Introduction
Liver transplantation is the standard treatment for
patients with end-stage liver failure. Despite immunosuppressive
drugs providing excellent short-term outcomes following
transplantation, acute and chronic rejection episodes still occur
and affect long-term graft function. Non-invasive monitoring of
graft-specific immune activation would allow for the early
diagnosis, and ultimately, the prediction and management of acute
or chronic liver rejection (1).
Therefore, novel methods are required in order to accurately
predict acute rejection following transplantation.
The Notch gene, originally identified in
Drosophila (2), is highly
conserved in both invertebrates and vertebrates (3). The Notch signaling pathway consists
of four Notch receptors (Notch 1–4), and five structurally similar
Notch ligands (Delta-like 1, 3, and 4, and Jagged 1 and 2)
(4,5). Notch signaling has an important role
in the regulation of cell fate, and influences the growth and
survival of progenitor cells (6).
The Notch signaling pathway regulates various aspects of embryonic
development, as well as differentiation processes and tissue
homeostasis in numerous adult organ systems (7).
A recent study demonstrated that Notch receptors and
ligands are highly expressed in the central and peripheral immune
systems (7). The Notch signaling
pathway has an important role in regulating the differentiation,
proliferation, and function of mature lymphocytes, including
peripheral T cells. Once naïve T cells migrate to the periphery in
response to antigens, in the presence of appropriate signals the
naïve T cells become activated and exert their function (7). Notch receptor expression has been
linked to T cell activation, proliferation, and cytokine production
(7), suggesting that these signals
may be closely associated with the immune reaction to allografts.
Following renal transplantation, Notch 1 expression was associated
with the immune state of recipients, indicating that the expression
levels of Notch 1 may predict long-term renal function. Notch 1 may
therefore serve as a marker of acute rejection and long-term renal
function following renal transplantation (8).
Peripheral blood is easily accessible and may be
used to assess biomarkers that accurately reflect or predict immune
responses to a graft. The present study established a rat liver
transplantation model in order to examine whether increased
expression levels of Notch 1 in the peripheral blood were
predictive of early acute immune rejection.
Materials and methods
Animals
Male Dark Agouti (DA) and Lewis rats (age, 8–10
weeks old; weight, 230±20 g) were purchased from the Laboratory
Animal Center of the Second Affiliated Hospital at Harbin Medical
University (Heilongjiang, China), and from the Shanghai SLAC
Laboratory Animal Co. Ltd. (Shanghai, China), respectively. All
rats were housed in microisolator cages in the barrier facility of
the Fujian Medical University (Fuzhou, China). The rats were housed
at 27°C in 45% humidity with 12 h light/dark cycle, with ad
libitum access to food and water. All experiments were approved
by the Ethics Committee of Fujian Medical University (Fuzhou,
China).
Establishment of a rat heterotopic liver
transplantation model
A heterotopic liver transplantation model was
established using DA rats (n=50) as donors and Lewis rats (n=25) as
recipients (DA/Lewis), with DA rats (DA/DA) (n=25) serving as
recipients in the control group (9–11).
Five rats from each group were sacrificed on days 3, 5, and 7
post-transplantation, prior to liver tissue sample harvesting. The
overall allograft survival rates were monitored in 10 rats from
each group, with allograft rejection being histologically
confirmed.
Liver function measurements
The serum concentration levels of total bilirubin
(TBIL) and alanine transaminase (ALT) were measured using the
caffeine method and rate method according to the manufacturer's
instructions (Cobas 8000 Biochemical Analyzer; Roche Diagnostics,
Basel, Switzerland) on days 3, 5, and 7 following liver
transplantation.
Notch 1 quantification by ELISA
The concentration levels of serum Notch 1 were
quantified on days 3, 5, and 7 following liver transplantation by
ELISA (450 nm) using Quantikine M kits (Yueyan Biotech, Shanghai,
China), according to the manufacturer's instructions. The Bio-Rad
550 ELISA Plate-Reader was used (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Histological analysis
Allografts were histologically examined following
fixing of allograft tissues in 4% paraformaldehyde and embedding in
paraffin (Sinopharm Chemical Reagent Co., Shanghai, China). on days
3, 5, and 7, and the overall rejection rates were assessed
according to the Banff schema (12). Paraffin sections (5 µm) were
cut, then dewaxed and rehydrated through reducing graded alcohols,
using a standard protocol: Three changes of xylene (Sinopharm
Chemical Reagent Co.), 3 min each; two changes of 100% ethanol, 2
min each; 95% ethanol, 1 min; 70% ethanol, 1 min. Tissue sections
were then stained with hematoxylin (Amresco, Solon, OH, USA) and
eosin (Sinopharm Chemical Reagent Co.) for histological examination
and were observed by microscopy (BX46; Olympus, Tokyo, Japan). The
pathological features of acute rejection included the presence of
inflammatory infiltrates in the portal tracts, bile duct damage,
and endotheliitis, with at least two of these three features
required for diagnosis (13,14).
All histological evaluations were performed in a double-blinded
manner by two researchers.
Statistical analysis
SPSS 12 (SPSS, Inc., Chicago, IL, USA) analytical
software was used for all statistical analyses. The results were
presented as the mean ± standard deviation, and compared by one-way
analysis of variance or Student's t-test. Graft survival was
analyzed by life table methods, with differences in survival
assayed by the log-rank test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Liver function evaluation
The concentration levels of normal serum ALT and
TBIL in the Lewis rats were 26.3±7.3 U/l and 11.36±4.35
µmol/l, respectively (data not shown). A total of 5 and 7
days following transplantation, the concentration levels of both
TBIL and ALT were significantly higher in the DA/Lewis group, as
compared with the DA/DA group (P<0.001) (Figs. 1A and B). These results indicate
that liver function in the DA/Lewis group was continuously being
restored following transplantation.
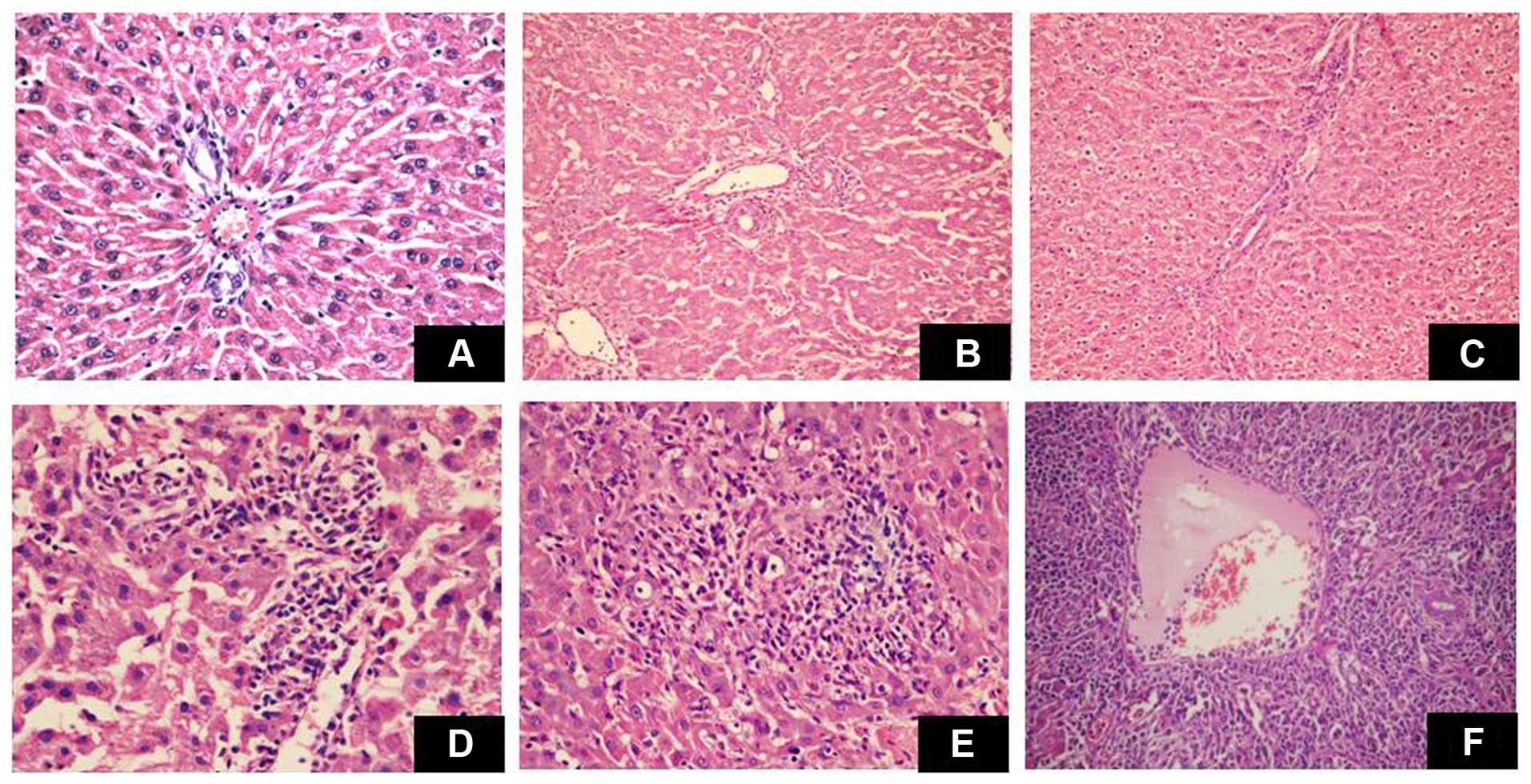
Histological assessment of donor liver
grafts
The liver grafts in the DA/Lewis group exhibited
moderate to severe acute rejection. Pathological changes included
mixed infiltrate, infiltration of most ducts by inflammatory cells,
and severe perivenular inflammation extending into the perivenular
parenchyma. Conversely, the liver grafts in the DA/DA group
exhibited no evidence of rejection (Fig. 2A-F).
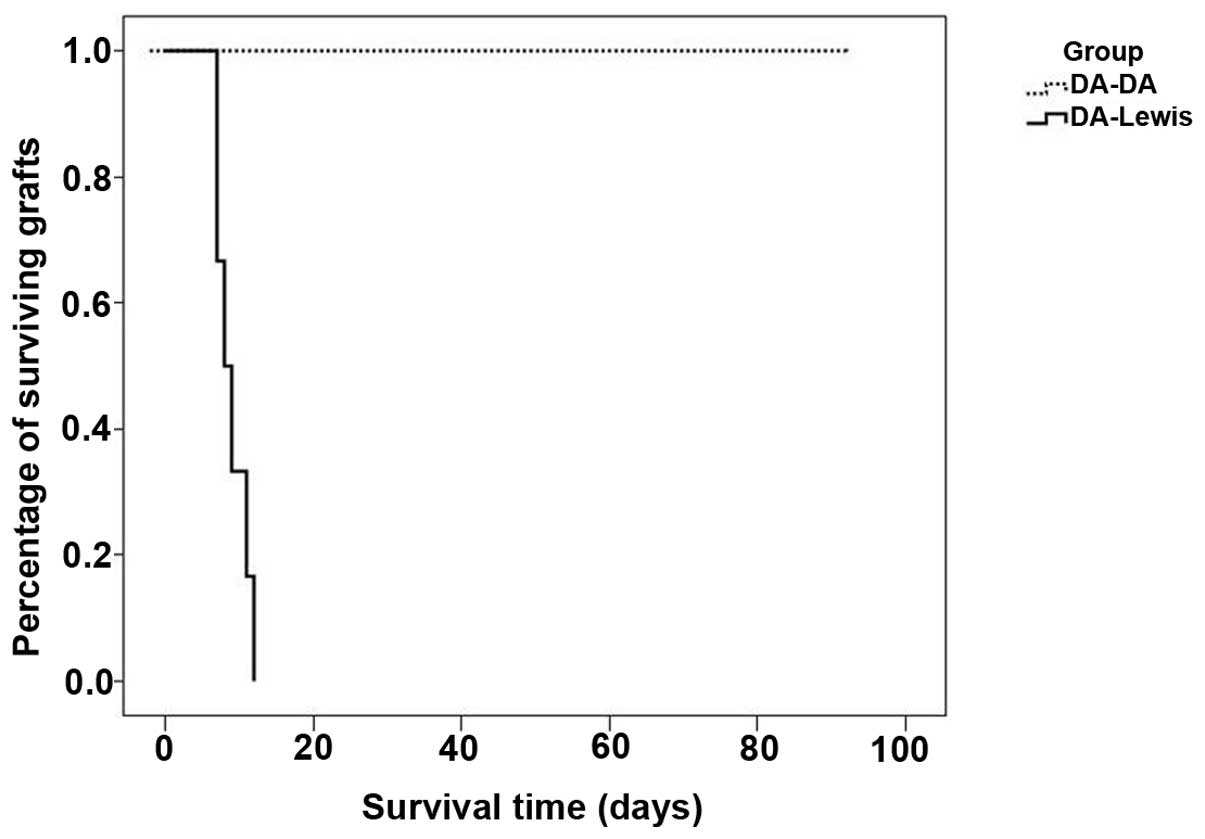
Survival of liver allografts in
recipients
Liver grafts survived >90 days in the DA/DA
group, but <10 days in the DA/Lewis group (P<0.0001);
(Fig. 3). These findings indicate
severe acute rejection in the DA/Lewis rats, whereas no rejection
was observed in the DA/DA group.
Serum Notch 1 levels
The concentration levels of serum Notch 1 were
significantly higher in the DA/Lewis group, as compared with the
DA/DA group on days 3, 5 and 7 (P<0.0001) (Table I). These concentrations increased
significantly over time in the DA/Lewis group (P<0.0001),
suggesting a correlation between Notch 1 concentration and the
progression of acute liver rejection.
 | Table IDetection of the concentration levels
of serum Notch 1 in the liver transplant recipients, as determined
by ELISA (µmol/l). |
Table I
Detection of the concentration levels
of serum Notch 1 in the liver transplant recipients, as determined
by ELISA (µmol/l).
| Group | Day 3 | Day 5 | Day 7 |
|---|
| DA/DA | 9.313±1.011 | 9.030±0.742 | 8.791±0.721 |
| DA/Lewis |
13.405±0.802a |
17.527±0.824a |
23.605±0.731b |
Discussion
The present study established a rat liver
transplantation model in order to examine the correlation between
immune allograft rejection and Notch 1 levels in peripheral blood.
The results indicated the presence of both acute rejection and
increasing serum concentration levels of Notch 1 over time in the
DA/Lewis group, suggesting that Notch 1 concentration may serve as
a marker for early acute rejection.
Non-invasive monitoring of graft-specific immune
activation would allow for the early diagnosis, and ultimately, the
prediction and pre-emptive management of acute or chronic rejection
(1). Peripheral blood is easily
accessible and may be used to identify and monitor biomarkers that
accurately reflect, detect, or predict detrimental immune responses
to grafts (1). In the present
study, livers were transplanted from DA rats into either Lewis or
DA rats (control), in order to evaluate liver function, histology,
and survival. The results demonstrated that the concentration
levels of TBIL and ALT were significantly higher in the DA/Lewis
group, as compared with the DA/DA group, 5 and 7 days following
transplantation (P<0.0001). The liver grafts in the DA/Lewis
group exhibited moderate to severe acute rejection, and overall
survival was significantly shorter in the DA/Lewis group, as
compared with the DA/DA group (P<0.0001). These findings
indicated that a model of acute rejection of liver transplants had
been successfully established.
Notch signaling pathways have important roles in
regulating the proliferation and function of mature lymphocytes
(6,7). Notch signaling has also been
implicated as an important regulator of peripheral T cell
activation and effector cell differentiation (15), and Notch signals may be closely
associated with immune reactions in the allograft. Notch 1
expression in peripheral blood mononuclear cells (PBMCs) has
previously been shown to correlate with acute rejection and
long-term renal function following renal transplantation, with the
expression of Notch 1 in PBMCs increasing prior to the increase in
the concentration levels of serum creatinine (8). Similarly, in the present study the
concentration levels of serum Notch 1 were higher in the DA/Lewis
group, as compared with the DA/DA group (P<0.0001), and the
concentration levels of Notch 1 increased significantly over time
in the DA/Lewis group, as compared with the DA/DA group
(P<0.0001).
Notch signaling has been reported to critically
influence the differentiation of activated T cells into T helper 1
cells, which control cellular immunity (15,16).
Furthermore, Notch gene expression was induced in primary
CD4+ T cells following specific peptide/antigen
stimulation (17). Notch activity
contributes to peripheral T cell responses, including
CD4+ T cells, via augmentation of a positive feedback
loop (17). T cell receptor
activation of peripheral T cells in vitro has been shown to
upregulate the expression of Notch 1, which was correlated with
increased T cell proliferation and interferon-γ cytokine production
(18).
Previous studies have reported that Notch signaling
may induce immune tolerance. Notch signaling has been implicated in
the induction of regulatory T cell (T reg) differentiation and
function (19–21). T reg cells are crucial for the
negative regulation of hyperactive T cells and immune tolerance,
via the suppression of T cell reactivity in peripheral tissues
(22–24). Notch ligands have been reported to
enhance T reg cell differentiation and function in vitro
(25,26); however, this activity has yet to be
evaluated using genetic approaches (15). The results of the present study
regarding the role of Notch 1 in the acute rejection model of liver
transplantation appear to differ from these previous reports.
Genetic approaches are required in order to clarify the importance
and mechanism underlying Notch signaling in liver
transplantation.
In conclusion, the present study demonstrated that
the concentration levels of serum Notch 1 are significantly
increased during liver allograft rejection. These results suggested
that Notch 1 is involved in the mechanisms underlying liver
allograft rejection. Therefore, Notch 1 may serve as a marker of
acute rejection in a rat liver transplantation model. These
findings suggest that the concentration levels of serum Notch 1 may
predict acute rejection in rat liver transplantation.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81202337),
the Fujian Provincial Natural Foundation (grant no. 2009D066), the
Fujian Medical University Foundation (grant no. 09ZD007), and the
Science and Technology Project of Fuzhou (grant no.
2013-S-125–9).
References
|
1
|
Heidt S, San Segundo D, Shankar S, Mittal
S, Muthusamy AS, Friend PJ, Fuggle SV and Wood KJ: Peripheral blood
sampling for the detection of allograft rejection: Biomarker
identification and validation. Transplantation. 92:1–9. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mohr OL: Character changes caused by
mutation of an entire region of a chromosome in Drosophila.
Genetics. 4:275–282. 1919.PubMed/NCBI
|
|
3
|
Chen L, Ashraf M, Wang Y, Zhou M, Zhang J,
Qin G, Rubinstein J, Weintraub NL and Tang Y: The role of notch 1
activation in cardiosphere derived cell differentiation. Stem Cells
Dev. 21:2122–2129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ladi E, Nichols JT, Ge W, Miyamoto A, Yao
C, Yang LT, Boulter J, Sun YE, Kintner C and Weinmaster G: The
divergent DSL ligand Dll3 does not activate Notch signaling but
cell autonomously attenuates signaling induced by other DSL
ligands. J Cell Biol. 170:983–992. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bray SJ: Notch signalling: A simple
pathway becomes complex. Nat Rev Mol Cell Biol. 7:678–689. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoyne GF: Notch signaling in the immune
system. J Leukoc Biol. 74:971–981. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Radtke F, Fasnacht N and Macdonald HR:
Notch signaling in the immune system. Immunity. 32:14–27. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng K, Sun X, Wu W, Yang S, Cai J and
Tan J: A new index for acute rejection after renal transplant:
Notch receptor-1. Exp Clin Transplant. 10:433–438. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Macomber CW and Shah SA: A technique of
recipient portal venoplasty and cuff insertion for portal
revascularization in orthotopic rat liver transplantation. J Surg
Res. 179:45–46. 2013. View Article : Google Scholar
|
|
10
|
Li N, Cai CJ, Wu YR and Lu MQ: A technique
of recipient portal venoplasty and cuff insertion for portal
revascularization in orthotopic rat liver transplantation. J Surg
Res. 176:317–320. 2012. View Article : Google Scholar
|
|
11
|
Peng Y, Gong JP, Yan LN, Li SB and Li XH:
Improved two-cuff technique for orthotopic liver transplantation in
rat. Hepatobiliary Pancreat Dis Int. 3:33–37. 2004.PubMed/NCBI
|
|
12
|
Höroldt BS, Burattin M, Gunson BK,
Bramhall SR, Nightingale P, Hübscher SG and Neuberger JM: Does the
Banff rejection activity index predict outcome in patients with
early acute cellular rejection following liver transplantation?
Liver Transpl. 12:1144–1151. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
No authors listed. Banff schema for
grading liver allograft rejection: An international consensus
document. Hepatology. 25:658–663. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hübscher SG: Transplantation pathology.
Semin Liver Dis. 29:74–90. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan JS, Kousis PC, Suliman S, Visan I and
Guidos CJ: Functions of notch signaling in the immune system:
Consensus and controversies. Annu Rev Immunol. 28:343–365. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amsen D, Antov A and Flavell RA: The
different faces of Notch in T-helper-cell differentiation. Nat Rev
Immunol. 9:116–124. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Adler SH, Chiffoleau E, Xu L, Dalton NM,
Burg JM, Wells AD, Wolfe MS, Turka LA and Pear WS: Notch signaling
augments T cell responsiveness by enhancing CD25 expression. J
Immunol. 171:2896–2903. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Palaga T, Miele L, Golde TE and Osborne
BA: TCR-mediated Notch signaling regulates proliferation and
IFN-gamma production in peripheral T cells. J Immunol.
171:3019–3024. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hoyne GF, Dallman MJ and Lamb JR: T-cell
regulation of peripheral tolerance and immunity: The potential role
for Notch signalling. Immunology. 100:281–288. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Asano N, Watanabe T, Kitani A, Fuss IJ and
Strober W: Notch 1 signaling and regulatory T cell function. J
Immunol. 180:2796–2804. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ostroukhova M, Qi Z, Oriss TB,
Dixon-McCarthy B, Ray P and Ray A: Treg-mediated immunosuppression
involves activation of the Notch-HES1 axis by membrane-bound
TGF-beta. J Clin Invest. 116:996–1004. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boks MA, Kager-Groenland JR, Haasjes MS,
Zwaginga JJ, van Ham SM and ten Brinke A: IL-10-generated
tolerogenic dendritic cells are optimal for functional regulatory T
cell induction - a comparative study of human clinical-applicable
DC. Clin Immunol. 142:332–342. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gregori S, Tomasoni D, Pacciani V,
Scirpoli M, Battaglia M, Magnani CF, Hauben E and Roncarolo MG:
Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic
DC-10 requires the IL-10-dependent ILT4HLA-G pathway. Blood.
116:935–944. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wing K, Fehérvári Z and Sakaguchi S:
Emerging possibilities in the development and function of
regulatory T cells. Int Immunol. 18:991–1000. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vigouroux S, Yvon E, Wagner HJ, Biagi E,
Dotti G, Sili U, Lira C, Rooney CM and Brenner MK: Induction of
antigen-specific regulatory T cells following overexpression of a
Notch ligand by human B lymphocytes. J Virol. 77:10872–10880. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kared H, Adle-Biassette H, Foïs E, Masson
A, Bach JF, Chatenoud L, Schneider E and Zavala F:
Jagged2-expressing hematopoietic progenitors promote regulatory T
cell expansion in the periphery through notch signaling. Immunity.
25:823–834. 2006. View Article : Google Scholar : PubMed/NCBI
|