletogenesis. Development.
134:2159–2169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haycraft CJ, Banizs B, Aydin-Son Y, Zhang
Q, Michaud EJ and Yoder BK: Gli2 and Gli3 localize to cilia and
require the intrafla-gellar transport protein polaris for
processing and function. PLoS Genet. 1:e532005. View Article : Google Scholar
|
|
13
|
Gu S, Wei N, Yu L, Fei J and Chen Y:
Shox2-deficiency leads to dysplasia and ankylosis of the
temporomandibular joint in mice. Mech Dev. 125:729–742. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Liang W, Ye H, Weng X, Liu F and Liu
X: Overexpression of Shox2 leads to congenital dysplasia of the
temporomandibular joint in mice. Int J Mol Sci. 15:13135–13150.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gkantidis N, Katsaros C and Chiquet M:
Detection of gelatinolytic activity in developing basement
membranes of the mouse embryo head by combining sensitive in situ
zymography with immunolabeling. Histochem Cell Biol. 138:557–571.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pessoa JI, Guimarães GN, Viola NV, da
Silva WJ, de Souza AP, Tjäderhane L, Line SR and Marques MR: In
situ study of the gelatinase activity in demineralized dentin from
rat molar teeth. Acta Histochem. 115:245–251. 2013. View Article : Google Scholar
|
|
17
|
Sakakura Y, Hosokawa Y, Tsuruga E, Irie K
and Yajima T: In situ localization of gelatinolytic activity during
development and resorption of Meckel's cartilage in mice. Eur J
Oral Sci. 115:212–223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mori-Akiyama Y, Akiyama H, Rowitch DH and
de Crombrugghe B: Sox9 is required for determination of the
chondrogenic cell lineage in the cranial neural crest. Proc Natl
Acad Sci USA. 100:9360–9365. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shibata S and Yokohama-Tamaki T: An in
situ hybridization study of Runx2, Osterix, and Sox9 in the anlagen
of mouse mandibular condylar cartilage in the early stages of
embryogenesis. J Anat. 213:274–283. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Su SC, Tanimoto K, Tanne Y, Kunimatsu R,
Hirose N, Mitsuyoshi, Okamoto Y and Tanne K: Celecoxib exerts
protective effects on extracellular matrix metabolism of mandibular
condylar chondrocytes under excessive mechanical stress.
Osteoarthritis Cartilage. 22:845–851. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu YD, Liao LF, Zhang HY, Lu L, Jiao K,
Zhang M, Zhang J, He JJ, Wu YP, Chen D, et al: Reducing dietary
loading decreases mouse temporomandibular joint degradation induced
by anterior crossbite prosthesis. Osteoarthritis Cartilage.
22:302–312. 2014. View Article : Google Scholar :
|
|
22
|
Burrage PS, Mix KS and Brinckerhoff CE:
Matrix metalloproteinases: Role in arthritis. Front Biosci.
11:529–543. 2006. View
Article : Google Scholar
|
|
23
|
Touaitahuata H, Cres G, de Rossi S, Vives
V and Blangy A: The mineral dissolution function of osteoclasts is
dispensable for hypertrophic cartilage degradation during long bone
development and growth. Dev Biol. 393:57–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Inada M, Wang Y, Byrne MH, Rahman MU,
Miyaura C, López-Otín C and Krane SM: Critical roles for
collagenase-3 (Mmp13) in development of growth plate cartilage and
in endochondral ossification. Proc Natl Acad Sci USA.
101:17192–17197. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang SY, Herber RP, Ho SP and Alliston T:
Matrix metallo-proteinase-13 is required for osteocytic perilacunar
remodeling and maintains bone fracture resistance. J Bone Miner
Res. 27:1936–1950. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Malemud CJ: Matrix metalloproteinases:
Role in skeletal development and growth plate disorders. Front
Biosci. 11:1702–1715. 2006. View
Article : Google Scholar
|
|
27
|
Jackson MT, Moradi B, Smith MM, Jackson CJ
and Little CB: Activation of matrix metalloproteinases 2, 9, and 13
by activated protein C in human osteoarthritic cartilage
chondrocytes. Arthritis Rheumatol. 66:1525–1536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Otero M, Plumb DA, Tsuchimochi K, Dragomir
CL, Hashimoto K, Peng H, Olivotto E, Bevilacqua M, Tan L, Yang Z,
et al: E74-like factor 3 (ELF3) impacts on matrix metalloproteinase
13 (MMP13) transcriptional control in articular chondrocytes under
proinflammatory stress. J Biol Chem. 287:3559–3572. 2012.
View Article : Google Scholar :
|
|
29
|
Zhang L, Yang M, Yang D, Cavey G, Davidson
P and Gibson G: Molecular interactions of MMP-13 C-terminal domain
with chondrocyte proteins. Connect Tissue Res. 51:230–239. 2010.
View Article : Google Scholar : PubMed/NCBI
|