Introduction
Mitogen-activated protein kinases (MAPKs) are
serine/threonine protein kinases. The conserved MAPK signaling
pathway exists in all eukaryotes and is considered a central hub in
the regulation of cellular processes, including growth,
proliferation, division, cell cycle progression, apoptosis,
necrosis and cell-cell interactions (1–3). The
signaling cascade includes no less than three enzymes that are
activated in sequence: An MAPK kinase kinase, an MAPK kinase and an
MAPK (1). These enzymes all
possess 11 conserved sub-domains and are activated via
phosphorylation of amino acid residues (4). Rossomando et al (5) identified the extracellular
signal-regulated kinase (ERK)-MAPK in mammalian cells in 1991,
while the stress-activated protein kinase (SAPK)-MAPK and p38-MAPK
were discovered subsequently (6,7).
Together, these three enzymes are the main members of the MAPK
family.
Osteoclasts are specialized multinucleated cells
that execute the catabolic phase of bone remodeling (8,9).
Bone remodeling is a persistent physiological process in healthy
humans and animals that is initiated by osteoclasts and is critical
for bone mass homeostasis (10,11).
Osteoclasts are responsible for bone resorption, while osteoblasts
are responsible for bone matrix generation and mineralization.
Together, these processes lead to whole bone remodeling (12). In instances where these processes
become unbalanced, bone mass may increase or decrease abnormally
and cause diseases including osteopetrosis, osteoporosis,
chondropathy and rheumatoid arthritis (13). Receptor activator of nuclear factor
κB ligand (RANKL) is a member of the tumor necrosis factor (TNF)
ligand superfamily and is an essential cytokine for
osteoclastogenesis (14). RANKL is
produced by osteoblasts/stromal cells and combines with its
receptor, RANK, which is expressed on the surface of osteoclast
precursors (14). RANKL/RANK
association triggers the signaling cascades involved in
differentiation and activation of osteoclasts (9). Osteoprotegerin (OPG), a protein
generated by osteoblast/stromal cells, acts as a decoy receptor for
RANKL and inhibits RANKL/RANK association, inhibiting the
development of osteoclasts (15).
The RANK/RANKL/OPG axis regulates bone metabolism and
osteoclastogenesis (14).
Previous studies have demonstrated that ERK-MAPK,
c-Jun N-terminal kinase (JNK)-MAPK and p38-MAPK are involved in the
RANKL/RANK signaling pathway, which regulates osteoclast
differentiation, maturation and survival (16–19).
It is, however, unknown whether the MAPK signaling pathway is
involved in OPG-induced inhibition of osteoclast development. The
present study attempted to examine the involvement of the MAPK
signaling pathway by utilizing specific inhibitors of the MAPK
pathway in order to test whether OPG or specific kinase inhibitors
may have potential use in the treatment of bone loss-associated
diseases resulting from dynamic bone resorption of osteoclasts.
Materials and methods
Cells and reagents
The murine monocyte/macrophage cell line RAW264.7
was purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA). The primary rabbit anti-phospho p38-MAPK
polyclonal antibody (cat. no. SAB4504095), Acid Phosphatase kit
387-A [tartrate-resistant acid phosphatase (TRAP) staining kit] and
the specific inhibitors U0126, SB202190, SP600125 were obtained
from Sigma-Aldrich (St. Louis, MO, USA). The primary rabbit
anti-ERK1/2 polyclonal antibody (cat. no. 06-642) was purchased
from Millipore Corporation (Billerica, MA, USA). The primary
anti-p38-MAPK polyclonal (cat. no. 9212S), rabbit anti-phospho
SAPK/JNK monoclonal (cat. no. 4671S), rabbit anti-phospho ERK1/2
monoclonal (cat. no. 9101S), rabbit anti β-actin polyclonal (cat.
no. 4970S) and rabbit anti-SAPK/JNK polyclonal (cat. no. 9258S)
antibodies were purchased from Cell Signaling Technology Inc.
(Beverly, MA, USA). The rabbit anti-sheep immunoglobulin G (IgG)
horseradish peroxidase (HRP)-conjugated secondary antibody (cat.
no. sc-2770) was obtained from Santa Cruz Biotechnology (Dallas,
TX, USA). Macrophage colony-stimulating factor (M-CSF), RANKL and
OPG were purchased from PeproTech Inc. (Rocky Hill, NJ, USA).
Bovine cortical bone was purchased from a slaughterhouse (Yangzhou,
China) and was sawed into slices by a saw microtome (SP1600; Leica
Microsystems, Wetzlar, Germany) at Shanghai Ninth People's Hospital
Affiliated to Shanghai JiaoTong University School of Medicine
(Shanghai, China).
TRAP-positive cell staining and bone
resorption activity assay
RAW264.7 cells were cultured in Dulbecco's modified
Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS;
Gibco-BRL, Carlsbad, CA, USA), 2 mM/l l-glutamine, 100 U/ml penicillin
and 100 µg/ml streptomycin at 37°C in a humidified
atmosphere of 5% CO2. For studies on osteoclast
differentiation, cells were adjusted to a concentration of
1.5×104 cells/ml in α-Minimum Essential Medum (α-MEM)
containing 10% FBS, 2 mM/l l-glutamine, 100 U/ml penicillin
and 100 µg/ml streptomycin. Cells were seeded in 96-well and
48-well plates with bovine cortical bone slices. After a 24 h of
incubation, the medium was replaced with serum-free α-MEM
containing M-CSF (25 ng/ml) and RANKL (30 ng/ml), and the cells
were cultured for another 48 h. At that time-point, in the presence
of M-CSF + RANKL, 0, 10, 20, 50 or 100 ng/ml OPG was added and
cells were incubated for an additional three days.
At the end of the incubation, TRAP staining of
osteoclasts and bone resorption activity assays was performed
according to the manufacturer's instructions. The number of
TRAP-positive cells in each group was counted and compared.
Briefly, TRAP-positive cells in ten randomized visual fields from
three random wells were counted using an inverted microscope (Leica
Microsystems GmbH, Wetzlar, Germany). At the same time, bone
resorption by differentiated osteoclasts in bovine cortical bone
slices was calculated in the different groups. The bone slices
co-cultured with differentiated osteoclasts were removed from the
plates. Any remaining cells on bone slices were removed by
ultrasonic cleaning. The resorption lacunae on bone slices were
observed using an environmental scanning electron microscope
(XL30-ESEM; Philips, Eindhoven, The Netherlands). The volume of
resorption lacunae was determined by professional image analysis
software (version 1.0; JEDA Technologies, Nanjing, China).
Western blot analysis
RAW264.7 cells were cultured in α-MEM containing 10%
FBS, 2 mM/l l-glutamine, 100 U/ml penicillin
and 100 µg/ml streptomycin in six-well plates for 24 h at a
concentration of 1.5×104 cells/ml. Medium was then
replaced with serum-free α-MEM with M-CSF (25 ng/ml) + RANKL (30
ng/ml) and the cells were cultured for a further 48 h. Subsequent
treatment was dependent on the assay type. For time-course studies,
100 ng/ml OPG was added in the presence of M-CSF + RANKL and cells
incubated for 15, 30, 60 or 120 min. For concentration gradient
studies, 0, 10, 20, 50 or 100 ng/ml OPG were added in the presence
of M-CSF + RANKL and cells were incubated for 30 min. For studies
on inhibition of the MAPK signaling pathway, complete medium was
removed and replaced with serum-free medium. The cells were
pre-treated with 100 ng/ml OPG and either 0.2 µM U0126 (a
specific inhibitor of the ERK-MAPK signaling pathway), 10 µM
SB202190 (a specific inhibitor of the p38-MAPK signaling pathway)
or 10 µM SP600125 (a specific inhibitor of the JNK-MAPK
signaling pathway) for 30 min. M-CSF + RANKL were then added for an
additional 30 min.
Following incubation, cells from each experimental
group were collected and lysed in 180 µl
radioimmunoprecipitation assay (RIPA) buffer with 1% (v/v) PMSF for
30 min with intermittent vibration. After sonication, the solution
of lysed cells was centrifuged (12,000 × g for 10 min at 4°C) and
the supernatants were extracted. The total protein concentration
was 6–15 mg/ml. The protein was loaded (60–150 µg per lane)
and were separated, transferred, blocked and incubated overnight at
4°C with primary anti-phospho p38-MAPK, anti-phospho JNK-MAPK,
anti-phospho ERK-MAPK, anti-p38-MAPK, anti-JNK-MAPK, anti-ERK-MAPK
or anti-β-actin antibodies diluted in 5% BSA-Tris-buffered saline
with Tween-20 (TBST). Following incubation with the primary
antibodies, the samples were incubated with anti-sheep IgG HRP
secondary antibody diluted in 5% BSA-TBST for 90 min at room
temperature. Immunoreactive proteins were visualized by enhanced
chemiluminescence (ECL) using ECL-plus detection reagents (Thermo
Fisher Scientific, Waltham, MA, USA). The experiments were repeated
three times.
Results
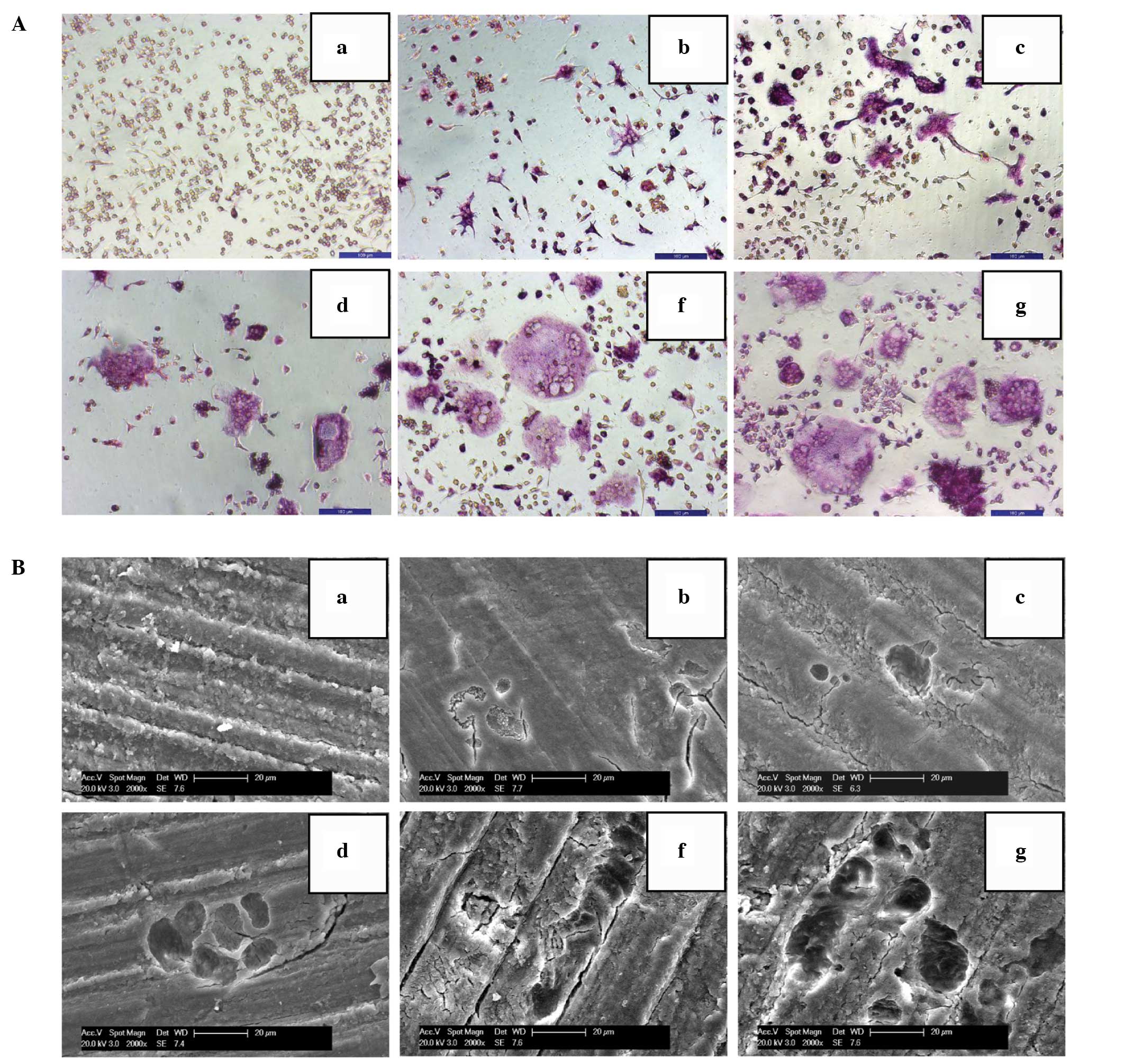
OPG inhibits the differentiation and bone
resorption activities of osteoclasts
Multinucleated TRAP-positive cells were observed in
M-CSF + RANKL-treated RAW264.7 cells after four days of incubation.
The differentiated cells showed characteristic bone resorption
activity, as indicated by the observation that cells corroded the
bone slices to produce cavities. OPG treatment reduced the number
of multinucleated TRAP-positive cells and inhibited the bone
resorption activity of differentiated cells in a
concentration-dependent manner as compared to those in the control
group (Fig. 1A and B).
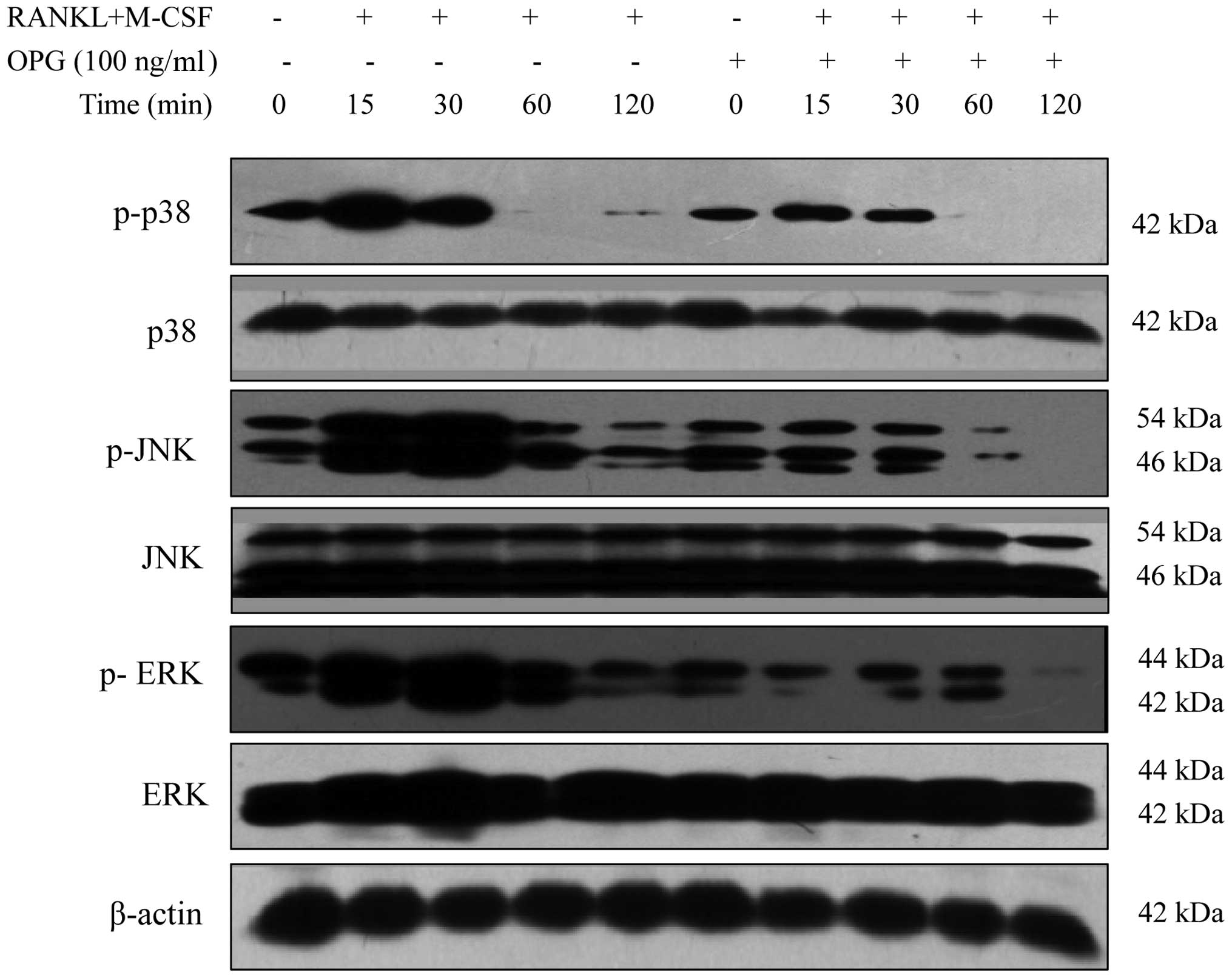
OPG affects the MAPK signaling pathway in
a time-dependent manner
All three branches of the MAPK signaling pathway,
namely p38-MAPK, JNK-MAPK and ERK-MAPK, were activated in the M-CSF
+ RANKL-treated RAW264.7 cells within 15 min of incubation.
Phosphorylation levels of p38-MAPK peaked within 15 min and
subsequently declined. Phosphorylation levels of JNK-MAPK and
ERK-MAPK peaked within 30 min and declined afterwards. The addition
of OPG decreased the phosphorylation levels of p38-MAPK, JNK-MAPK
and ERK-MAPK in a time-dependent manner (Fig. 2).
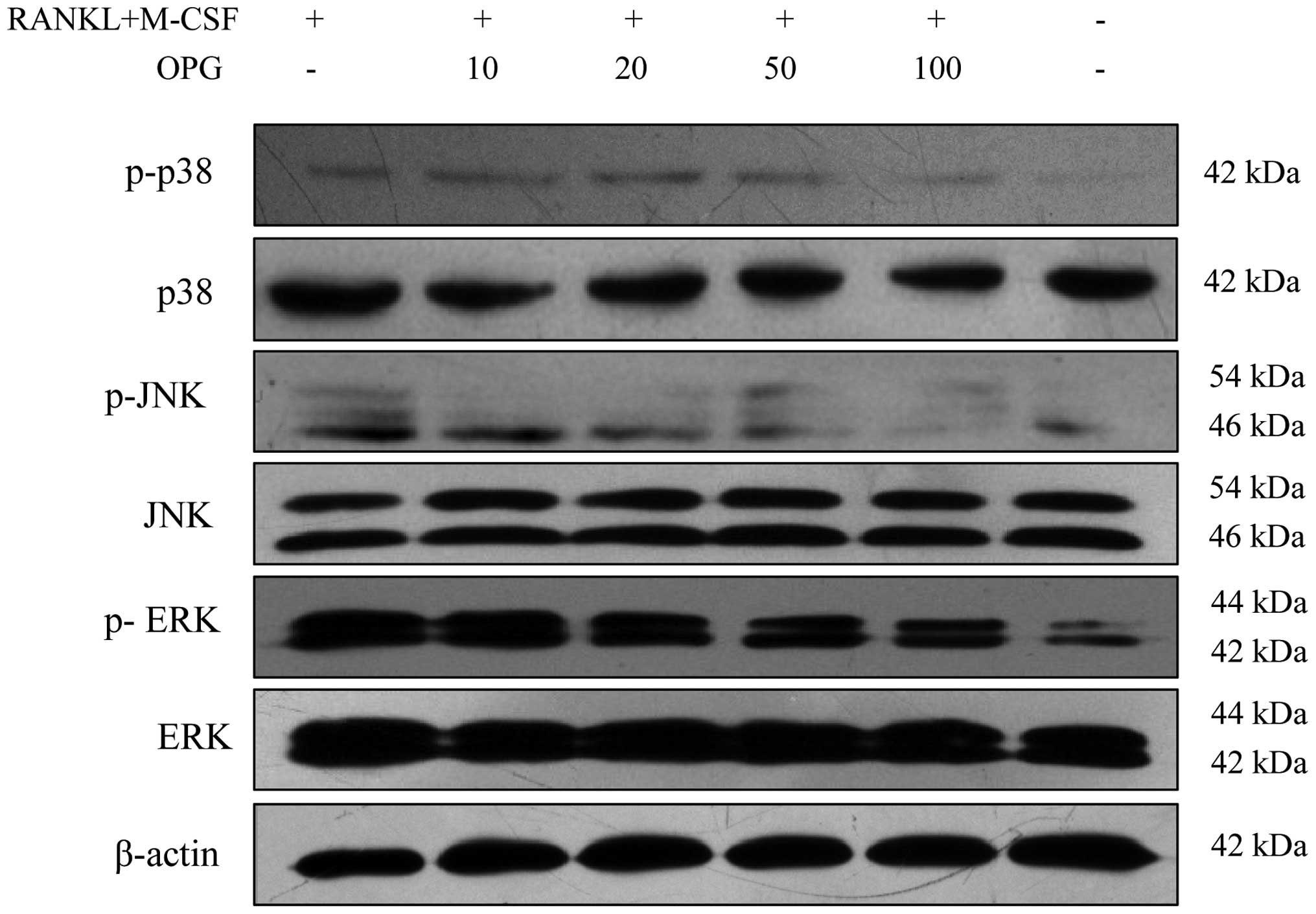
OPG inhibits the MAPK signaling pathway
in a concentration-dependent manner
Compared to those in the RAW264.7 control group, the
phosphorylation levels of p38-MAPK, JNK-MAPK and ERK-MAPK increased
with M-CSF + RANKL treatment. Of note, OPG decreased the
phosphorylation levels of p38-MAPK, JNK-MAPK and ERK-MAPK in a
concentration-dependent manner (Fig.
3).
OPG inhibits osteoclast differentiation
via the MAPK signaling pathway
Specific inhibitors of p38-MAPK, JNK-MAPK and
ERK-MAPK were employed in the present study in order to test the
involvement of the MAPK signaling pathway in the effects of OPG on
osteoclast differentiation. The results further confirmed that the
three signaling pathways are involved in the differentiation and
activation of osteoclasts and that OPG affects the differentiation
and activation of osteoclasts.
Discussion
In view of the fact that the amounts of purified
primary osteoclasts required for signal transduction studies were
prohibitive, RAW264.7 cells were used to study the MAPK signaling
pathway. These cells are considered optimal osteoclast precursors
and have been extensively applied in in vitro studies on
osteoclasts (20–22). A previous study by our group
demonstrated that M-CSF + RANKL induces RAW264.7-cell
differentiation into osteoclasts on a morphological as well as
molecular basis; and showed that this process was inhibited by OPG
(23). These results laid a
foundation for the present study, which aimed to investigate the
involvement of the MAPK signaling pathway in this process. Over the
previous decades, multiple studies have demonstrated that OPG
inhibits the differentiation and activation of osteoclasts
(24–26). Few of these studies, however, have
focused on the associated signal transduction mechanisms. The
present study demonstrated that the MAPK signaling pathway is
involved in the M-CSF + RANKL-induced differentiation and
activation of osteoclasts, which can be modulated by OPG.
ERK-MAPK, JNK-MAPK and p38-MAPK in osteoclasts and
osteoclast precursors are activated by the combination of RANKL and
RANK (16–18,27).
p38-MAPK is activated by MAPK kinase 6, which is activated by the
combination of RANKL and RANK, and subsequently phosphorylates
microphthalmia-associated transcription factor (MITF), promoting
the differentiation of osteoclasts (28). Lee et al (17) and Li et al (18) further confirmed that p38-MAPK is
involved in the differentiation of osteoclasts through studies
using specific signaling inhibitors (17,18).
Previous studies suggested that ERK-MAPK was involved in survival
of osteoclasts rather than bone resorption activities (29,30).
Tumor necrosis factor receptor-associated factor (TRAF)-6 is an
adapter protein that is the binding site of the RANK cytoplasmic
motif PFQEP369-373 (31). RANK
regulates the development of osteoclasts using TRAF-6 as an
intermediary (32). Transforming
growth factor beta-activated kinase 1/MAP3K7 binding protein 2 are
proteins downstream of TRAF-6, which activate JNK-MAPK and p38-MAPK
(33). Downstream transcription
factors of ERKs and JNKs include activator protein (AP)-1, the Fos
dipolymer family (c-Fos, FosB, Fra-1, and Fra-2), and the Jun
family (c-Jun, JunB, and JunD) (25). These transcription factors are
master regulators of osteoclast differentiation (25,34).
ERK-MAPK activates c-Fos, while JNK-MAPK enhances the
transcriptional activity of AP-1 through the phosphorylation of
c-Jun (35). Furthermore, AP-1
triggers the gene-encoding matrix metalloproteinase and alkaline
phosphatase, promoting the differentiation, survival and fusion of
osteoclast precursors, and advancing the activation of mature
osteoclasts as well (36,37). Activated ERK enters the nucleus of
mature macrophages and sequentially activates the transcription
factor Elk, associating with the cis-regulating element located in
the promoter region of the c-Fos gene, and driving the
differentiation of the mature macrophages into osteoclast
precursors (38). M-CSF
specifically stimulates the growth of macrophage colonies (39). M-CSF has an important role in the
differentiation and survival of osteoclast precursors as well as
the survival of mature osteoclasts (40,41).
Weilbaecher et al (42)
suggested that M-CSF impacts the development of osteoclasts through
MAPK signaling pathway activation, further activating MITF and
transcription factor binding to immunoglobulin heavy constant mu
enhancer 3, and promoting the differentiation of osteoclasts and
the formation of TRAP-positive multinucleated cells. In addition,
the present study confirmed that ERK-MAPK, JNK-MAPK and p38-MAPK
were phosphorylated during M-CSF + RANKL-induced
osteoclastogenesis.
Simonet et al (24) reported that OPG is involved in the
regulation of bone density by specifically inhibiting
osteoclastogenesis. Subsequent studies suggested that the
differentiation and activation of osteoclasts were blocked by OPG
through direct and indirect effects. OPG binds to RANKL in a
competitive manner with respect to RANK, impeding the RANKL-RANK
signaling cascade (25), by an
indirect mechanism. In addition, OPG can induce apoptosis in mature
osteoclasts by damaging cellular structures such as the F-actin
ring (43,44), via a direct mechanism. In the
present study, OPG was added to differentiating osteoclast
precursors rather than differentiated osteoclasts. It appears,
therefore, that OPG interfered with the differentiation of
osteoclasts via the indirect mechanism. Theoleyre et al
(20) investigated the direct
influences of OPG on osteoclasts and demonstrated that OPG induces
the phosphorylation of ERK1/2 and p38 in differentiated
osteoclasts. The study suggested that OPG had specific inhibitory
activity on the development of osteoclasts via ERK1/2
phosphorylation (20). In the
present study, however, ERK1/2 phosphorylation levels were found to
be decreased alongside the inhibition of osteoclastogenesis by OPG.
A possible interpretation of this discrepancy is that ERK1/2
phosphorylation levels were increased during apoptosis of
osteoclasts in the study by Theoleyre et al (20).
In conclusion, the findings of the present study
indicated that the MAPK signaling pathway is involved in the
regulation of osteoclastogenesis as well as in the OPG-mediated
inhibition of osteoclast differentiation and activation.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (nos. 31172373, 31372495 and
31302154), a Project Funded by the Priority Academic Program
Development of Jiangsu Higher Education Institutions, the National
Research Foundation for the Doctoral Program of Higher Education of
China (no. 20113250110003) and the Anhui Provincial Natural Science
Foundation (no. 1508085QH172). Furthermore, the authors would like
to thank the ACCDON LLC (Woburn, MA, USA) for the professional
English editing service.
References
|
1
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seger R and Krebs EG: The MAPK signaling
cascade. FASEB J. 9:726–735. 1995.PubMed/NCBI
|
|
3
|
Sompallae R, Stavropoulou V, Houde M and
Masucci MG: The MAPK signaling cascade is a central hub in the
regulation of cell cycle, apoptosis and cytoskeleton remodeling by
tripeptidyl-peptidase II. Gene Regul Syst Bio. 2:253–265.
2008.PubMed/NCBI
|
|
4
|
Robinson MJ and Cobb MH: Mitogen-activated
protein kinase pathways. Curr Opin Cell Biol. 9:180–186. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rossomando AJ, Sanghera JS, Marsden LA,
Weber MJ, Pelech SL and Sturgill TW: Biochemical characterization
of a family of serine/threonine protein kinases regulated by
tyrosine and serine/threonine phosphorylations. J Biol Chem.
266:20270–20275. 1991.PubMed/NCBI
|
|
6
|
Brewster JL, de Valoir T, Dwyer ND, Winter
E and Gustin MC: An osmosensing signal transduction pathway in
yeast. Science. 259:1760–1763. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kyriakis JM and Avruch J: pp54
microtubule-associated protein 2 kinase. A novel serine/threonine
protein kinase regulated by phosphorylation and stimulated by
poly-L-lysine. J Biol Chem. 265:17355–17363. 1990.PubMed/NCBI
|
|
8
|
Jansen ID, Vermeer JA, Bloemen V, Stap J
and Everts V: Osteoclast fusion and fission. Calcif Tissue Int.
90:515–522. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Honma M, Ikebuchi Y, Kariya Y and Suzuki
H: Regulatory mechanisms of RANKL presentation to osteoclast
precursors. Curr Osteoporos Rep. 12:115–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakashima T: Regulation mechanism of bone
remodeling. Kokubyo Gakkai Zasshi. 80:75–80. 2013.In Japanese.
PubMed/NCBI
|
|
11
|
Lemaire V, Tobin FL, Greller LD, Cho CR
and Suva LJ: Modeling the interactions between osteoblast and
osteoclast activities in bone remodeling. J Theor Biol.
229:293–309. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boyce BF and Xing L: Functions of
RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem
Biophys. 473:139–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wright HL, McCarthy HS, Middleton J and
Marshall MJ: RANK, RANKL and osteoprotegerin in bone biology and
disease. Curr Rev Musculoskelet Med. 2:56–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pérez-Sayáns M, Somoza-Martín JM,
Barros-Angueira F, Rey JM and García-García A: RANK/RANKL/OPG role
in distraction osteogenesis. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 109:679–686. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fu YX, Gu JH, Zhang YR, Tong XS, Zhao HY,
Yuan Y, Liu XZ, Bian JC and Liu ZP: Osteoprotegerin influences the
bone resorption activity of osteoclasts. Int J Mol Med.
31:1411–1417. 2013.PubMed/NCBI
|
|
16
|
Jimi E, Akiyama S, Tsurukai T, Okahashi N,
Kobayashi K, Udagawa N, Nishihara T, Takahashi N and Suda T:
Osteoclast differentiation factor acts as a multifunctional
regulator in murine osteoclast differentiation and function. J
Immunol. 163:434–442. 1999.PubMed/NCBI
|
|
17
|
Lee SE, Woo KM, Kim SY, Kim HM, Kwack K,
Lee ZH and Kim HH: The phosphatidylinositol 3-kinase, p38, and
extracellular signal-regulated kinase pathways are involved in
osteoclast differentiation. Bone. 30:71–77. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Udagawa N, Itoh K, Suda K, Murase Y,
Nishihara T, Suda T and Takahashi N: p38 MAPK-mediated signals are
required for inducing osteoclast differentiation but not for
osteoclast function. Endocrinology. 143:3105–3113. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Junttila MR, Li SP and Westermarck J:
Phosphatase-mediated crosstalk between MAPK signaling pathways in
the regulation of cell survival. FASEB J. 22:954–965. 2008.
View Article : Google Scholar
|
|
20
|
Theoleyre S, Wittrant Y, Couillaud S,
Vusio P, Berreur M, Dunstan C, Blanchard F, Rédini F and Heymann D:
Cellular activity and signaling induced by osteoprotegerin in
osteoclasts: Involvement of receptor activator of nuclear factor
kappaB ligand and MAPK. Biochim Biophys Acta. 1644:1–7. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mladenović Ž, Johansson A, Willman B,
Shahabi K, Björn E and Ransjö M: Soluble silica inhibits osteoclast
formation and bone resorption in vitro. Acta Biomater. 10:406–418.
2014. View Article : Google Scholar
|
|
22
|
Chen X, Zhu G, Jin T, Gu S, Xiao H and Qiu
J: Cadmium induces differentiation of RAW264.7 cells into
osteoclasts in the presence of RANKL. Food Chem Toxicol.
49:2392–2397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu YX, Gu JH, Zhang YR, Tong XS, Zhao HY,
Yuan Y, Liu XZ, Bian JC and Liu ZP: Inhibitory effects of
osteoprotegerin on osteoclast formation and function under
serum-free conditions. J Vet Sci. 14:405–412. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simonet WS, Lacey DL, Dunstan CR, Kelley
M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et
al: Osteoprotegerin: A novel secreted protein involved in the
regulation of bone density. Cell. 89:309–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hofbauer LC: Osteoprotegerin ligand and
osteoprotegerin: Novel implications for osteoclast biology and bone
metabolism. Eur J Endocrinol. 141:195–210. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wong BR, Besser D, Kim N, Arron JR,
Vologodskaia M, Hanafusa H and Choi Y: TRANCE, a TNF family member,
activates Akt/PKB through a signaling complex involving TRAF6 and
c-Src. Mol Cell. 4:1041–1049. 1999. View Article : Google Scholar
|
|
28
|
Shih J, Bauer D, Orloff J, Capizzi T,
Thompson D, Oppenheimer L and Ross PD: Proportion of fracture risk
reduction explained by BMD changes using Freedman analysis depends
on choice of predictors. Osteoporos Int. 13:S38–S39. 2002.
|
|
29
|
Miyazaki T, Katagiri H, Kanegae Y,
Takayanagi H, Sawada Y, Yamamoto A, Pando MP, Asano T, Verma IM,
Oda H, et al: Reciprocal role of ERK and NF-kappaB pathways in
survival and activation of osteoclasts. J Cell Biol. 148:333–342.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
David JP, Rincon M, Neff L, Horne WC and
Baron R: Carbonic anhydrase II is an AP-1 target gene in
osteoclasts. J Cell Physiol. 188:89–97. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ye H, Arron JR, Lamothe B, Cirilli M,
Kobayashi T, Shevde NK, Segal D, Dzivenu OK, Vologodskaia M, Yim M,
et al: Distinct molecular mechanism for initiating TRAF6
signalling. Nature. 418:443–447. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Blair HC, Robinson LJ and Zaidi M:
Osteoclast signalling pathways. Biochem Biophys Res Commun.
328:728–738. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mizukami J, Takaesu G, Akatsuka H, Sakurai
H, Ninomiya-Tsuji J, Matsumoto K and Sakurai N: Receptor activator
of NF-kappaB ligand (RANKL) activates TAK1 mitogen-activated
protein kinase kinase kinase through a signaling complex containing
RANK, TAB2, and TRAF6. Mol Cell Biol. 22:992–1000. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi HJ, Park YR, Nepal M, Choi BY, Cho
NP, Choi SH, Heo SR, Kim HS, Yang MS and Soh Y: Inhibition of
osteoclastogenic differentiation by Ikarisoside A in RAW 264.7
cells via JNK and NF-kappaB signaling pathways. Eur J Pharmacol.
636:28–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cano E and Mahadevan LC: Parallel signal
processing among mammalian MAPKs. Trends Biochem Sci. 20:117–122.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grigoriadis AE, Wang ZQ, Cecchini MG,
Hofstetter W, Felix R, Fleisch HA and Wagner EF: c-Fos: A key
regulator of osteoclast-macrophage lineage determination and bone
remodeling. Science. 266:443–448. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chapurlat RD, Palermo L, Ramsay P and
Cummings SR: Risk of fracture among women who lose bone density
during treatment with alendronate. The Fracture Intervention Trial.
Osteoporos Int. 16:842–848. 2005. View Article : Google Scholar
|
|
38
|
Hong SY, Jeon YM, Lee HJ, Kim JG, Baek JA
and Lee JC: Activation of RhoA and FAK induces ERK-mediated
osteopontin expression in mechanical force-subjected periodontal
ligament fibroblasts. Mol Cell Biochem. 335:263–272. 2010.
View Article : Google Scholar
|
|
39
|
Stanley ER, Berg KL, Einstein DB, Lee PS,
Pixley FJ, Wang Y and Yeung YG: Biology and action of
colony–stimulating factor-1. Mol Reprod Dev. 46:4–10. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tsurukai T, Udagawa N, Matsuzaki K,
Takahashi N and Suda T: Roles of macrophage-colony stimulating
factor and osteoclast differentiation factor in osteoclastogenesis.
J Bone Miner Metab. 18:177–184. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fuller K, Owens JM, Jagger CJ, Wilson A,
Moss R and Chambers TJ: Macrophage colony-stimulating factor
stimulates survival and chemotactic behavior in isolated
osteoclasts. J Exp Med. 178:1733–1744. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Weilbaecher KN, Motyckova G, Huber WE,
Takemoto CM, Hemesath TJ, Xu Y, Hershey CL, Dowland NR, Wells AG
and Fisher DE: Linkage of M-CSF signaling to Mitf, TFE3, and the
osteoclast defect in Mitf(mi/mi) mice. Mol Cell. 8:749–758. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shiotani A, Takami M, Itoh K, Shibasaki Y
and Sasaki T: Regulation of osteoclast differentiation and function
by receptor activator of NFkB ligand and osteoprotegerin. Anat Rec.
268:137–146. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hakeda Y, Kobayashi Y, Yamaguchi K, Yasuda
H, Tsuda E, Higashio K, Miyata T and Kumegawa M: Osteoclastogenesis
inhibitory factor (OCIF) directly inhibits bone-resorbing activity
of isolated mature osteoclasts. Biochem Biophys Res Commun.
251:796–801. 1998. View Article : Google Scholar : PubMed/NCBI
|