Introduction
Schizophrenia (SCZ) is considered a heritable
disorder that affects brain responses to external stimuli (1,2).
This abnormality may cause certain psychological disorders and
cause a patient to withdraw from society. The precise etiological
factors associated with SCZ remain unknown. In clinical practice,
SCZ diagnosis predominantly depends on symptoms that are numerous
and debilitating (3). A number of
SCZ sub-types are distinguishable according to the symptoms that a
patient exhibits. Paranoid and undifferentiated SCZ are the most
common SCZ sub-types (4). However,
the causes of these two SCZ sub-types have remain elusive.
Studies have demonstrated that a number of
susceptibility genes may be associated with specific illness
sub-types (4). For example, the
dystrobrevin-binding protein 1 gene has been shown to be associated
with negative and cognitive symptoms in patients with SCZ (4). SCZ is a complex disorder that may be
caused by genetic and epigenetic factors (5). The symptoms associated with SCZ are
variable. Therefore, there is a requirement to identify biomarkers
that may indicate a patients' genetic pre-disposition to SCZ and
the possible environmental exposure risks associated with SCZ.
DNA methylation, one of the most common and
important epigenetic factors, is influenced by environmental
factors and regulates gene expression (6). Numerous studies have demonstrated
that DNA methylation is involved in SCZ (7–18).
For example, Reelin is a protein which is responsible for neuronal
connectivity and synaptic plasticity (19). Abnormal expression of Reelin may
result in cognitive deficits in SCZ (19). In brain samples from patients with
SCZ, mRNA and protein levels of Reelin were shown to be reduced by
~50% in response to Reelin promoter hypermethylatation compared to
those in control samples (8).
However, to the best of our knowledge, studies on DNA methylation
in SCZ have predominantly focussed on protein-coding genes. Few
studies have investigated the involvement of long non-coding RNAs
(lncRNAs) in SCZ (20,21).
lncRNA is a type of non-coding RNA that is >200
nucleotides long. Sequence characteristics are similar to those of
mRNAs; however, they lack encoding capacity. In mammals, a number
lncRNAs have been shown to be associated with SCZ (22,23).
The functions of the majority of lncRNAs are unknown. However,
studies have demonstrated that lncRNAs are regulators of
expression, sub-cellular location and activity of certain
protein-coding genes. lncRNAs are associated with a number of
biological processes via protein-coding gene regulation, including
genomic imprinting control, cell differentiation, immune responses,
human diseases and tumorigenesis (24–26).
Therefore, alterations in lncRNA gene methylation may result in
differential expression patterns and, therefore, affect the
expression of target protein-coding genes. Usually, these lncRNAs
are anti-sense transcripts and their promoters are located in the
intronic region of the corresponding protein coding genes. DNA
methylation in lncRNA gene promotor regions may affect the
expression of their corresponding target genes by influencing
histone modification, which is mediated by binding to histone
modification proteins, including polycomb repressive complex 2 and
protein regulator of cytokinesis 1 (27). DNA methylation patterns of lncRNA
genes have been investigated in developmental biology and in
diseases, such as cancer (28,29).
However, to the best of our knowledge, no studies on DNA
methylation patterns of lncRNA genes in SCZ have been preformed. In
the present study, it was hypothesized that epigenetic alterations
of lncRNAs may be associated with SCZ.
In the present study, methylated DNA-binding
domain-sequencing (MBD-Seq) was used in order to identify DNA
hyper-methylated regions in male patients with paranoid or
undifferentiated SCZ. DNA methylation levels of protein-coding
genes and lncRNAs were measured in peripheral blood samples in
order to explore epigenetic biomarkers for diagnosis and provide a
foundation for the development of novel anti-psychotic drugs for
SCZ.
Materials and methods
Sample collection
Peripheral blood samples were obtained from Ningbo
Kangning Hospital (Zhejiang, China) in 2011. Patients were
clinically diagnosed by trained psychiatrists using the Diagnostic
and Statistical Manual of Mental Disorders, 4th Ed. (30). Case 1 refers to a male patient with
paranoid SCZ and case 2 refers to a male patient with
undifferentiated SCZ. Patients were verified to be without any
serious or unstable medical illness. A healthy male individual was
used as a negative control. Table
I lists the demographic and clinical characteristics of the
subjects in the present study. The negative control male and the
supervisors of SCZ patients provided their written informed consent
for the study. The present study was approved by the Human Research
Ethics Committees of Ningbo University and Ningbo Kangning Hospital
(Zhejiang, China).
 | Table IDemographic and clinical data. |
Table I
Demographic and clinical data.
| Characteristic | Control | Case 1 | Case 2 |
|---|
| Gender | Male | Male | Male |
| Age (years) | 25 | 34 | 22 |
| Diagnostic
subtype | NA | Paranoid |
Undifferentiated |
| Age at onset | NA | 19 | 11 |
| Family history | NA | Negative | Positive |
| Antipsychotic
drug | NA | Quetiapine | Clozapine |
| Psychotic
trauma | NA | Negative | Negative |
MBD enrichment and next-generation
sequencing
Methods for MBD-seq were similar to those described
in a previous study (31). Genomic
DNA was isolated from peripheral blood samples of the three
individuals using Qiagen Puregene kit (Qiagen, Hiden, Germany). DNA
was then sonicated into 200- to 400-bp fragments by 20 cycles of 30
sec sonication, using a Bioruptor sonicator (Diagenode, Liège,
Belgium). Subsequently, the active motif methyl collector kit
(Qiagen) was used to complete MBD2 enrichment. According to the
manufacturer's protocol, ~1 mg of sonicated genomic DNA was
incubated with MBD2-His-conjugated protein and magnetic beads. DNA
was purified for each biological replicate, and then pooled
post-enrichment. After enrichment, DNA purification columns
(Qiagen) were used to purify both the methylated fraction and
supernatant fractions, as reported previously (31). Finally, MBD2-enriched genomic DNA
from each sample was submitted for high-throughput sequencing
analysis. Libraries were sequenced twice using an Illumina Hiseq
2000 platform (Illumina, San Diego, CA, USA), obtaining two
datasets of 51- and 101-bp single-end reads, which were used
separately in order to identify the MBD2-bound fraction of the
human genome, respectively.
Data processing of MBD-seq
MBD-seq reads were filtered using FASTX-Toolkit
software with a cutoff of <Q20 in order to obtain high-quality
reads. Remaining reads were then mapped to the human genome (hg19;
ensembl) using bowtie and <2 mismatches (32). DNA methylation peaks for each
sample were identified using MACS software (Miltenyi Biotech,
Bergisch Gladbach, Germany) (33)
with a threshold of P<10–5. Reads from the healthy sample were
used as a negative control for the experiments.
Annotation of DNA methylation peaks
Genomic regions of each peak were compared with the
known genes in the hg19 reference genome, including coding and
lncRNAs, using the RefSeq database (http://www.ncbi.nlm.nih.gov/refseq/). The regions were
assigned to one of six classes associated with known genes using
HOMER software (http://homer.salk.edu/homer/microarray/) (34) The six classes of genome regions
were intergenic, 3′ untranslated regions (UTR), intron, exon,
5′UTR, promoter-transcript start site (promoter-TSS) and transcript
termination site (TTS). Promoter-TSS were identified from −1000 bp
to +100 bp, while TTS was identified from −100 bp to +1000 bp.
Subsequently, intergenic peaks identified using the RefSeq database
were compared with genomic regions of lncRNAs from NONCODE version
4.0 database (http://www.noncode.org/). Images of
peak visualization were obtained using Drompa 2.3.1 version
software (35).
CpG island analysis
Genomic locations of CpG islands were downloaded
from UCSC (https://genome.ucsc.edu/) (36). Genomic regions of each peak were
compared with those of CpG islands in order to identify peaks
overlapping with or containing CpG islands. The number of CG sites
was counted and compared with that in random genomic regions with
the same length distribution.
Gene ontology (GO) and pathway enrichment
analysis
GO terms and their corresponding depth levels in the
GO tree were obtained from the GO database (http://geneontology.org/page/go-database). For each GO
term, the P-value was calculated using a hyper-geometric test and
adjusted with false rate discovery using the 'multtest' package in
R statistics. Enriched GO terms with adjusted P-values <0.05
were then obtained. Enriched Kyoto Encyclopedia of Genes and
Genomes (http://www.genome.jp/kegg/) pathways
were obtained.
Results
Statistical information from the MBD-seq
dataset
Sequencing was repeated twice and two datasets of
MBD-seq were obtained for each SCZ sample. Sample reads were
filtered using a cutoff of Q20, which resulted in 40–75% of the
original reads, of which 65–90% matched with hg19 human genome
reads (Table II). Using MACS
software for peak calling, 1,236 and 1,552 peaks were obtained in
the first dataset from patients with paranoid and undifferentiated
SCZ, respectively. In the second dataset, 1,397 and 1,437 peaks
were obtained from patients with paranoid and undifferentiated SCZ,
respectively. The results of two datasets were compared by HOMER
software with default parameters. Compared with the second dataset,
there were 12.14 and 12.37% different peaks in the first dataset of
paranoid and undifferentiated SCZ, respectively. Peak regions from
the two datasets were then merged and peaks shared by the two
datasets were selected using HOMER software. Finally, 757 and 871
peaks were obtained from patients with paranoid and
undifferentiated SCZ, respectively. The average peak length was 288
and 431 bp in patients with paranoid SCZ and undifferentiated SCZ,
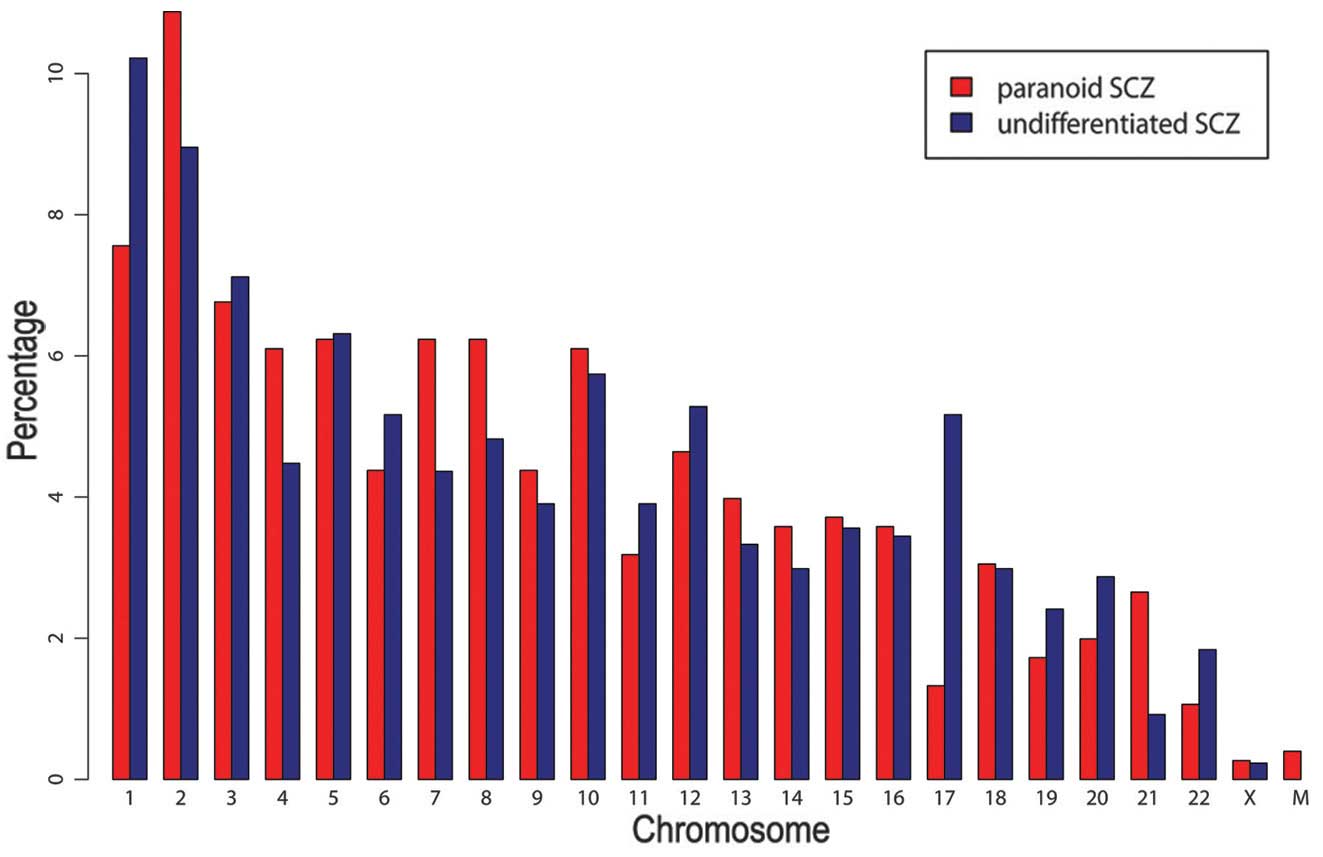
respectively. As demonstrated in Fig.
1, chromosome distributions in the two SCZ subtypes were
similar (pearson correlation test: r=0.84, P<0.01). G+C contents
and CG percentages were similar in the patient with paranoid SCZ
(G+C=49.0%; CG=1.9%) and that with undifferentiated SCZ (G+C=51.5%;
CG=2.7%). By contrast, G+C and CG contents of random sequences with
the same length distribution were markedly lower in the patients
with paranoid SCZ (G+C=37.3%; CG=0.8%) and undifferentiated SCZ
(G+C=38.4%; CG=0.9%), compared with those in the control patient.
Three and 19 peaks overlapped with CpG islands in the paranoid and
the undifferentiated SCZ sample. Among them, four peaks in the
undifferentiated SCZ sample were located in the gene promotor
regions, including tripartite motif containing 45 (TRIM45), major
histocompatibility complex, class II, DP α 1 (HLA-DPA1),
thioesterase super-family member 6 (THEM6) and chromosome 6 open
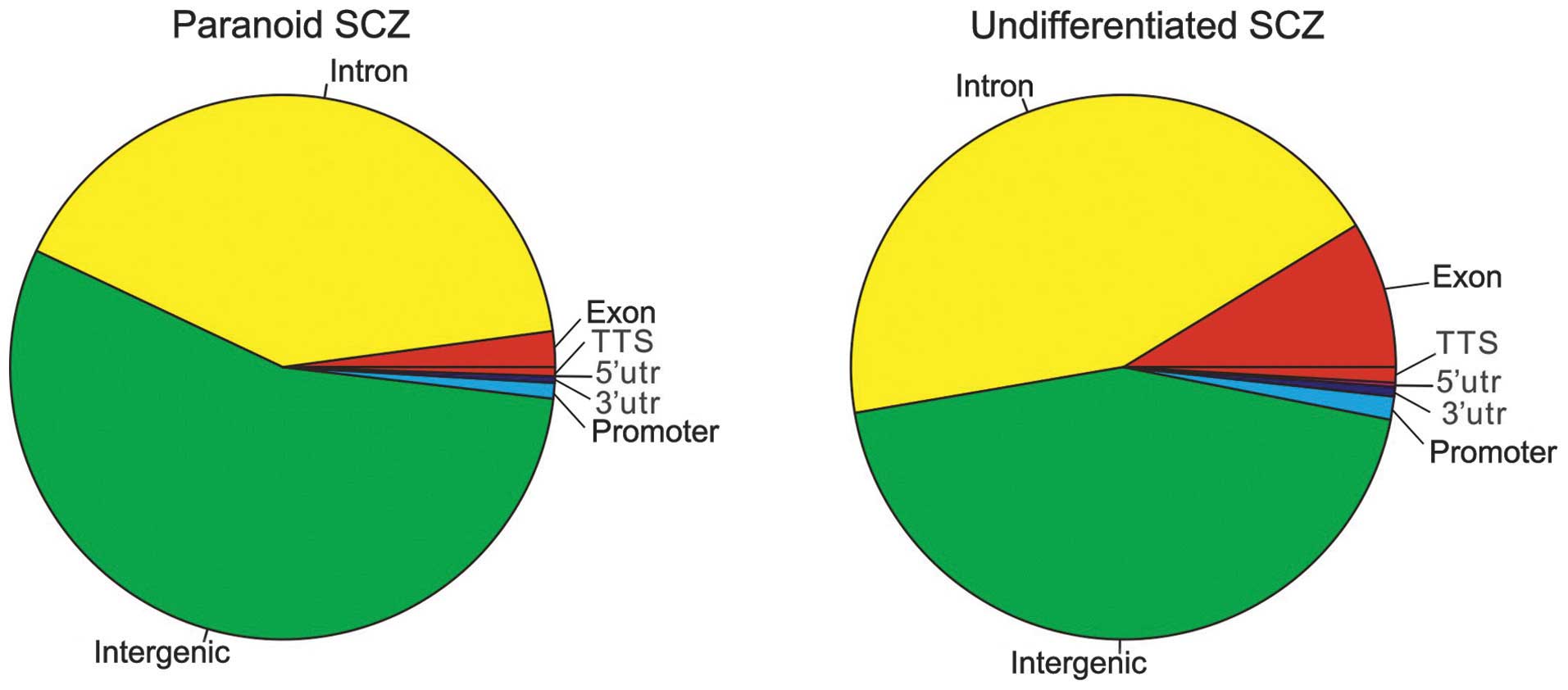
reading frame 123 (C6orf123). As demonstrated in Fig 2, the intergenic region was the most
enriched (paranoid SCZ, 54.96%; undifferentiated SCZ, 44.20%),
followed by the intron region (paranoid SCZ, 40.69%;
undifferentiated SCZ, 43.97%).
 | Table IIMethylated DNA-binding
domain-sequencing datasets. |
Table II
Methylated DNA-binding
domain-sequencing datasets.
| Data | Control | Paranoid SCZ | Undifferentiated
SCZ |
|---|
| Total reads
(n) | 30,901,427 | 8,720,205 | 20,384,242 |
| Reads post-Q20
filter, n (% of total) | 13,800,627
(45) | 4,017,085 (47) | 9,003,647 (45) |
| Read/match ratio
(%) | 75.69 | 81.43 | 76.34 |
| Unique read/match
ratio (%) | 66.48 | 72.83 | 67.61 |
| Peaks (n) | | 1,236 | 1,552 |
Hypermethylation peaks in SCZ are
predominantly enriched in genes characterized by neuron
function
There were 291 coding genes, 18 non-coding genes and
1 pseudogene in the paranoid SCZ sample, whilst 411 coding genes,
21 non-coding genes and 10 pseudogenes were observed in the
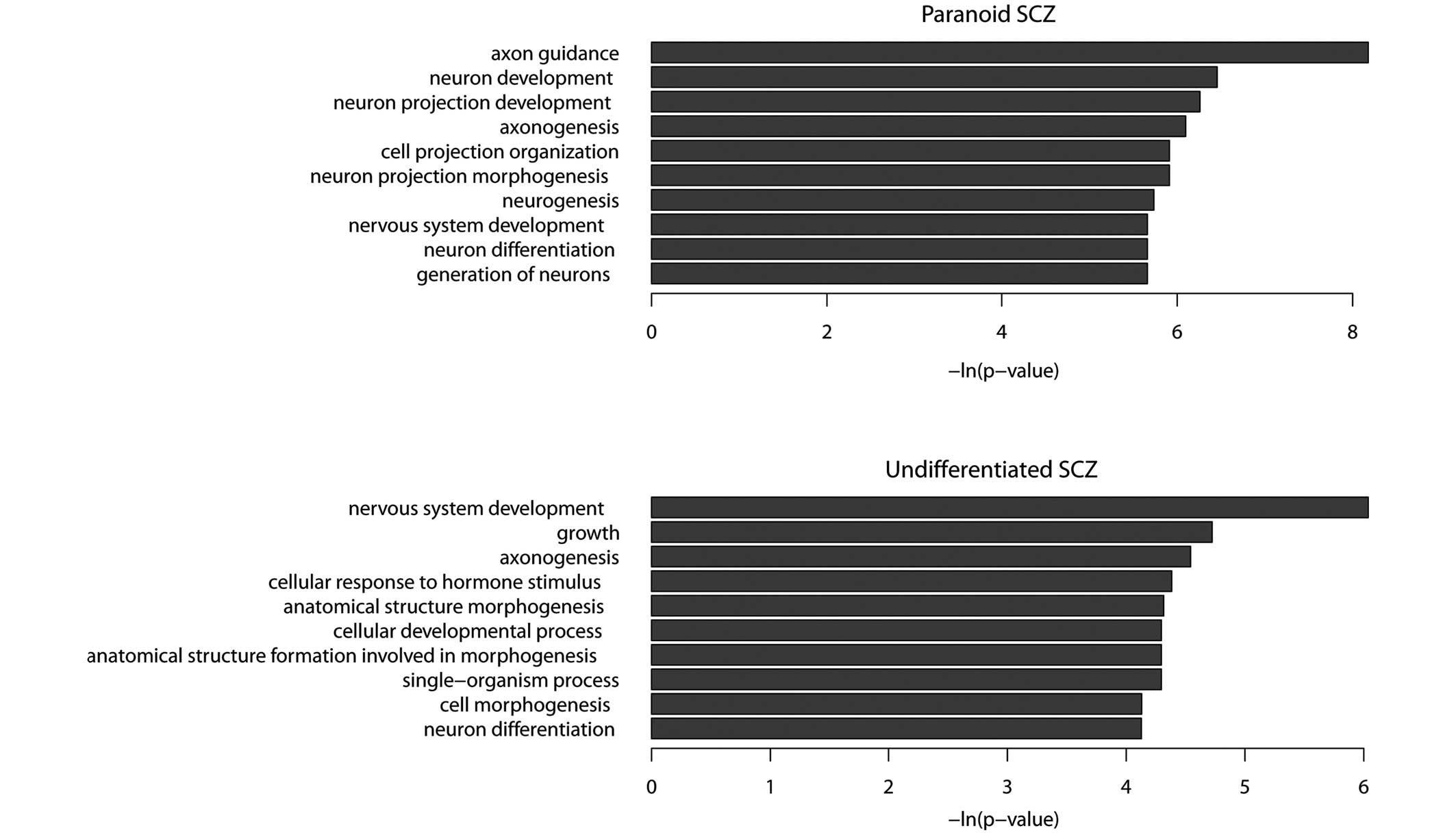
undifferentiated SCZ sample. GO term enrichment analysis
demonstrated that the functions of protein-coding genes in the
paranoid SCZ sample were predominantly associated with the nervous
system. The five most enriched GO terms were axon guidance, neuron
development, neuron projection development, axonogenesis and neuron
projection morphogenesis (P<0.01; Fig. 3A). A number of these GO terms are
associated with SCZ. For example, contactin-associated protein-like
2 has been shown to mediate cell-cell interactions in the nervous
system and is associated with SCZ (37,38)
and synapsin III, a member of the synaptic vesicle-associated
protein family, was shown to be a candidate gene for SCZ (39). Furthermore, hyper-methylated genes
have been shown to be enriched in several important pathways,
including the Wnt signaling pathway (40), calcium signaling pathway (41) and a number of pathways associated
with cancer (P<0.05).
The functions of hypermethylated protein-coding
genes observed in the undifferentiated SCZ sample were associated
with the nervous system, including nervous system development,
axonogenesis, neuron differentiation and cell morphogenesis
(Fig. 3B). There were 32
hypermethylated genes shared by paranoid and undifferentiated SCZ
data-sets (Table III). GO term
enrichment analysis demonstrated that these shared hypermethylated
genes were enriched in the following functions: Axon guidance,
axonogenesis, neuron projection morphogenesis and neuron projection
development. The results of the present study suggested that SCZ
may be caused by aberrant hypermethylation of a number of genes
associated with the nervous system.
 | Table IIIHypermethylation genes shared between
paranoid and undifferentiated SCZ samples. |
Table III
Hypermethylation genes shared between
paranoid and undifferentiated SCZ samples.
| Gene ID | Gene name | Gene
definition |
|---|
| 2139 | EYA2 | Eyes absent homolog
2 (Drosophila) |
| 2272 | FHIT | Fragile histidine
triad |
| 11240 | PADI2 | Peptidyl arginine
deiminase, type II |
| 100507421 | TMEM178B | Transmembrane
protein 178B |
| 5332 | PLCB4 | Phospholipase C, β
4 |
| 5184 | PEPD | Peptidase D |
| 84691 | FAM71F1 | Family with
sequence similarity 71, member F1 |
| 400941 | LINC00487 | Long intergenic
non-protein coding RNA 487 |
| 23261 | CAMTA1 | Calmodulin binding
transcription activator 1 |
| 84131 | CEP78 | Centrosomal protein
78kDa |
| 29994 | BAZ2B | Bromodomain
adjacent to zinc finger domain, 2B |
| 121256 | TMEM132D | Transmembrane
protein 132D |
| 57221 | KIAA1244 | KIAA1244 |
| 3680 | ITGA9 | Integrin, α 9 |
| 643650 | LOC643650 | Uncharacterized
LOC643650 |
| 3899 | AFF3 | AF4/FMR2 family,
member 3 |
| 10395 | DLC1 | Deleted in liver
cancer 1 |
| 3786 | KCNQ3 | Potassium
voltage-gated channel, KQT-like subfamily, member 3 |
| 29119 | CTNNA3 | Catenin
(cadherin-associated protein), α 3 |
| 1002 | CDH4 | Cadherin 4, type 1,
R-cadherin (retinal) |
| 54715 | RBFOX1 | RNA binding
protein, fox-1 homolog (Caenorhabditis elegans) 1 |
| 27086 | FOXP1 | Forkhead box
P1 |
| 169044 | COL22A1 | Collagen, type
XXII, α 1 |
| 256380 | SCML4 | Sex comb on
midleg-like 4 (Drosophila) |
| 25771 | TBC1D22A | TBC1 domain family,
member 22A |
| 10426 | TUBGCP3 | Tubulin, gamma
complex associated protein 3 |
| 124540 | MSI2 | Musashi homolog 2
(Drosophila) |
| 51151 | SLC45A2 | Solute carrier
family 45, member 2 |
| 10404 | CPQ | Carboxypeptidase
Q |
| 125336 | LOXHD1 | Lipoxygenase
homology domains 1 |
| 80216 | ALPK1 | α-kinase 1 |
| 92293 | TMEM132C | Transmembrane
protein 132C |
Protein-coding genes with hypermethylated
promoters
There were 7 (0.92%) and 12 (1.38%) peaks observed
in the promoter regions in the paranoid and undifferentiated SCZ
samples, respectively. The 7 genes in the paranoid SCZ sample were
zinc finger protein 641, RNA binding protein, fox-1 homolog
(RBFOX1), solute carrier family 45 member 2, ubiquitin-conjugating
enzyme E2Q family-like 1, cluster of differentiation 8a, retinoic
acid early transcript 1K pseudogene and GRAM domain containing 3.
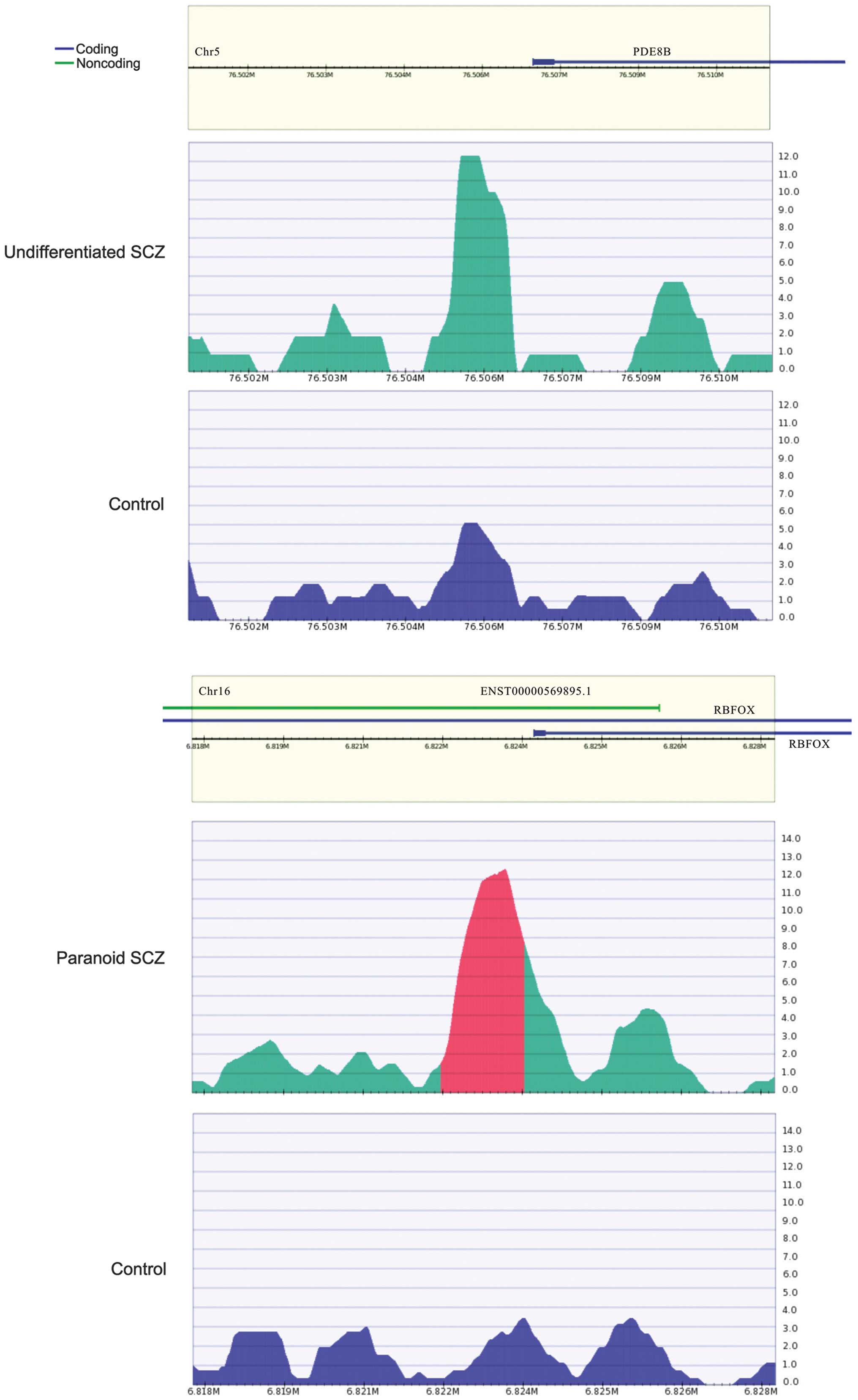
RBFOX1 (Fig. 4) is a type of RNA
binding protein that is capable of regulating alternative splicing.
RBFOX1 binds with ataxin-2, which causes familial neurodegenerative
diseases. Therefore, RBFOX1 is involved in neuron development
(42). It was hypothesized that
the hypermethylated RBFOX1 promoter may affect RBFOX1 expression,
thereby regulating biological processes involved in SCZ.
For the undifferentiated SCZ sample, genes with
hypermethylated promoters included claudin 5, prostaglandin
reductase 1, HLA-DPA1, C6ORF123, THEM6, phosphodiesterase 8B
(PDE8B), zinc finger protein 622, TRIM45, C11ORF16, polymerase
(RNA) I polypeptide E, tensin like C1 domain containing phosphatase
and potassium voltage-gated channel, delayed-rectifier, subfamily
S, member 2. Among these, PDE8B (Fig.
4) has been reported to be associated with SCZ (43). A study has demonstrated that PDE8B
expression is upregulated following treatment with olanzapine and
down-regulated following treatment with lithium, which are two
drugs that are used to treat schizoaffective disorders (44).
lncRNA genes and DNA
hypermethylation
A total of 17 hyper-methylated lncRNAs were
identified in the paranoid SCZ sample. Among them, 3 were shown to
be associated with cancer (disrupted in renal carcinoma 3, cancer
susceptibility candidate 2 and plasmacytoma variant translocation
1; data available on request). SCZ patients are more likely to
suffer from cancer compared with the general population. Therefore,
SCZ may lead to alterations in epigenetic modifications for a
number of cancer-associated genes and, therefore, increase the risk
of cancer (45). In the
undifferentiated SCZ sample, 20 lncRNAs were identified, including
5 anti-sense RNAs to protein-coding genes, which were shown to be
associated with SCZ. Sodium leak channel non-selective-anti-sense
RNA 1 (NALCN-AS1; data available on request) is the anti-sense of
NALCN that has been reported as a SCZ candidate gene (46–48).
Microtubule-associated protein tau-AS1 (MAPT-AS1; data available on
request) is the anti-sense lncRNA gene of MAPT that has been shown
to be associated with neurodegenerative disorders and SCZ (49). Guanine nucleotide binding protein
(G protein) α stimulating activity polypeptide 1-AS (GNAS-AS; data
available on request) is the anti-sense lncRNA gene of GNAS1 that
may influence a patients' susceptibility to SCZ (50). POU domain, class 4, transcription
factor 1-AS1 (POU4F1-AS1; data available on request) is the
anti-sense of POU4F1 that exhibits functions associated with the
nervous system (51).
The development of DNA sequencing technology has
enabled the identification of an increasing number of human
lncRNAs. Although their functions are currently unknown, lncRNAs
are likely to be transcribed and be involved in a number of
biological processes. The present study has identified a number of
peaks in the intergenic regions that may correspond to novel
lncRNAs. Therefore, lncRNAs were obtained from NONCODE v4.0 and
their genomic regions were compared with the peaks of the
intergenic regions identified in the present study. The results
identified 131 out of 416 (31.5%) and 114 out of 385 (29.6%)
intergenic peaks in the paranoid and undifferentiated SCZ,
respectively, corresponding to 434 and 320 lncRNAs from NONCODE
database for paranoid and undifferentiated SCZ, respectively. The
results of the present study suggested that intergenic
hypermethylation may influence lncRNA expression and function. The
results of the present study showed that lncRNA n373859 was
hypermethylated in the promoter region in paranoid SCZ. As shown in
the NONCODE database, lncRNA n373859 is highly expressed in brain
tissue. Further study is warranted to investigate whether the
aberrant promoter hypermethylation of lncRNA n373859 may lead to
downregulation in patients with paranoid SCZ.
Discussion
DNA methylation is associated with epigenetic
modifications and may be an important factor for SCZ. Aberrant
hypermethylation may contribute to abnormal gene expression and
influence the development of SCZ. Although a number of studies have
reported the effects of numerous important coding genes on DNA
methylation in SCZ, including reelin (8,9),
catechol-O-methyltransferase (10,11),
sex determining region Y (SRY)-box 10 (12), brain-derived neurotrophic factor
(16), dopamine receptor D4
(17) and cytotoxic
T-lymphocyte-associated protein 4 (17), there are limited studies on
lncRNAs. In the present study, a number of hypermethylated loci
were identified in protein-coding genes and lncRNAs in male
patients with SCZ. The results have provided novel insights for the
understanding of SCZ etiology.
In the present study, male patients were selected in
order to investigate candidate hypermethylated lncRNAs associated
with SCZ. Gender differences are common for a number of aspects of
SCZ, such as incidence and prevalence, the presentation and course
of the illness, and the various effects on brain development
(52). The onset of SCZ is delayed
in females and there is typically a second peak in female patients
at >45 years of age (53).
Estrogen level reduction is considered to be associated with this
process (54). Males typically
suffer from SCZ more severely and are less responsive to medication
than females (52). A number of
reasons to explain the early onset in men compared with women have
been proposed, including slower maturation and differences in
lateralization of the brain, greater incidence of head trauma and
the lack of neuroprotective effects from female hormones (52). Therefore, males are more
susceptible to SCZ than females (52,55–57).
Studies of hormones in female mammals have revealed that psychotic
symptoms may vary throughout the menstrual cycle, and that estrogen
may enhance neurocognitive performance and improves symptoms of SCZ
(58,59). Due to the gender differences
associated with SCZ, gender-specific characteristics and endocrine
gland side effects require further research in order to develop
anti-psychotic treatments. Advances in epigenetic research in SCZ
will provide novel insights into SCZ pathogenesis and the
development of medication for patients with SCZ.
SCZ pathophysiology research is often based on
individual genes and the environment (60). An epidemiological study suggested
that interactions between genes and the environment are involved in
the molecular mechanisms underlying the onset of SCZ (60). Epigenetic factors, including DNA
methylation, genomic imprinting, histone modifications and
non-coding RNA expression are involved in SCZ pathogenesis
(60–63). These factors may contribute to the
mechanisms associated with gender-specific genes and gender
dimorphism in SCZ (61).
DNA extracted from peripheral blood samples has been
shown to be informative for SCZ research. Dysregulation levels of a
number of SCZ candidate genes in peripheral blood samples are
associated with a number of clinical symptoms of SCZ (63,64).
In the present study, which used genome-wide methylation analyses,
DNA methylation levels of protein-coding genes and lncRNAs in
peripheral blood samples were investigated. Epigenetic biomarkers
were identified that may be used for the diagnosis and development
of anti-psychotic drugs for patients with SCZ.
The number of peaks that exhibited DNA
hypermethylation were similar between the different SCZ subtypes
(paranoid and undifferentiated). Hypermethylated genes were shown
to exhibit functions associated with the nervous system in paranoid
and undifferentiated SCZ. This suggested that DNA methylation may
affect the activity or expression of neuron-associated genes, and
thereby influence the development of SCZ. Hypermethylation of
promoters may affect the transcription of corresponding genes. In
the present study, 7 and 12 peaks were identified in the promoter
regions of Refseq genes in paranoid and undifferentiated SCZ
samples, respectively. The expression of these genes may be
affected by changes to DNA methylation and, therefore, influence
biological processes associated with SCZ. With the exception of the
peaks corresponding to coding genes, a number of peaks were shown
to correspond with known lncRNAs. Furthermore, ~30% of the
intergenic peaks that were identified corresponded with novel
lncRNAs, according to the RefSeq database. These results suggested
that lncRNAs may represent an important regulator for SCZ
pathogenesis.
In conclusion, hypermethylated coding and non-coding
genes were identified in samples from males with paranoid and
undifferentiated SCZ. The results of the present study may clarify
the mechanisms underlying SCZ pathogenesis.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 31100919,
81371469 and 31301084), Natural Science Foundation of Zhejiang
Province (grant nos. LR13H020003 and LQ13C060002), Natural Science
Foundation of Ningbo (grant no. 2013A610249), K.C. Wong Magna Fund
in Ningbo University, and Ningbo social development research
projects (grant no. 2012C50032).
References
|
1
|
Hall MH, Schulze K, Rijsdijk F, Picchioni
M, Ettinger U, Bramon E, Freedman R, Murray RM and Sham P:
Heritability and reliability of P300, P50 and duration mismatch
negativity. Behav Genet. 36:845–857. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uhlhaas PJ and Singer W: Abnormal neural
oscillations and synchrony in schizophrenia. Nat Rev Neurosci.
11:100–113. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tamminga CA and Holcomb HH: Phenotype of
schizophrenia: A review and formulation. Mol Psychiatry. 10:27–39.
2005. View Article : Google Scholar
|
|
4
|
Fanous AH and Kendler KS: Genetics of
clinical features and subtypes of schizophrenia: A review of the
recent literature. Curr Psychiatry Rep. 10:164–170. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stefansson H, Ophoff RA, Steinberg S,
Andreassen OA, Cichon S, Rujescu D, Werge T, Pietiläinen OP, Mors
O, Mortensen PB, et al: Genetic Risk and Outcome in Psychosis
(GROUP): Common variants conferring risk of schizophrenia. Nature.
460:744–747. 2009.PubMed/NCBI
|
|
6
|
Davies W: Genomic imprinting on the X
chromosome: Implications for brain and behavioral phenotypes. Ann
NY Acad Sci. 1204(Suppl): E14–E19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sharma RP, Grayson DR and Gavin DP:
Histone deactylase 1 expression is increased in the prefrontal
cortex of schizophrenia subjects: Analysis of the National Brain
Databank microarray collection. Schizophr Res. 98:111–117. 2008.
View Article : Google Scholar :
|
|
8
|
Grayson DR, Jia X, Chen Y, Sharma RP,
Mitchell CP, Guidotti A and Costa E: Reelin promoter
hypermethylation in schizophrenia. Proc Natl Acad Sci USA.
102:9341–9346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abdolmaleky HM, Cheng KH, Russo A, Smith
CL, Faraone SV, Wilcox M, Shafa R, Glatt SJ, Nguyen G, Ponte JF, et
al: Hypermethylation of the reelin (RELN) promoter in the brain of
schizophrenic patients: A preliminary report. Am J Med Genet B
Neuropsychiatr Genet. 134B:60–66. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abdolmaleky HM, Cheng KH, Faraone SV,
Wilcox M, Glatt SJ, Gao F, Smith CL, Shafa R, Aeali B, Carnevale J,
et al: Hypomethylation of MB-COMT promoter is a major risk factor
for schizophrenia and bipolar disorder. Hum Mol Genet.
15:3132–3145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nohesara S, Ghadirivasfi M, Mostafavi S,
Eskandari MR, Ahmadkhaniha H, Thiagalingam S and Abdolmaleky HM:
DNA hypomethylation of MB-COMT promoter in the DNA derived from
saliva in schizophrenia and bipolar disorder. J Psychiatr Res.
45:1432–1438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iwamoto K, Bundo M, Yamada K, Takao H,
Iwayama-Shigeno Y, Yoshikawa T and Kato T: DNA methylation status
of SOX10 correlates with its downregulation and oligodendrocyte
dysfunction in schizophrenia. J Neurosci. 25:5376–5381. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tolosa A, Sanjuán J, Dagnall AM, Moltó MD,
Herrero N and de Frutos R: FOXP2 gene and language impairment in
schizophrenia: Association and epigenetic studies. BMC Med Genet.
11:1142010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pun FW, Zhao C, Lo WS, Ng SK, Tsang SY,
Nimgaonkar V, Chung WS, Ungvari GS and Xue H: Imprinting in the
schizophrenia candidate gene GABRB2 encoding GABA(A) receptor β(2)
subunit. Mol Psychiatry. 16:557–568. 2011. View Article : Google Scholar
|
|
15
|
Abdolmaleky HM, Yaqubi S, Papageorgis P,
Lambert AW, Ozturk S, Sivaraman V and Thiagalingam S: Epigenetic
dysregulation of HTR2A in the brain of patients with schizophrenia
and bipolar disorder. Schizophr Res. 129:183–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ikegame T, Bundo M, Sunaga F, Asai T,
Nishimura F, Yoshikawa A, Kawamura Y, Hibino H, Tochigi M, Kakiuchi
C, et al: DNA methylation analysis of BDNF gene promoters in
peripheral blood cells of schizophrenia patients. Neurosci Res.
77:208–214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kordi-Tamandani DM, Vaziri S, Dahmardeh N
and Torkamanzehi A: Evaluation of polymorphism, hypermeth-ylation
and expression pattern of CTLA4 gene in a sample of Iranian
patients with schizophrenia. Mol Biol Rep. 40:5123–5128. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng J, Wang Y, Zhou K, Wang L, Li J,
Zhuang Q, Xu X, Xu L, Zhang K, Dai D, et al: Male-specific
association between dopamine receptor D4 gene methylation and
schizophrenia. PLoS One. 9:e891282014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Folsom TD and Fatemi SH: The involvement
of Reelin in neuro-developmental disorders. Neuropharmacology.
68:122–135. 2013. View Article : Google Scholar :
|
|
20
|
Vučićević D, Schrewe H and Orom UA:
Molecular mechanisms of long ncRNAs in neurological disorders.
Front Genet. 5:482014.
|
|
21
|
Barry G, Briggs JA, Vanichkina DP, Poth
EM, Beveridge NJ, Ratnu VS, Nayler SP, Nones K, Hu J, Bredy TW, et
al: The long non-coding RNA Gomafu is acutely regulated in response
to neuronal activation and involved in schizophrenia-associated
alternative splicing. Mol Pyschiatry. 19:486–494. 2014. View Article : Google Scholar
|
|
22
|
Okazaki Y, Furuno M, Kasukawa T, Adachi J,
Bono H, Kondo S, Nikaido I, Osato N, Saito R, Suzuki H, et al:
Analysis of the mouse transcriptome based on functional annotation
of 60,770 full-length cDNAs. Nature. 420:563–573. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ota T, Suzuki Y, Nishikawa T, Otsuki T,
Sugiyama T, Irie R, Wakamatsu A, Hayashi K, Sato H, Nagai K, et al:
Complete sequencing and characterization of 21,243 full-length
human cDNAs. Nat Genet. 36:40–45. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mercer TR, Dinger ME, Sunkin SM, Mehler MF
and Mattick JS: Specific expression of long noncoding RNAs in the
mouse brain. Proc Natl Acad Sci USA. 105:716–721. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wilusz JE, Sunwoo H and Spector DL: Long
noncoding RNAs: Functional surprises from the RNA world. Genes Dev.
23:1494–1504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taft RJ, Pang KC, Mercer TR, Dinger M and
Mattick JS: Non-coding RNAs: Regulators of disease. J Pathol.
220:126–139. 2010. View Article : Google Scholar
|
|
27
|
Pandey RR, Mondal T, Mohammad F, Enroth S,
Redrup L, Komorowski J, Nagano T, Mancini-Dinardo D and Kanduri C:
Kcnq1ot1 antisense noncoding RNA mediates lineage-specific
transcriptional silencing through chromatin-level regulation. Mol
Cell. 32:232–246. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ibala-Romdhane S, Al-Khtib M, Khoueiry R,
Blachère T, Guérin JF and Lefèvre A: Analysis of H19 methylation in
control and abnormal human embryos, sperm and oocytes. Eur J Hum
Genet. 19:1138–1143. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chiesa N, De Crescenzo A, Mishra K, Perone
L, Carella M, Palumbo O, Mussa A, Sparago A, Cerrato F, Russo S, et
al: The KCNQ1OT1 imprinting control region and non-coding RNA: New
properties derived from the study of Beckwith-Wiedemann syndrome
and Silver-Russell syndrome cases. Hum Mol Genet. 21:10–25. 2012.
View Article : Google Scholar
|
|
30
|
American Psychiatric Association:
Diagnostic and statistical manual of mental disorders, text
revision (DSM-IV-TR). American Psychiatric Association; 4th
Edition. 2000
|
|
31
|
Hogart A, Lichtenberg J, Ajay SS, Anderson
S and Margulies EH: Genome-wide DNA methylation profiles in
hematopoietic stem and progenitor cells reveal overrepresentation
of ETS transcription factor binding sites. Genome Res.
22:1407–1418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Langmead B, Trapnell C, Pop M and Salzberg
SL: Ultrafast and memory-efficient alignment of short DNA sequences
to the human genome. Genome Biol. 10:R252009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Y, Liu T, Meyer CA, Eeckhoute J,
Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, et
al: Model-based analysis of ChIP-Seq (MACS). Genome Biol.
9:R1372008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Heinz S, Benner C, Spann N, Bertolino E,
Lin YC, Laslo P, Cheng JX, Murre C, Singh H and Glass CK: Simple
combinations of lineage-determining transcription factors prime
cis-regulatory elements required for macrophage and B cell
identities. Mol Cell. 38:576–589. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakato R, Itoh T and Shirahige K: DROMPA:
Easy-to-handle peak calling and visualization software for the
computational analysis and validation of ChIP-seq data. Genes
Cells. 18:589–601. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Karolchik D, Hinrichs AS, Furey TS, Roskin
KM, Sugnet CW, Haussler D and Kent WJ: The UCSC Table Browser data
retrieval tool. Nucleic Acids Res. 32:D493–D496. 2004. View Article : Google Scholar :
|
|
37
|
Ji W, Li T, Pan Y, Tao H, Ju K, Wen Z, Fu
Y, An Z, Zhao Q, Wang T, et al: CNTNAP2 is significantly associated
with schizophrenia and major depression in the Han Chinese
population. Psychiatry Res. 207:225–228. 2013. View Article : Google Scholar
|
|
38
|
Rodenas-Cuadrado P, Ho J and Vernes SC:
Shining a light on CNTNAP2: Complex functions to complex disorders.
Eur J Hum Genet. 22:171–178. 2014. View Article : Google Scholar :
|
|
39
|
Kao HT, Porton B, Czernik AJ, Feng J, Yiu
G, Häring M, Benfenati F and Greengard P: A third member of the
synapsin gene family. Proc Natl Acad Sci USA. 95:4667–4672. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bousman CA, Glatt SJ, Chandler SD, Lohr J,
Kremen WS, Tsuang MT and Everall IP: Negative symptoms of psychosis
correlate with gene expression of the Wnt/β-catenin signaling
pathway in peripheral blood. Psychiatry J. 2013:8529302013.
View Article : Google Scholar
|
|
41
|
Berridge MJ: Dysregulation of neural
calcium signaling in Alzheimer disease, bipolar disorder and
schizophrenia. Prion. 7:2–13. 2013. View Article : Google Scholar :
|
|
42
|
Fogel BL, Wexler E, Wahnich A, Friedrich
T, Vijayendran C, Gao F, Parikshak N, Konopka G and Geschwind DH:
RBFOX1 regulates both splicing and transcriptional networks in
human neuronal development. Hum Mol Genet. 21:4171–4186. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fatemi SH, Folsom TD, Reutiman TJ and
Vazquez G: Phosphodiesterase signaling system is disrupted in the
cerebella of subjects with schizophrenia, bipolar disorder, and
major depression. Schizophr Res. 119:266–267. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fatemi SH, Folsom TD, Reutimann TJ, Braun
NN and Lavergne LG: Levels of phosphodiesterase 4A and 4B are
altered by chronic treatment with psychotropic medications in rat
frontal cortex. Synapse. 64:550–555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Irwin KE, Henderson DC, Knight HP and Pirl
WF: Cancer care for individuals with schizophrenia. Cancer.
120:323–334. 2014. View Article : Google Scholar
|
|
46
|
Wang KS, Liu XF and Aragam N: A
genome-wide meta-analysis identifies novel loci associated with
schizophrenia and bipolar disorder. Schizophr Res. 124:192–199.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Teo C, Zai C, Borlido C, Tomasetti C,
Strauss J, Shinkai T, Le Foll B, Wong A, Kennedy JL and De Luca V:
Analysis of treatment-resistant schizophrenia and 384 markers from
candidate genes. Pharmacogenet Genomics. 22:807–811. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Al-Sayed MD, Al-Zaidan H, Albakheet A,
Hakami H, Kenana R, Al-Yafee Y, Al-Dosary M, Qari A, Al-Sheddi T,
Al-Muheiza M, et al: Mutations in NALCN cause an
autosomal-recessive syndrome with severe hypotonia, speech
impairment, and cognitive delay. Am J Hum Genet. 93:721–726. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dobson-Stone C, Polly P, Korgaonkar MS,
Williams LM, Gordon E, Schofield PR, Mather K, Armstrong NJ, Wen W,
Sachdev PS and Kwok JB: GSK3B and MAPT polymorphisms are associated
with grey matter and intracranial volume in healthy individuals.
PLoS One. 8:e717502013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Minoretti P, Politi P, Coen E, Di Vito C,
Bertona M, Bianchi M and Emanuele E: The T393C polymorphism of the
GNAS1 gene is associated with deficit schizophrenia in an Italian
population sample. Neurosci Lett. 397:159–163. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zou M, Li S, Klein WH and Xiang M:
Brn3a/Pou4f1 regulates dorsal root ganglion sensory neuron
specification and axonal projection into the spinal cord. Dev Biol.
364:114–127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Abel KM, Drake R and Goldstein JM: Sex
differences in schizophrenia. Int Rev Psychiatry. 22:417–428. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lindamer LA, Lohr JB, Harris MJ and Jeste
DV: Gender, estrogen, and schizophrenia. Psychopharmacol Bull.
33:221–228. 1997.PubMed/NCBI
|
|
54
|
Mellios N, Galdzicka M, Ginns E, Baker SP,
Rogaev E, Xu J and Akbarian S: Gender-specific reduction of
estrogen-sensitive small RNA, miR-30b, in subjects with
schizophrenia. Schizophr Bull. 38:433–443. 2012. View Article : Google Scholar :
|
|
55
|
Boksa P: Animal models of obstetric
complications in relation to schizophrenia. Brain Res Brain Res
Rev. 45:1–17. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Missios S, Harris BT, Dodge CP, Simoni MK,
Costine BA, Lee YL, Quebada PB, Hillier SC, Adams LB and Duhaime
AC: Scaled cortical impact in immature swine: Effect of age and
gender on lesion volume. J Neurotrauma. 26:1943–1951. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wilson C and Terry AV Jr:
Neurodevelopmental animal models of schizophrenia: Role in novel
drug discovery and development. Clin Schizophr Relat Psychoses.
4:124–137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cosimo Melcangi R and Garcia-Segura LM:
Sex-specific therapeutic strategies based on neuroactive steroids:
In search for innovative tools for neuroprotection. Horm Behav.
57:2–11. 2010. View Article : Google Scholar
|
|
59
|
Kulkarni J, Gurvich C, Lee SJ, Gilbert H,
Gavrilidis E, de Castella A, Berk M, Dodd S, Fitzgerald PB and
Davis SR: Piloting the effective therapeutic dose of adjunctive
selective estrogen receptor modulator treatment in postmenopausal
women with schizophrenia. Psychoneuroendocrinology. 35:1142–1147.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Roth TL, Lubin FD, Sodhi M and Kleinman
JE: Epigenetic mechanisms in schizophrenia. Biochim Biophys Acta.
1790:869–877. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Feng J, Sun G, Yan J, Noltner K, Li W,
Buzin CH, Longmate J, Heston LL, Rossi J and Sommer SS: Evidence
for X-chromosomal schizophrenia associated with microRNA
alterations. PLoS One. 4:e61212009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Beveridge NJ and Cairns MJ: MicroRNA
dysregulation in schizophrenia. Neurobiol Dis. 46:263–271. 2012.
View Article : Google Scholar
|
|
63
|
Melas PA, Rogdaki M, Ösby U, Schalling M,
Lavebratt C and Ekström TJ: Epigenetic aberrations in leukocytes of
patients with schizophrenia: Association of global DNA methylation
with antipsychotic drug treatment and disease onset. FASEB J.
26:2712–2718. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lai CY, Yu SL, Hsieh MH, Chen CH, Chen HY,
Wen CC, Huang YH, Hsiao PC, Hsiao CK and Liu CM: MicroRNA
expression aberration as potential peripheral blood biomarkers for
schizophrenia. PLoS One. 6:e216352011. View Article : Google Scholar : PubMed/NCBI
|