Introduction
Ewing's sarcoma (ES) is the second most common type
of primary bone and soft malignant tumor, often occurs in children
and adolescents, and is characterized by a pathognomonic
chromosomal translocation known as t(11;22) (q24;q12) or t(21;22)
(q22;q12) (1,2). Despite rapid advances in modern
biomedicinal therapy, the 5-year survival rate of patients with ES
has only reached 50–60% (3,4).
Numerous studies have focused on investigating the underlying
mechanism of ES, as well as therapeutic targets for patients with
ES; however, the process of ES carcinogenesis remains largely
unknown (5–9). Therefore, developing an effective
therapeutic strategy for the treatment of ES is critical for young
patients.
Insulin-like growth factor 1 receptor (IGF-1R)
represents an important therapeutic target in the pathogenesis of
ES cells, and is regarded to be an effective biological therapy for
ES (10–12). A previous study reported that
mutations in IGF-1R may induce apoptosis and inhibit tumorigenesis,
as well as enhance chemosensitivity in ES cells (13). Previous studies involving the
inhibition of IGF-1R to regulate cell proliferation and apoptosis
were also conducted (14,15). Increasing evidence suggests that
the IGF-1R inhibitor NVP-AEW541 may possess the ability to enhance
cell apoptosis, inhibit proliferation or arrest the cell cycle in
ES (16,17). Therefore, identifying more
effective inhibitors of IGF-1R may prove to be advantageous in the
prevention of ES.
Picropodophyllin (PPP), an epimer of
podophyllotoxin, may be a novel selective inhibitor of IGF-1R. PPP
strongly inhibits cell growth in various types of cancer, including
lymphoma, asopharyngeal carcinoma and colorectal carcinoma
(18–21). Although PPP induces apoptosis in
cultured IGF-1R-positive tumor cells, the mechanism underlying
these effects remains to be elucidated (22). In addition, its effect on ES and
the underlying mechanism also remain to be clarified. In the
present study, the effects of PPP on the proliferation and
apoptosis of ES cell lines was investigated, along with the
associated signaling pathway.
Materials and methods
Cells and culture
A673 and SK-ES-1 human ES cell lines were obtained
from the American Type Culture Collection (Manassas, VA, USA). The
A673 cells (5×105) were maintained in RPMI-1640 medium
(Gibco Life Technologies, Carlsbad, CA, USA) supplemented with 10%
fetal bovine serum (FBS; Invitrogen Life Technologies, Carlsbad,
CA, USA), streptomycin (100 mg/ml; Shanghai Sangon Biological
Engineering Technology & Services Co., Ltd., Shanghai, China),
and penicillin (100 U/ml; Shanghai Sangon Biological Engineering
Technology & Services Co., Ltd.) in a 5% (v/v) CO2
incubator at 37°C. The SK-ES-1 cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Invitrogen Life Technologies)
supplemented with 15% FBS. The cells were also incubated in a
humidified atmosphere containing 5% (v/v) CO2 at
37°C.
Cell viability assay
An MTT assay was used to determine the effects of
PPP or fumonisin B1 (FB1) on the viability of the ES cell lines.
Briefly, the cells were seeded in 96-well plates (3×103
cells/200 µl) for 24 h. The cells were treated with various
concentrations (0.05, 0.1, 0.2, 0.4 and 0.8 µM) of PPP (cat.
no. UNO-000037; 99% pure; UNO, Zhongshan, China) or 50 µM
FB1 (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 48 h. MTT
solution (Sigma-Aldrich, St. Louis, MO, USA) at a final
concentration of 0.5 mg/ml was subsequently added and the samples
were incubated for a further 4 h at 37°C. The medium (RMPI-1640 or
DMEM) was then discarded, and 200 µl dimethyl sulfoxide
(Gibco Life Technologies) was added to dissolve the formazan dye
crystals for 15 min. Absorbance was finally measured at 570 nm
using a microplate reader (Molecular Devices, LLC, Sunny Vale, CA,
USA) with a reference wavelength of 630 nm. The results were
expressed as a percentage of the MTT reduction, and assumed that
the absorbance of the control cells was 100%. Each experiment was
performed in triplicate.
Cell proliferation analysis
A bromodeoxyuridine (BrdU) Cell Staining kit
(Invitrogen Life Technologies) was used to investigate the effects
of PPP on ES cell proliferation, according to the manufacturer's
instructions. Briefly, the cells were seeded in 96-well plates
(5×103 cells/well) for 24 h, and exposed to various
concentrations of PPP for a further 48 h. The cells were then fixed
with 10 µl BrdU for 5 h, and the medium (RMPI-1640 or DMEM)
was discarded prior to the addition of 100 µl/well
fixing/denaturing solution (Beyotime Institute of Biotechnology,
Nantong, China), incubated at room temperature for 15 min. The
solution was then removed and 100 µl/well prepared detection
antibody solution (mouse anti-human BrdU monoclonal antibody) was
added and incubated for 1 h at room temperature. The plates were
then washed three times with phosphate-buffered saline (PBS),
followed by the addition of 100 µl/well horseradish
peroxidase (HRP)-conjugated secondary antibody solution, incubated
for 30 min at room temperature. The plates were further washed
three times with washing buffer, and 100 µl
3,3′,5,5′-tetramethylbenzidine substrate was added, and incubated
for 30 min at room temperature. The quantity of BrdU incorporated
into the cells was determined at 450 nm using a microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Apoptosis analysis
The cell lines cultured in RPMI-1640 were seeded in
96-well plates (2×104 cells/well), treated with PPP or
FB1 for 24 h, and harvested with trypsin (Gibco Life Technologies).
Following two washes with PBS on ice, the cells were incubated with
fluorescein isothiocyanate-conjugated Annexin V (Sigma-Aldrich) in
binding buffer (50 mM HEPES, 700 mM NaCl, 12.5 mM CaCl2,
pH 7.4) for 20 min at 37°C in the dark. The cells were then washed
with PBS, and incubated with 10 ml phosphatidylinositol (PI)
solution (1 mg/ml; Sigma-Aldrich) for 20 min at room temperature in
the dark. The cells were then analyzed at 488 nm and 633 nm using a
FACScan Flow Cytometer (BD Biosciences, Franklin Lakes, NJ, USA) to
determine the relative apoptosis levels.
Western blot analysis
For western blot analysis, the cells were lysed with
2X lysis buffer containing 250 mM Tris-HCl (pH 6.5), 2% SDS, 4%
β-mercaptoethanol, 0.02% bromphenol blue and 10% glycerol. Protein
concentration was determined using a Bicinchoninic Acid Protein
Assay kit (Bio-Rad Laboratories, Inc.) and equal quantities of
protein were analyzed by SDS-PAGE (20 mg/lane) on a 5% stacking gel
and a 10% separating gel (Beyotime Institute of Biotechnology),
prior to being transferred onto polyvinylidene difluoride membranes
(EMD Millipore, Billerica, MA, USA) at 10 V for 30 min. The
membranes were blocked for 2 h with 5% non-fat dry milk in
Tris-buffered saline containing 0.1% Tween-20 (Beyotime Institute
of Biotechnology) (TBST), and incubated at 4°C overnight with the
following primary antibodies: Rabbit anti-human Akt (cat. no. 9272;
Cell Signaling Technology, Inc., Danvers, MA, USA; 1:1,000), rabbit
anti-human phosphorylated (p)-Akt (cat. no. SAB4301497;
Sigma-Aldrich; 1:800), rabbit anti-human p-IGF-1R (cat. no. I2033;
Sigma-Aldrich; 1:500), rabbit anti-human IGF-1R (cat. no. 3027;
Cell Signaling Technology, Inc.; 1:800) and mouse anti-human
β-actin (cat. no. 3700; Cell Signaling Technology, Inc.; 1:1,000).
Following washing with TBST, the membranes were incubated with
HRP-conjugated goat anti-rabbit IgG (cat. no. A0545; Sigma-Aldrich;
1:80,000) or HRP-conjugated horse anti-mouse IgG (cat. no. 7076;
Cell Signaling Technology, Inc.; 1:20,000) secondary antibodies
targeting rabbit or mouse in TBST for 45 min at room temperature.
Following three washes with TBST, the proteins were developed using
an Enhanced Chemiluminescence kit (GE Healthcare Life Sciences,
Chalfont, UK). Detection was performed using an Enhanced
Chemiluminescence system (EMD Millipore).
Statistical analysis
The results are expressed as the mean ± standard
deviation of at least three independent experiments. Statistical
analysis was conducted using SPSS 16.0 (SPSS, Inc., Chicago, IL,
USA). The statistical significance of the differences between the
control and drug-treated groups were evaluated using an unpaired
Student's t-test. P<0.05 was considered to indicated a
statistically significant result.
Results
PPP inhibits cell viability in a dose-
and time-dependent manner
Cell viability evaluation is one of the most
important steps in the quality control process for therapeutic drug
use (23). To investigate the
effects of PPP on ES, an MTT assay was first used to assess the
influence of PPP on the cell growth of the A673 and SK-ES-1 ES cell
lines. As shown in Fig. 1A, PPP
inhibited A673 and SK-ES-1 cell viability in a dose-dependent
manner, and the half maximal inhibitory concentration
(IC50) values for the A673 and SK-ES-1 cell lines were
0.42 and 0.48 µM, respectively. Further analysis determined
that PPP exhibited time-dependent inhibitory effects on A673 and
SK-ES-1 cell viability. As shown in Fig. 1B, the percentage of A673 and
SK-ES-1 cell viability declined significantly at 24 h, and the
rates of cell viability were 49 and 52%, respectively, as compared
with the control (P<0.05). These results suggest that PPP
inhibits cell viability in a dose- and time-dependent manner.
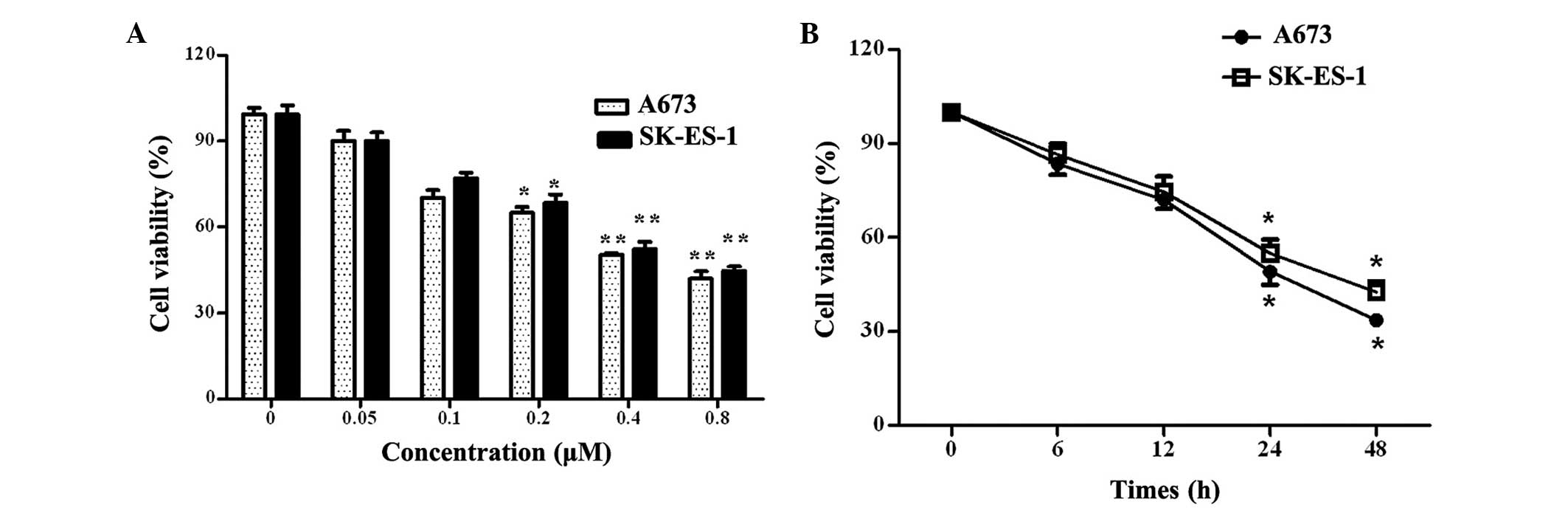 | Figure 1Effects of PPP on Ewing's sarcoma cell
viability in vitro. (A) A673 and SK-ES-1 cell lines were
treated with various concentrations of PPP (0, 0.05, 0.1, 0.2, 0.4
and 0.8 µM). A total of 48 h later, an MTT assay was
performed to analyze cell viability. The error bars represent the
mean ± standard deviation. (B) Following treatment with 0.4
µM PPP for 6, 12, 24 or 48 h, cell viability was detected
using an MTT assay. *P<0.05 and
**P<0.01, vs. the control group. PPP,
picropodophyllin. |
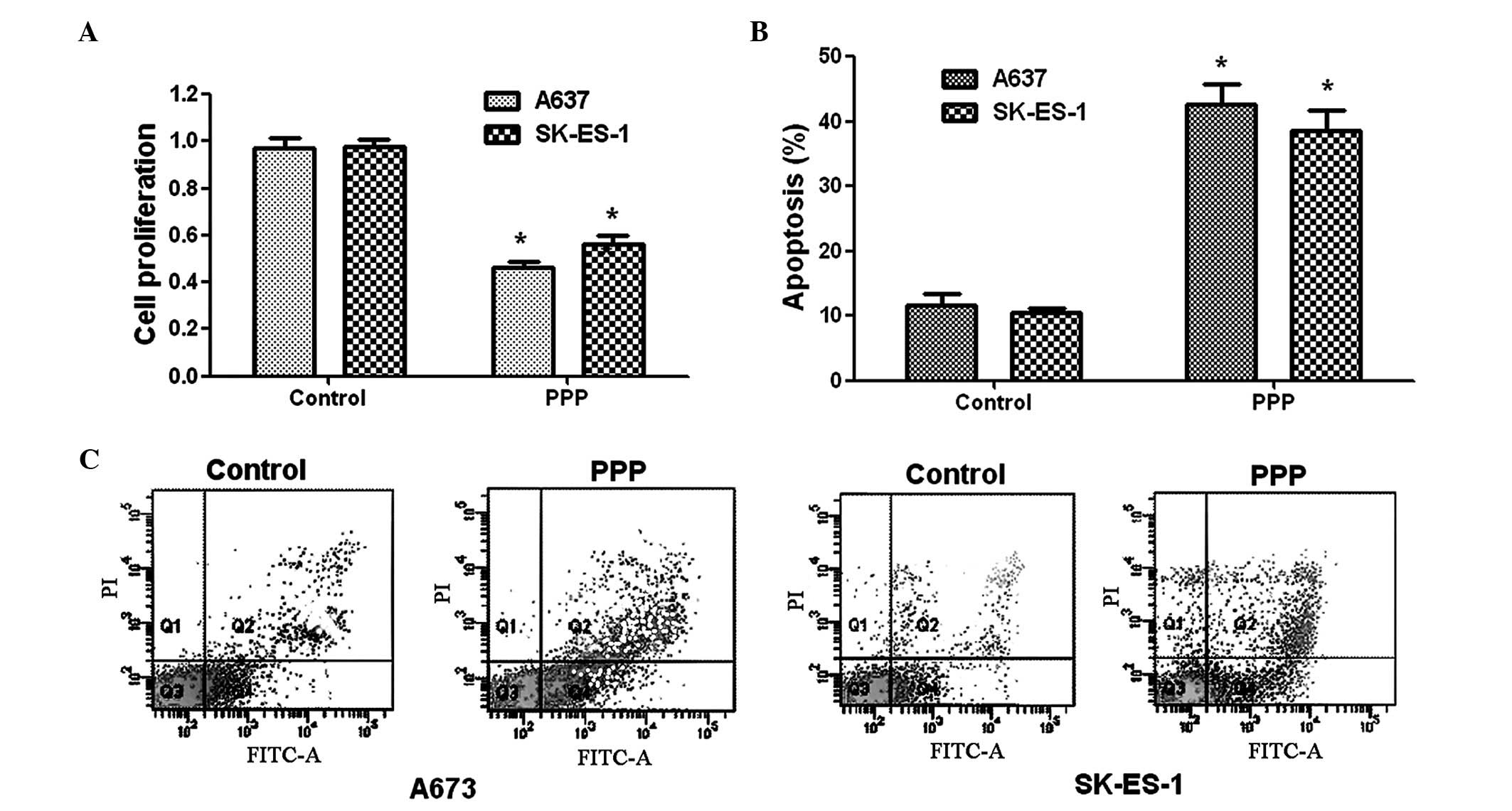
Effects of PPP on cell proliferation
To evaluate the effects of PPP on ES cell
proliferation, a BrdU cell staining kit was used. As shown in
Fig. 2A, PPP was able to inhibit
>50% of A673 cells at an value IC50 of 0.42
µM. Although the effect of PPP on the SK-ES-1 cells was more
marked than that on the A673 cells, cell viability still decreased
by 44% in the A673 cells. These results suggest that PPP is able to
inhibit ES cell survival.
PPP treatment induces ES cell
apoptosis
To further evaluate the effect of PPP on cell
apoptosis, Annexin V/PI double staining was performed on the A673
and SK-ES-1 cells. The SK-ES-1 cell apoptotic rates were
significantly increased from 10.1 (control) to 38.5% (PPP-treated)
(Fig. 2B). The apoptotic rates
induced by PPP are shown in Fig.
2C. The apoptotic rates in the A673 cells were significantly
increased from 11.2% (control) to 40.8% (PPP-treated) following
treatment with 0.42 µM PPP. These data demonstrated the
effect of PPP on cell apoptosis in the A673 and SK-ES-1 cells.
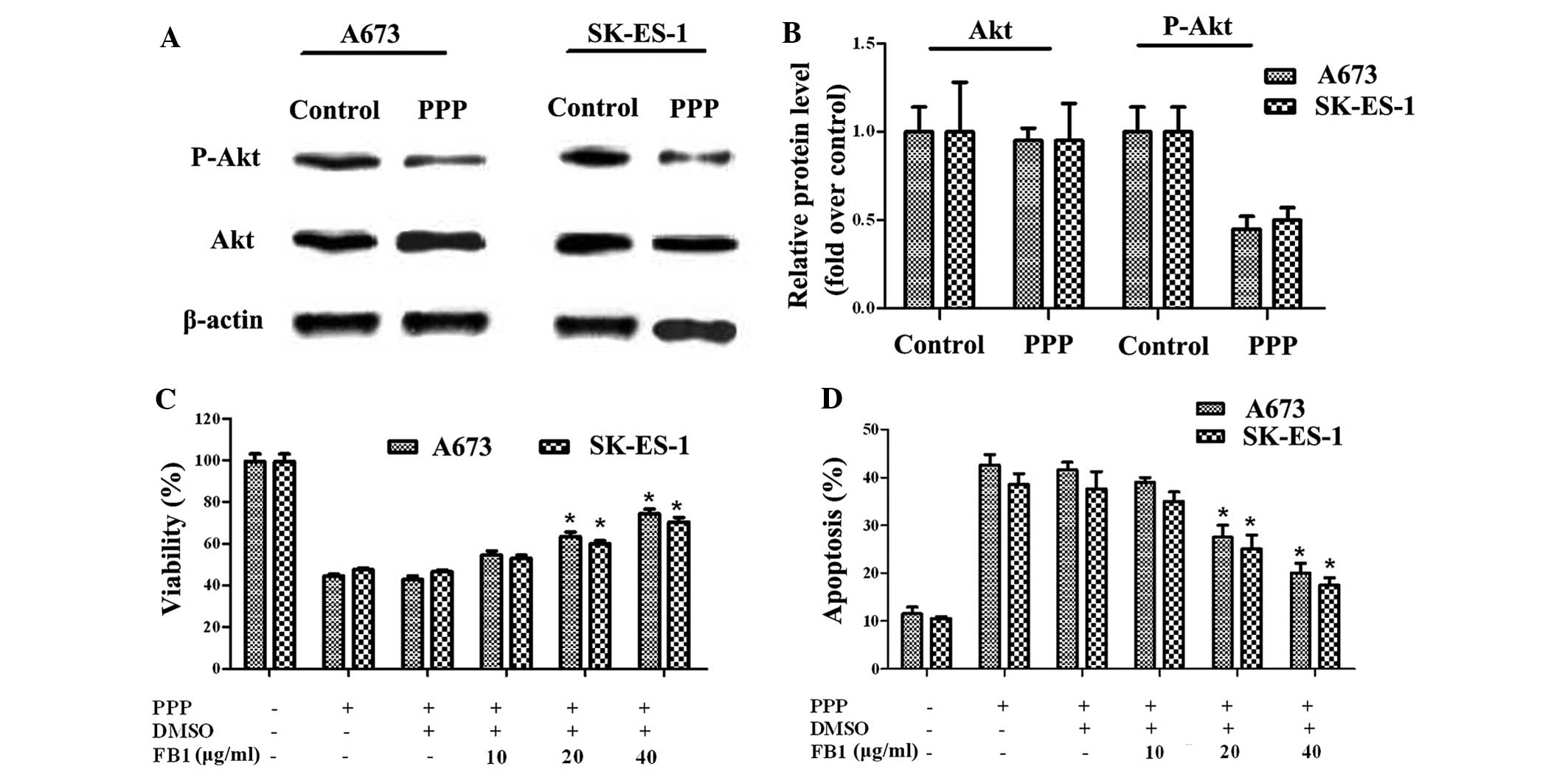
PPP blocks ES cell growth through Akt
signaling
The Akt signaling pathway has an important role in
cell progression, including proliferation and apoptosis. The
activation of Akt improves the survival of ES cell lines (24). To explore the mechanism underlying
the effects of PPP on cell growth, the effects of PPP on Akt
expression were detected in the A673 and SK-ES-1 cell lines. As
shown in Fig. 3A and B, the total
Akt levels remained unchanged, whereas the phosphorylation levels
of Akt markedly decreased in the two cell lines. Therefore, PPP may
inhibit ES cell growth via Akt signaling. To further verify this
hypothesis, FB1, a specific activator of Akt, was used for
subsequent study. Following pre-treatment with FB1, the viability
of the A673 and SK-ES-1 cells was significantly increased. The
viability of the A673 cells increased from 42.8 to 63.5%, following
the addition of 20 µg/ml FB1. In addition, PPP-inhibited
cell viability in the SK-ES-1 cells increased from 44 to 53, 60 and
70.5%, respectively, following treatment with various doses of FB1
(Fig. 3C). Conversely, PPP-induced
cell apoptosis was decreased from 42.55 to 20% (40 µg/ml
FB1-treated groups) in the A673 cells, following treatment with
FB1. Furthermore, apoptotic rates in the SK-ES-1 cells also
decreased from 38.55 (PPP-treated) to 17.5% (40 µg/ml
FB1-treated; Fig. 3D). These
results suggest that PPP markedly inhibits A673 and SK-ES-1 cell
growth by blocking Akt signaling.
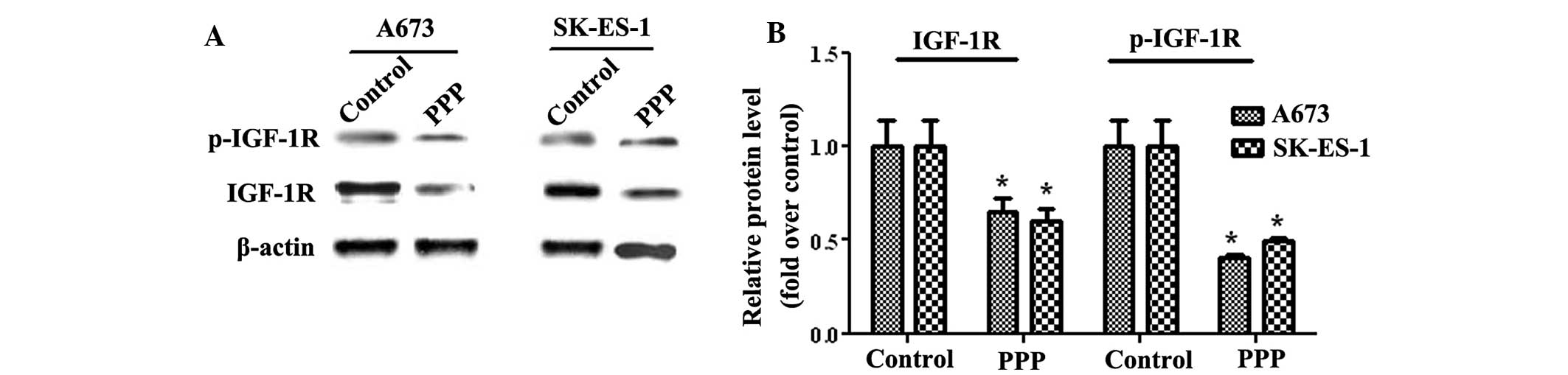
Effects of PPP on IGF-1R activation
IGF-1R is overexpressed in various tumors, including
breast tumors, prostate tumors and myeloma (25). In addition, IGF-1R has an important
role in the prevention of apoptosis by inducing the Akt signaling
transduction cascade (26). To
further investigate the PPP-regulated Akt signaling pathway in ES,
the expression levels of IGF-1R were analyzed. Total IGF-1R
expression levels in the A673 cells decreased by ~35% compared with
untreated cells, whereas SK-ES-1 cells exhibited a 40% decrease in
IGF-1R expression (Fig. 4).
Similarly, the phosphorylation levels of IGF-1R decreased by
>50%, as compared with those of the control cells in the two
cell lines. The A673 cells exhibited a 59% decrease in the
phosphorylation levels of IGF-1R, and the SK-ES-1 cells a 50.5%
decrease, as compared with the control group. As an inhibitor of
IGF-1R, PPP significantly reduced the expression and
phosphorylation levels of IGF-1R. These results suggest that PPP
regulates ES cell growth via the IGF-1R/Akt signaling pathway.
Discussion
ES is a relatively rare type of malignancy
predominantly occurring between the ages of four and 25 (27,28).
The aim of current research is to acquire a greater understanding
of the biological pathogenesis of ES, and to identify an effective
drug for the treatment of ES (29). Previous studies demonstrated that
PPP inhibits numerous types of cancers, including osteosarcoma and
human multiple myeloma (30,31);
however, no research has been performed to date on ES. To the best
of our knowledge, the present study is the first to investigate the
function of PPP in ES. The results of the present study
demonstrated that PPP induces proliferation inhibition and
apoptotic enhancement in human ES cell lines. Therefore, PPP may be
effective in the inhibition of ES, and merits further
investigation.
Numerous molecular studies have demonstrated that
the Akt signal transduction cascade usually participates in ES cell
progression, cell apoptosis, cell proliferation and drug
susceptibility (32–34). As an IFG-1R inhibitor, PPP was
found to have Akt inhibitory effects in neuroblastoma cell lines
(35). Furthermore, the efficacy
of PPP against multiple myeloma has also been demonstrated
(31). To further investigate the
mechanism underlying the cell growth inhibitory effects of PPP, the
present study investigated the Akt signaling pathway in ES. The
results indicated that PPP induced downregulation of p-Akt
expression by ~50%. The inhibitory effect of PPP on p-Akt was
recovered following treatment with an Akt-specific activator, FB1.
These results demonstrated that PPP was indeed able to inhibit
human ES survival by blocking the Akt signaling pathway.
Recently, IGF signaling has become a potential
target for novel anticancer agents (36,37).
IGF-1R is an activator of the Akt signaling pathway. The
IGF-1R-mediated Akt signaling pathway exhibited anticancer effects
in various types of cancer (38,39).
Baumgarten et al (40)
suggested that IGF-1R signaling may be required for Akt activation.
Previous studies have also demonstrated the association between
IFG-1R and Akt (41,42). In the present study, PPP appeared
to block IGF-1R phosphorylation. Consequently, it was hypothesized
that PPP may inhibit ES growth by inhibiting the IGF-1R-Akt
signaling pathway.
In conclusion, the present study demonstrated the
effectiveness of PPP in human ES. The inhibition of cell survival,
as well as the effective induction of apoptosis, led to further
investigation of the underlying mechanism. The IGF-1R/Akt signaling
pathway was shown to involve the PPP-induced survival in ES cells.
Therefore, the application of PPP may provide a novel therapeutic
strategy for the treatment of ES.
References
|
1
|
Iwamoto Y: Diagnosis and treatment of
Ewing's sarcoma. Jpn J Clin Oncol. 37:79–89. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Delattre O, Zucman J, Melot T, Garau XS,
Zucker JM, Lenoir GM, Ambros PF, Sheer D, Turc-Carel C and Triche
TJ: The Ewing family of tumors-a subgroup of small-round-cell
tumors defined by specific chimeric transcripts. N Engl J Med.
331:294–299. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Alava E and Gerald WL: Molecular
biology of the Ewing's sarcoma/primitive neuroectodermal tumor
family. J Clin Oncol. 18:204–213. 2000.PubMed/NCBI
|
|
4
|
Burgert EO Jr, Nesbit ME, Garnsey LA,
Gehan EA, Herrmann J, Vietti TJ, Cangir A, Tefft M, Evans R and
Thomas P: Multimodal therapy for the management of nonpelvic,
localized Ewing's sarcoma of bone: Intergroup study IESS-II. J Clin
Oncol. 8:1514–1524. 1990.PubMed/NCBI
|
|
5
|
Kontny U: Regulation of apoptosis and
proliferation in Ewing's sarcoma-opportunities for targeted
therapy. Hematol Oncol. 24:14–21. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanaka K, Iwakuma T, Harimaya K, Sato H
and Iwamoto Y: EWS-Fli1 antisense oligodeoxynucleotide inhibits
proliferation of human Ewing's sarcoma and primitive
neuroectodermal tumor cells. J Clin Invest. 99:239–247. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kinsey M, Smith R and Lessnick SL: NR0B1
is required for the oncogenic phenotype mediated by EWS/FL1 in
Ewing's sarcoma. Mol Cancer Res. 4:851–859. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kauer M, Ban J, Kofler R, Walker B, Davis
S, Meltzer P and Kovar H: A molecular function map of Ewing's
sarcoma. PLoS One. 4:e54152009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rocchi A, Manara MC, Sciandra M, Zambelli
D, Nardi F, Nicoletti G, Garofalo C, Meschini S, Astolfi A, Colombo
MP, et al: CD99 inhibits neural differentiation of human Ewing
sarcoma cells and thereby contributes to oncogenesis. J Clin
Invest. 120:668–680. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scotlandi K, Maini C, Manara MC, Benini S,
Serra M, Cerisano V, Strammiello R, Baldini N, Lollini PL, Nanni P,
et al: Effectiveness of insulin-like growth factor I receptor
antisense strategy against Ewing's sarcoma cells. Cancer Gene Ther.
9:296–307. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang HG, Jenabi JM, Liu XF, Reynolds CP,
Triche TJ and Sorensen PH: Inhibition of the insulin-like growth
factor I receptor by epigallocatechin gallate blocks proliferation
and induces the death of Ewing tumor cells. Mol Cancer Ther.
9:1396–1407. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Prieur A, Tirode F, Cohen P and Delattre
O: EWS/FLI-1 silencing and gene profiling of Ewing cells reveal
downstream oncogenic pathways and a crucial role for repression of
insulin-like growth factor binding protein 3. Mol Cell Biol.
24:7275–7283. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scotlandi K, Avnet S, Benini S, Manara MC,
Serra M, Cerisano V, Perdichizzi S, Lollini PL, De Giovanni C,
Landuzzi L and Picci P: Expression of an IGF-I receptor dominant
negative mutant induces apoptosis, inhibits tumorigenesis and
enhances chemosensitivity in Ewing's sarcoma cells. Int J Cancer.
101:11–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Benini S, Manara MC, Baldini N, Cerisano
V, Massimo Serra, Mercuri M, Lollini PL, Nanni P, Picci P and
Scotlandi K: Inhibition of insulin-like growth factor I receptor
increases the antitumor activity of doxorubicin and vincristine
against Ewing's sarcoma cells. Clin Cancer Res. 7:1790–1797.
2001.PubMed/NCBI
|
|
15
|
Scotlandi K, Benini S, Sarti M, Serra M,
Lollini PL, Maurici D, Picci P, Manara MC and Baldini N:
Insulin-like growth factor I receptor-mediated circuit in Ewing's
sarcoma/peripheral neuroectodermal tumor: A possible therapeutic
target. Cancer Res. 56:4570–4574. 1996.PubMed/NCBI
|
|
16
|
Scotlandi K, Manara MC, Nicoletti G,
Lollini PL, Lukas S, Benini S, Croci S, Perdichizzi S, Zambelli D,
Serra M, et al: Antitumor activity of the insulin-like growth
factor-I receptor kinase inhibitor NVP-AEW541 in musculoskeletal
tumors. Cancer Res. 65:3868–3876. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Manara MC, Landuzzi L, Nanni P, Nicoletti
G, Zambelli D, Lollini PL, Nanni C, Hofmann F, García-Echeverría C,
Picci P and Scotlandi K: Preclinical in vivo study of new
insulin-like growth factor-I receptor-specific inhibitor in Ewing's
sarcoma. Clin Cancer Res. 13:1322–1330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang Z, Fang Z, Zhen H, Zhou L, Amin HM
and Shi P: Inhibition of type I insulin-like growth factor receptor
tyrosine kinase by picropodophyllin induces apoptosis and cell
cycle arrest in T lymphoblastic leukemia/lymphoma. Leuk Lymphoma.
55:1876–1883. 2014. View Article : Google Scholar
|
|
19
|
Yin SC, Guo W and Tao ZZ: Picropodophyllin
inhibits tumor growth of human nasopharyngeal carcinoma in a mouse
model. Biochem Biophys Res Commun. 439:1–5. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Q, Wei F, Lv G, Li C, Liu T,
Hadjipanayis CG, Zhang G, Hao C and Bellail AC: The association of
TP53 mutations with the resistance of colorectal carcinoma to the
insulin-like growth factor-1 receptor inhibitor picropodophyllin.
BMC Cancer. 13:5212013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu X, Wang L, Mei J, Wang X, Zhu X, Zhang
Q and Lv J: Picropodophyllin inhibits epithelial ovarian cancer
cells in vitro and in vivo. Biochem Biophys Res Commun.
435:385–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hixon ML, Paccagnella L, Millham R,
Perez-Olle R and Gualberto A: Development of inhibitors of the
IGF-1R/PI3K/Akt/mTOR pathway. Rev Recent Clin Trials. 5:189–208.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martin-Piedra MA, Garzon I, Oliveira AC,
Alfonso-Rodriguez CA, Carriel V, Scionti G and Alaminos M: Cell
viability and proliferation capability of long-term human dental
pulp stem cell cultures. Cytotherapy. 16:266–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li J, You T and Jing J: MiR-125b inhibits
cell biological progression of Ewing's sarcoma by suppressing the
PI3K/Akt signalling pathway. Cell Prolif. 47:152–160. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bertrand FE, Steelman LS, Chappell WH,
Abrams SL, Shelton JG, White ER, Ludwig DL and McCubrey JA: Synergy
between an IGF-1R antibody and Raf//MEK//ERK and PI3K//Akt//mTOR
pathway inhibitors in suppressing IGF-1R-mediated growth in
hematopoietic cells. Leukemia. 20:1254–1260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shelton JG, Steelman LS, White ER and
McCubrey JA: Synergy between PI3K/Akt and Raf/MEK/ERK pathways in
IGF-1R mediated cell cycle progression and prevention of apoptosis
in hematopoietic cells. Cell Cycle. 3:372–379. 2004.PubMed/NCBI
|
|
27
|
Yang Y, Li H, Zhang F, Shi H, Zhen T, Dai
S, Kang L, Liang Y, Wang J and Han A: Clinical and biological
significance of hepatoma-derived growth factor in Ewing's sarcoma.
J Pathol. 231:323–334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gorelik N, Dickson BC, Wunder JS and
Bleakney R: Ewing's sarcoma of the patella. Skeletal Radiol.
42:729–733. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sankar S and Lessnick SL: Promiscuous
partnerships in Ewing's sarcoma. Cancer Genet. 204:351–365. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Duan Z, Choy E, Harmon D, Yang C, Ryu K,
Schwab J, Mankin H and Hornicek FJ: Insulin-like growth factor-I
receptor tyrosine kinase inhibitor cyclolignan picropodophyllin
inhibits proliferation and induces apoptosis in multidrug resistant
osteosarcoma cell lines. Mol Cancer Ther. 8:2122–2130. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Menu E, Jernberg-Wiklund H, De Raeve H, De
Leenheer E, Coulton L, Gallagher O, Van Valckenborgh E, Larsson O,
Axelson M, Nilsson K, et al: Targeting the IGF-1R using
picropodophyllin in the therapeutical 5T2MM mouse model of multiple
myeloma: beneficial effects on tumor growth, angio-genesis, bone
disease and survival. Int J Cancer. 121:1857–1861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Krishnan K, Bruce B, Hewitt S, Thomas D,
Khanna C and Helman LJ: Ezrin mediates growth and survival in
Ewing's sarcoma through the AKT/mTOR, but not the MAPK, signaling
pathway. Clin Exp Metastasis. 23:227–236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kilic-Eren M, Boylu T and Tabor V:
Targeting PI3K/Akt represses Hypoxia inducible factor-1alpha
activation and sensitizes Rhabdomyosarcoma and Ewing's sarcoma
cells for apoptosis. Cancer Cell Int. 13:362013. View Article : Google Scholar
|
|
34
|
Li J, You T and Jing J: MiR-125b inhibits
cell biological progression of Ewing's sarcoma by suppressing the
PI3K/Akt signalling pathway. Cell Prolif. 47:152–160. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qi L, Toyoda H, Shankar V, Sakurai N,
Amano K, Kihira K, Iwasa T, Deguchi T, Hori H, Azuma E, et al:
Heterogeneity of neuroblastoma cell lines in insulin-like growth
factor 1 receptor/Akt pathway-mediated cell proliferative
responses. Cancer Sci. 104:1162–1171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bähr C and Groner B: The insulin like
growth factor-1 receptor (IGF-1 R) as a drug target: Novel
approaches to cancer therapy. Growth Horm IGF Res. 14:287–295.
2004. View Article : Google Scholar
|
|
37
|
Bianco R, Melisi D, Ciardello F and
Tortora G: Key cancer cell signal transduction pathways as
therapeutic targets. Eur J Cancer. 42:290–294. 2006. View Article : Google Scholar
|
|
38
|
Fabian J, Lodrini M, Oehme I, Schier MC,
Thole TM, Hielscher T, Kopp-Schneider A, Opitz L, Capper D, von
Deimling A, et al: GRHL1 acts as tumor suppressor in neuroblastoma
and is negatively regulated by MYCN and HDAC3. Cancer Res.
74:2604–2616. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Estañ MC, Calviño E, de Blas E,
Boyano-Adanez Mdel C, Mena ML, Gómez-Gómez M, Rial E and Aller P:
2-Deoxy-D-glucose cooperates with arsenic trioxide to induce
apoptosis in leukemia cells: Involvement of IGF-1R-regulated
Akt/mTOR, MEK/ERK and LKB-1/AMPK signaling pathways. Biochem
Pharmacol. 84:1604–1616. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Baumgarten SC, Convissar SM, Fierro MA,
Winston NJ, Scoccia B and Stocco C: IGF-1R signaling is necessary
for FSH-induced activation of AKT and differentiation of human
cumulus granulosa cells. J Clin Endocrinol Metab. 99:2995–3004.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen C, Xu Y and Song Y: IGF-1
gene-modified muscle-derived stem cells are resistant to oxidative
stress via enhanced activation of IGF-1R/PI3K/AKT signaling and
secretion of VEGF. Mol Cell Biochem. 386:167–175. 2014. View Article : Google Scholar
|
|
42
|
Jiang YY, Huang H, Wang HJ, Wu D, Yang R,
Tashiro S, Onodera S and Ikejima T: Interruption of mitochondrial
complex IV activity and cytochrome c expression activated
O2·-mediated cell survival in silibinin-treated human
melanoma A375-S2 cells via IGF-1R-PI3K-Akt and IGF-1R-PLC gamma-PKC
pathways. Eur J Pharmacol. 668:78–87. 2011. View Article : Google Scholar : PubMed/NCBI
|