Introduction
Colorectal cancer (CRC) is the second leading cause
of cancer-associated mortality (1). However, early diagnosis of CRC is
challenging, as marked symptoms only appear in the period of
disease progression. Therefore, most patients are diagnosed at a
late stage (2). As a result, only
radical resection of the tumor improves the clinical prognosis and
provides a chance to prolong survival. However, up to 50% of the
post-operative patients suffer from a disease relapse within the
first 3–5 years after operation (3). To reduce the risk of cancer
recurrence as well as metastasis, post-operative patients must be
closely monitored and their treatments adjusted to their condition.
In general, post-operative assessments are based on clinical
symptoms, serum biomarkers and imaging results (4). However, these relatively dated
methods, including examination of serum tumor markers and
computerized tomography, often lack sufficient sensitivity and
specificity for guiding timely and appropriate therapies.
Therefore, the development of more effective methods for the
surveillance the disease process is required.
Small changes in the body can lead to large changes
in metabolite levels (5).
Metabonomics, one of the '-omics' technologies, provides
quantitative measures to detect those large changes in the
metabolic profiles of individuals responding to pathophysiological
stimuli or genetic modification (6). Due to its high throughput,
sensitivity and non-invasiveness, metabonomic analysis has already
been used as A diagnostic tools in numerous human diseases,
including diabetes (7), cancer
(8), cardiovescular disease
(9) and respiratory disease
(10). Although a growing amount
of research has provided experimental and clinical evidence for the
close association between specific biomarkers and diagnosis of
certain types of cancer, including CRC (1,2,4), the
application of metabonomic analysis to post-operative monitoring of
CRC has rarely been reported (11).
Ultra-performance liquid chromatography coupled with
quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS) has been
applied to evaluate the levels of certain disease-associated
factors and biomarkers in biofluids which can be obtained by
non-invasive techniques (including serum, urine or feces) as well
as tissues (11). Due to the
combination of high operating pressures and 1.7-µm porous
particles, UPLC is more sensitive and has a higher peak capacity
and a better resolution compared with those of the traditional high
performance liquid chromatography (HPLC) technique (12). Thus, this novel technique is
considered to be suitable for large-scale untargeted metabonomics.
The present study aimed to utilize UPLC-QTOF-MS for the
post-operative monitoring of CRC patients by detecting changes in
their serum metabolite patterns.
Materials and methods
Patients
All patients and healthy volunteers provided written
informed consent in accordance with the institutional guidelines.
The present study was approved by the Human Ethics Committee of the
First Affiliated Hospital (School of Medicine, Zhejiang University,
Hangzhou, China). Twenty patients with CRC who underwent radical
resection, comprising nine cases of colon cancer and eleven cases
of rectal cancer, as well as twenty age- and gender-matched healthy
volunteers (individuals with no recorded tumors or other metabolic
diseases) were recruited between March 2013 and August 2014 at the
First Affiliated Hospital (School of Medicine, Zhejiang University,
Hangzhou, China). Clinical and demographic characteristics of these
individuals are listed in Table
I.
 | Table IClinical and demographic data on
healthy volunteers (n=20) and patients with CRC (n=20). |
Table I
Clinical and demographic data on
healthy volunteers (n=20) and patients with CRC (n=20).
| Characteristic | Value |
|---|
| Healthy
volunteers |
| Mean age ± SD
(years) | 60.95±5.81 |
| Age range
(years) | 52–71 |
| Gender
(male/female) | 14/6 |
| CRC patients |
| Mean age ± SD
(years) | 61.10±7.55 |
| Age range
(years) | 51–73 |
| Gender
(male/female) | 14/6 |
| Primary tumor site,
n (%) |
| Colon | 9 (45) |
| Rectum | 11 (55) |
| Duke staging, n
(%) |
| A | 1 (5) |
| B | 5 (25) |
| C | 14 (70) |
| D | 0 |
| Differentiation
grading, n (%) |
| Well | 0 (0) |
| Moderate | 14 (70) |
| Poor | 6 (30) |
The inclusion criteria were as follows: i) Positive
diagnosis with stage A-C CRC according to the Duke classification
(13); ii) histopathological
confirmation of adenocarcinoma of the colon or rectum; iii) no
prior enterectomy, radiotherapy and systemic chemotherapy; iv) no
other metabolic diseases, such as diabetes mellitus; v) no
inflammatory conditions. The exclusion criteria were as follows: i)
Extra-abdominal metastasis or stage D Duke Classification; ii)
concomitance of other malignant neoplasm; iii) application of any
drugs in the previous two weeks; iv) drop-out during the study
period.
Reagents and materials
HPLC-grade acetonitrile, leucine-enkephalin and
formic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Deionized water was produced using the Milli-Q ultrapure water
purification system (Millipore, Bedford, MA, USA). The internal
standards PC (17:0/0:0), PC (17:0/17:0), PE (17:0/17:0), PG
(17:0/17:0), PS (17:0/17:0) and PA (17:0/17:0) were obtained from
Avanti Polar Lipids Inc. (Alabaster, AL, USA), while DG
(17:0/17:0/0:0), TG (17:0/17:0/17:0), MG (17:0/0:0/0:0), PC
(16:1/0:0-D3), PC (16:1/16:1-D6) and TG (16:0/16:0/16:0-13C3) were
obtained from Larodan Fine Chemicals (Malmo, Sweden).
Sample collection and preparation
Blood samples were obtained healthy controls and
from CRC patients prior to radical resection as well as one month
after surgery prior to any additional medical treatments, such as
chemotherapy. All samples were collected from participants under
fasting conditions. In all instances, 5 ml blood from each proband
was collected in tubes which did not contain any anti-coagulant
substances. The collected blood was left on the laboratory bench
for 30 min at room temperature prior to being centrifuged (3,000 ×
g, 5 min, 4°C). The serum was carefully aspirated, transferred to a
clean Eppendorf tube and subsequently stored at −80°C.
Prior to metabonomics analysis, the serum sample was
thawed at room temperature. In order to precipitate the protein in
serum sample, 200 µl sample was added into 600 µl
ice-cold acetonitrile. The mixture was vortexed and centrifuged
(12,000 × g, 15 min, 4°C). Finally, the supernatant was transferred
into a HPLC sample glass vial and stored at 4°C for UPLC-MS
analysis. To evaluate the reproducibility and stability of the
UPLC-MS system, 10 µl of each sample was added into one vial
to generate a pooled quality control (QC) sample, which was
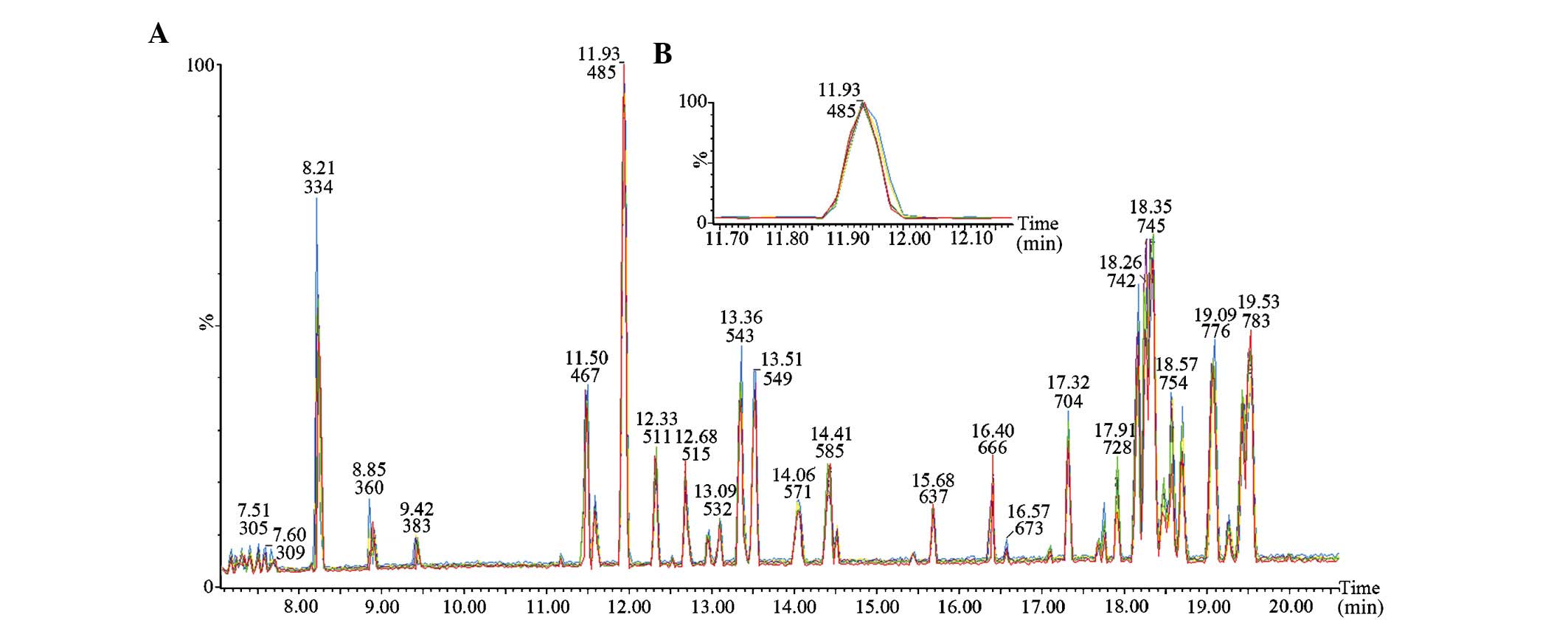
measured every eight samples throughout the experiment. The results
of five consecutive runs of the QC sample are shown in Fig. 1; the stable retention times and
tight overlap of the peaks demonstrate high repeatability and
stability of the analytical system.
Blood biochemical parameter analysis
All blood samples were subjected to analysis using
the Beckman-Coulter HMX automated system (Beckman-Coulter, Brea,
CA, USA) to analyze the biochemical blood parameters, including
hemoglobin (Hb), red blood cells (RBC) and white blood cells (WBC).
Serum carcinoembryonic antigen (CEA) was measured using a
chemiluminescent technique (CEA Access; Beckman Coulter).
UPLC-QTOF-MS analysis
UPLC was performed using a Waters Acquity UPLC
system (Waters Co., Milford, MA, USA) with a conditioned
autosampler at 4°C. A 5-µl sample was injected into a Waters
BEH C8 column (inner diameter × length, 2.1×100 mm; 1.7 µm
particle size), maintained at 50°C. Mobile phase A contained 0.1%
formic acid and 99.9% water or and mobile phase B contained 0.1%
formic acid and 99.9% acetonitrile. The gradient elution program
was as follows: 97% A in the initial 0–7 min, decreasing to 20% A
at 8 min and to 2% A at 16 min at a flow rate of at 300
µl/min. The conditions were kept constant for 5 min and then
changed to 100% B within 50 sec, which was maintained for 3 min.
The column was equilibrated to 97% A over 25 min, which was
maintained for 5 min.
Mass spectrometric analysis was performed using a
Waters Q-TOF Premier mass spectrometer (Waters Co.) in positive
electrospray ionization mode. The apparatus was previously
calibrated using sodium formate (Sigma-Aldrich) and a lock mass of
leucine enkephalin (0.5 ng/µl) was used for an accurate mass
determination setting at m/z 556.2771 in positive ion
mode. The detection parameters were optimized as follows: Capillary
voltage, 3 kV; and cone voltage, 40 V. The scanning time was 0.3
sec covering the 50–1,000 Dalton mass range. The source temperature
was set as 120°C and the desolvation gas temperature was 350°C.
Nitrogen (purchased as liquid nitrogen; Merriam-Graves Corp., White
River Junction, VT, USA) was used as the nebulizer gas at a flow
rate of 600 l/h.
Data analysis
The UPLC-MS data collected in positive ion mode were
pre-processed using Masslynx 4.1 software (Waters Co.). This
application was used for peak alignment to obtain a list containing
the m/z, retention time and intensities for all peaks
detected. The pre-processed data were exported and analyzed using
SIMCA-P+12.0 (Umetrics AB, Umea, Sweden). All data were normalized
and Pareto scaled prior to multivariate statistical analysis.
Unsupervised principal component analysis (PCA) was first used to
separate treatment groups from the control group. If the separation
between different groups was observed in the PCA plot, supervised
partial least-squares latent structure discriminate analysis
(PLS-DA) was then used to highlight the difference and obtain
metabolites that contributed to the classification. Potential
biomarkers were identified according to 'variable of importance in
projection' (VIP) values and S-plots.
To further identify the potential biomarkers, the
Human Metabolome Database (HMDB; http://hmdb.ca/),
PubChem compound (http://www.ncbi.nlm.nih. gov/) and Kyoto Encyclopedia
of Genes and Genomes (KEGG, http://www.genome.jp/kegg/) databases were searched to
match the selected ion spectra with those of the metabolites
obtained from databases. MS analysis of the experimental samples in
comparison with the standards was further performed for the
validation of the potential biomarkers.
Statistical analysis
Blood biochemical parameters and serum metabolic
biomarkers are expressed as the mean ± standard deviation. The
homogeneity of variances was verified using Bartlett's test.
One-way analysis of variance with Bonferroni's post-hoc test
was then performed to compare the spectral variables among
different serum samples. P≤0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed using GraphPad Prism 5 (GraphPad Inc. La Jolla, CA,
USA).
Results
Clinical biochemical features of CRC
patients
The clinical biochemical serum parameters of
patients prior to surgery (group 1), subsequent to surgery (group
2) and healthy controls (group 3) are presented in Fig. 2. Serum levels of CEA were
significantly elevated in group 1 compared with those in group 3
(P<0.001). Following surgery, the CEA levels were markedly
decreased by ~8-fold in group 2 (Fig.
2A). Two distinct populations with high and low levels of CEA
were observed in group 1, which may be due to the expression of CEA
depending on the location of the tumor (colon or rectal). By
contrast, a significant increase was noted in Hb levels following
surgery (P<0.001), while no statistically significant difference
was present between groups 2 and 3, thereby exhibiting a different
pattern of alteration from that of CEA (Fig. 2B). Furthermore, the red blood cell
count was slightly decreased in group 1 compared to that in groups
2 and 3, while the white blood cell count in group 1 was slightly
increased compared with that in groups 2 and 3 (Fig. 2C and D), indicating that the anemia
of the patients improved with surgical treatment. However, there
was no statistically significant difference in the red and white
blood cell count between groups 1–3 (P>0.05).
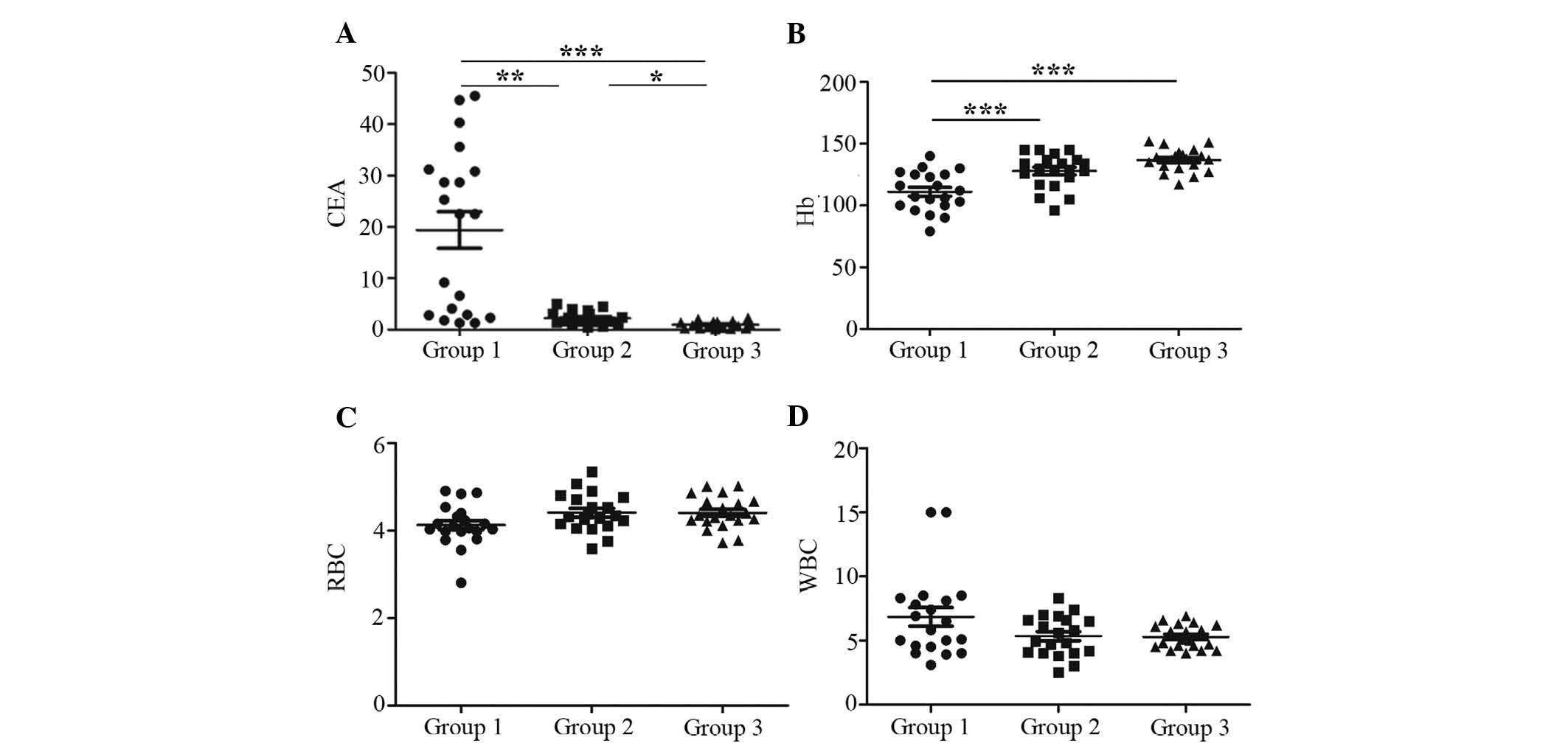 | Figure 2Clinical biochemical characteristics
of CRC patients and healthy controls. The serum levels of (A) CEA
(B) Hb (C) RBC (D) WBC in CRC patients prior and following
operation and in healthy controls. The scatter diagrams show that
serum levels of CEA in group 1 were markedly increased compared
with those in group 3 (P<0.001). After the operation, CEA levels
in group 2 decreased significantly (P<0.01) but remained higher
than the levels in group 3 (P<0.05). Unlike CEA, following
operation, serum levels of Hb in group 2 increased when compared to
those in group 1 (P<0.001), and no statistically significant
difference was noted between group 2 and group 3. Furthermore, the
RBC count was slightly lower and the WBC count was slightly higher
in group 1 compared with that in groups 2 and 3, while RBC and WBC
counts showed no statistically significant difference among the
three groups (P>0.05). Each data point represents the value for
one patient. Horizontal lines represent the mean values and bars
represent the standard deviation. *P<0.05,
**P<0.01, ***P<0.001. Groups: group 1,
CRC patients prior to operation; group 2, CRC patients posterior to
operation; group 3, healthy controls. CEA, carcinoembryonic
antigen; Hb, hemoglobin; RBC, red blood cell; WBC, white blood
cell; CRC, colorectal cancer. |
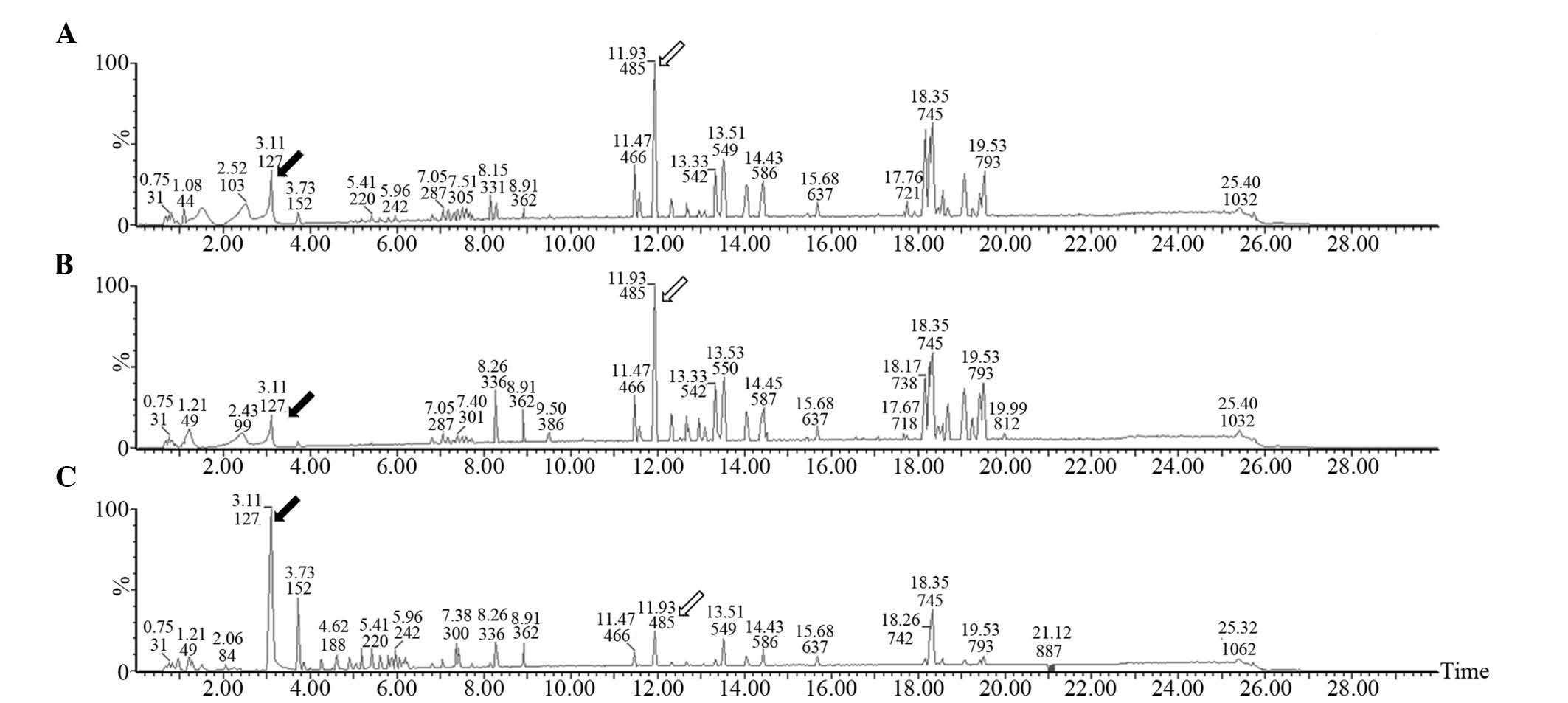
UPLC-MS analysis
Low-molecular-weight metabolites in the serum of CRC
patients prior to and following operation and healthy controls are
presented in base peak intensity chromatograms (Fig. 3). Compared with the healthy control
group, the levels of certain metabolites were increased (hollow
arrow) in the CRC patients, while others were decreased (black
arrow). The peak patterns in the pre-operative group were similar
to those in the post-operative group, suggesting a relatively small
effect of surgical operation on the patients' metabolite patterns.
However, the peak patterns in the healthy control group were
obviously different from those in the pre-operative and
post-operative groups.
PCA and PLS-DA analysis of UPLC-MS
data
In order to illustrate the differences in the
metabolic profiles among the three groups, an unsupervised PCA was
first used to analyze the multivariate data. In the PCA score plot,
data of the control and CRC serum samples did not cluster
sufficiently, as shown in Fig. 4A.
Therefore, a supervised PLS-DA was performed, which was better at
distinguishing the variation compared with the PCA method; it was
therefore used in order to discriminate the three groups according
to their the metabolic differences. The PLS-DA plot is shown in
Fig. 4B and C, where each data
point represents an independent sample. In spite of certain
overlaps between data points, the PLS-DA method was more suitable
for clustering of the three groups, producing a distinguished
classification. Similarities were observed within each group, and
the three distinct clusters clearly represented the pre-operative,
post-operative and healthy control groups in the PLS-DA scoring
plot, suggesting the presence of significant metabolic differences
between the three groups.
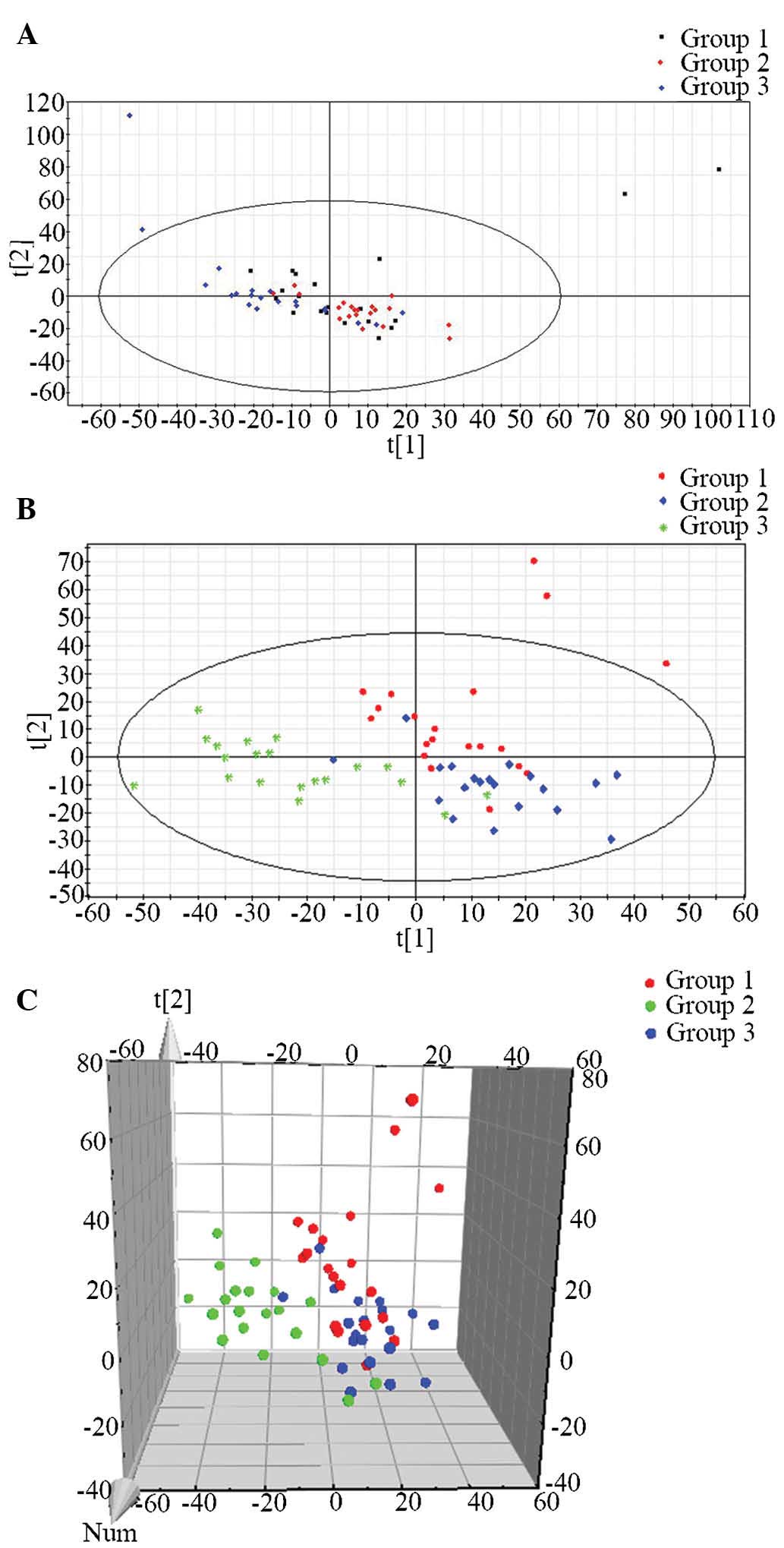 | Figure 4Unsupervised PCA and supervised
PLS-DA. (A) PCA score plot in positive ion mode. Green dots
represent samples of group 1, red diamonds represent samples of
group 2 and blue diamonds represent samples of group 3. (B) PLS-DA
score plot in positive ion mode based on UPLC-MS analysis data,
showing that the three groups were clearly distinguished from each
other. Red dots represent samples of group 1, blue diamonds
represent samples of group 2 and green stars represent samples of
group 3. (C) PLS-DA score 3D plot in positive ion mode based on
ultra-performance liquid chromatography quadrupole time-of-flight
mass spectrometry data. Red represents samples of group 1, blue
represents samples of group 2 and green represents samples of group
3 Group 1, patients with CRC prior to operation; group 2, patients
with CRC following operation; group 3, healthy controls. PCA,
principal component analysis; PLS-DA, partial least-squares
discriminant analysis; CRC, colorectal cancer; t1, largest
variation in the UPLC-MS data; t2, second-largest variation in the
UPLC-MS data; Num, number; UPLC-MS, ultra-performance liquid
chromatography coupled with quadrupole time-of-flight mass
spectrometry. |
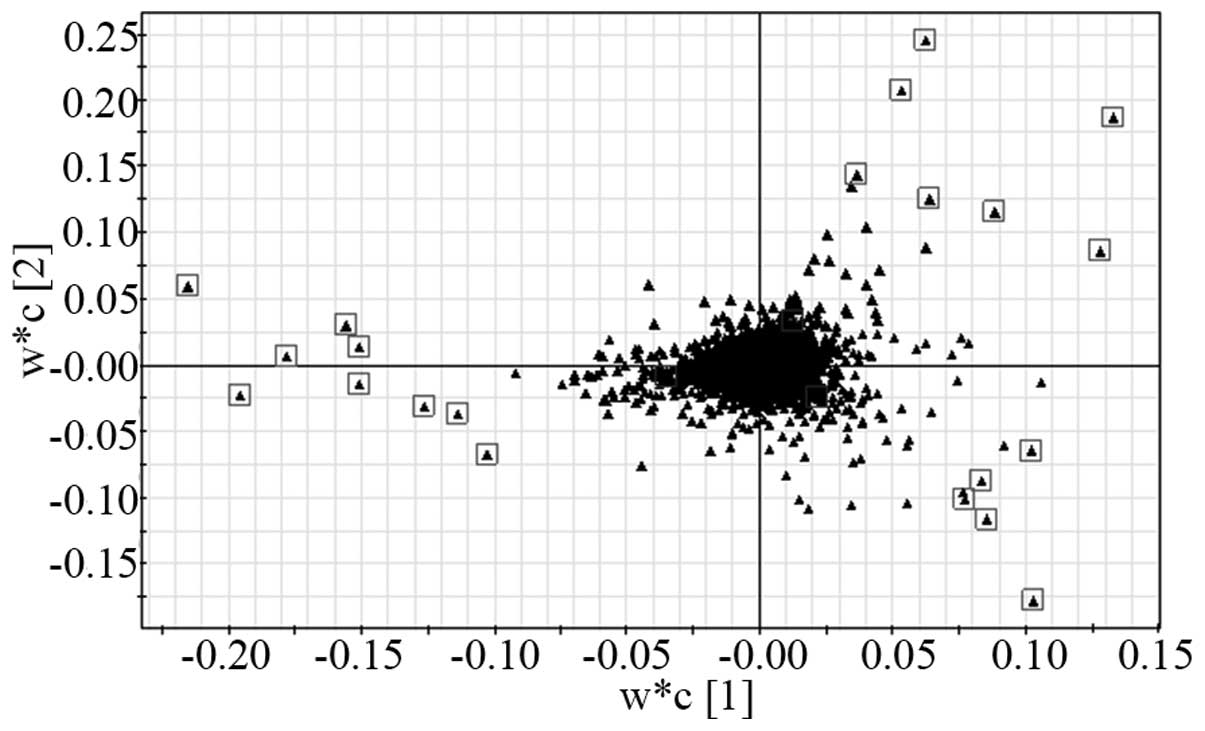
Candidate biomarker identification
According to the VIP values (the top 20 VIP) and the
S-plot, 20 metabolites were selected as candidate biomarkers
(Table II). In the S-plot,
significantly different metabolites were identified among the
pre-operative, post-operative and control groups, which may be
utilized for discriminating between the three groups. The boxes
indicate the candidate biomarker which are most suitable for the
discrimination of groups in the S-plot (Fig. 5).
 | Table IISignificant serum biomarkers
associated with colorectal cancer. |
Table II
Significant serum biomarkers
associated with colorectal cancer.
| Rt | m/z | VIP | Adduct | Identity | Significance
|
|---|
| Group 1–2 | Group 1–3 | Group 2–3 |
|---|
| 18.32 | 758.5718 | 10.3999 |
[M+H]+ | 16:0/18:2-PC | ns | ↓ | ↓↓↓ |
| 17.91 | 782.5719 | 9.13802 |
[M+H]+ | 18:2/18:2-PC | ns | ↓↓↓ | ↓↓↓ |
| 11.93 | 496.3411 | 8.91459 |
[M+H]+ | LPC16:0 | ns | ↑↑ | ns |
| 19.52 | 786.6036 | 8.31944 |
[M+H]+ | 18:0/18:2-PC | ns | ↓ | ↓↓↓ |
| 18.31 | 780.5537 | 7.41249 |
[M+Na]+ | 16:0/18:2-PC | ns | ↓ | ↓↓ |
| 19.51 | 808.5862 | 7.10026 |
[M+Na]+ | 18:0/18:2-PC | ns | ↓↓ | ↓↓↓ |
| 17.92 | 804.5538 | 7.03144 |
[M+Na]+ | 18:2/18:2-PC | ns | ↓↓↓ | ↓↓↓ |
| 18.62 | 806.5697 | 6.58736 |
[M+Na]+ | 18:1/18:2-PC | ns | ↓ | ns |
| 11.48 | 542.3235 | 5.95541 |
[M+Na]+ | LPC18:2 | ns | ↓↓ | ↓↓ |
| 11.92 | 991.6754 | 5.70262 |
[2M+H]+ | LPC16:0 | ns | ↑↑↑ | ↑↑ |
| 18.16 | 806.5722 | 5.42741 |
[M+H]+ | 16:0/22:6-PC | ns | ns | ns |
| 11.48 | 520.3411 | 5.40934 |
[M+H]+ | LPC18:2 | ns | ↓ | ↓ |
| 11.93 | 518.3238 | 5.26510 |
[M+Na]+ | LPC16:0 | ns | ↑ | ns |
| 14.41 | 603.4681 | 5.11722 |
[M+H]+ | DAG | ns | ns | ↑↑ |
| 18.62 | 784.5876 | 4.98453 |
[M+H]+ | 16:0/20:3-PC | ns | ns | ns |
| 13.34 | 524.3726 | 4.42997 |
[M+H]+ | LPC18:0 | ns | ↑ | ns |
| 11.58 | 518.3226 | 4.17220 |
[M+Na]+ | LPC16:0 isomer | ns | ↑ | ns |
| 18.25 | 782.5720 | 3.93874 |
[M+H]+ | 16:0/20:4-PC | ns | ↓ | ns |
| 19.43 | 810.6032 | 3.75328 |
[M+H]+ | 18:0/20:4-PC | ns | ↓ | ns |
| 17.76 | 780.5553 | 3.63848 |
[M+H]+ | 16:0/20:5-PC | ns | ns | ns |
The HMDB, PubChem compound and KEGG databases were
then searched to compare the MS data with chemical standards in
order to identify the potential biomarkers, which are listed in
Table II. Levels of three groups
of biomarkers, lysophosphatidylcholines (LPCs),
phosphatidylcholines (PCs) and diacylglycerols (DAGs), were
significantly different between the patients with CRC and the
healthy controls. Among them, PCs and LPCs which contain
polyunsaturated fatty acids were decreased, whereas LPCs and DAGs
which contain saturated fatty acids were increased in CRC patients
compared with those in healthy individuals. However, the top 20
discriminating metabolites were not significantly different between
pre-operative and post-operative CRC patients (P>0.05);
therefore, they are not suitable for discriminating between pre-
and post-operative patients.
Discussion
Metabonomics is a rapidly developing discipline that
provides a broad scope as well as direct information on complex
cellular responses with a low requirement of material and sample
preparation (14). It has been
extensively applied in human diseases and has significantly
contributed to the discovery of novel biomarkers of diseases. CRC
represents a major cause of cancer-associated mortality worldwide
(1). Metabonomics has offered a
novel perspective regarding the genesis of CRC as well as an
approach towards cancer diagnosis. Recently, multiple biomarkers
were identified in the tissues and biofluids of CRC patients
(1,2,5).
However, most of these markers have also been discovered in several
other metabolic disorders (15–17).
Therefore, specific biomarkers for monitoring CRC remain to be
discovered.
The present study utilized UPLC-MC in order to
detect serum metabolites in patients with CRCs. Due to the large
amount of data obtained, multivariate statistical analysis models,
including PCA and PLS-DA, were used to discriminate between
pre-operative and post-operative groups as well as healthy
controls. By using PLS-DA, in spite of certain overlaps between
data-points, three distinct clusters representing the three
different groups were obtained. Within each group, in-group
similarities were observed, which distinguished them from the other
groups. Candidate biomarkers for CRC patients at different
time-points of treatment were selected according to VIP values and
the S-plot. Metabolites that significantly contributed to the
discrimination of CRC patients were identified as LPCs, PCs and
DAGs. Most of the PCs were decreased in CRC patients, while the
majority of the LPCs and DAGs were markedly increased. Of note,
LPCs which contain saturated fatty acids [e.g. LPC (16:0) and LPC
(18:0)] were increased, whereas LPCs containing polyunsaturated
fatty acids [e.g. LPC (18:2)] were decreased in CRC patients.
The mechanisms by which PCs decrease in the serum of
CRC patients may depend on several factors. One of them may be
associated with the decrease in PC synthesis. PCs are major lipid
components of biomembranes, produced by two pathways: The de
novo pathway (Kennedy pathway) and the re-modeling pathway
(Land's pathway) (18). In the
Kennedy pathway, PCs are synthesized by CDP-choline and
diacylglycerol under the catalysis of PC synthase. Almost all
cancer types, including CRC (19),
lung cancer (20), heptocellular
carcinoma (21), are characterized
by specific shift in energy metabolism. A predominance of aerobic
glycolysis over oxidative phosphorylation (Warburg effect) is
usually present in cancer cells (22). As a result, the insufficient
formation of adenosine triphosphate and CDP-choline induce a
reduction in PC synthesis. Another mechanism is based on the
susceptibility of lipids containing polyunsaturated fatty acids to
free radicals and enzymes (23).
Due to oxidative stress, the integrity of cellular membranes is
destroyed and the generated lipid hydroperoxide becomes a major
reaction product (24). Lipid
hydroperoxide has been detected in a diverse range of diseases,
including diabetes (15), cancer
(16), arthritis (17) and Alzheimer's disease (25). The results of the present study
showed that the PCs which were decreased in CRC patients were
mainly polyunsaturated PCs. Thus, the other possible reason for the
reduction of PCs is the peroxidation and transformation of PCs. In
addition, following surgery, compared with the healthy controls,
the serum levels of PCs decreased even more significantly than
those in the pre-operative group, suggesting that the decreased
levels of PCs following surgery may be the result of injury-induced
elevation of aerobic glycolysis. This hypothesis is required to be
explored by further studies.
LPCs are the hydrolysis products of PCs. The present
study showed that LPCs containing polyunsaturated fatty acids were
downregulated in CRC patients, which was in line with the opposite
trend for PCs containing polyunsaturated fatty acids. Phospholipase
A2, a critical enzyme in catalyzing the hydrolysis of PCs into
LPCs, has an anti-tumorigenic role in various types of cancer
(26–28), and its low activity in CRC patients
may contribute to the decrease of LPCs. LPCs act as a bioactive
mediator in various biological processes, including injury and
inflammatory responses (29,30),
cellular motility, growth and regulation of differentiation
(31). Numerous clinical
experiments have shown that serum LPCs levels are lower in patients
with advanced cancer (32–34). However, the results of the present
study also showed that LPCs containing saturated fatty acids were
upregulated in CRC patients. A possible explanation may be the
different roles of various LPC sub-types in tumorigenesis. For
example, certain sub-types of LPCs and other lysophospholipids have
been demonstrated to elevate the production of multiple growth
factors in breast cancer cells, including interleukin-6 and -8,
which are regulators of neovascularization (35).
DAGs are well-known secondary messengers in
signaling pathways regulating cell proliferation and apoptosis
(36,37). DAGs are also involved in the
structural regulation of organelle morphology. Acute DAG deletion
disables nuclear membrane assembly and causes alterations of
endoplasmic reticulum morphology (38). In the present study, the levels of
DAGs were not significantly different between the pre-operative
group and the healthy control group. However, following surgery,
the serum levels of DAGs were markedly increased when compared to
those in the healthy controls, indicating that DAGs may function as
structural components of organelles to repair cells damaged during
the operation.
In conclusion, the present metabonomics study used
the novel UPLC-QTOF-MS method, which is a sensitive and effective
tool for biomarker discovery, to identify three groups of
biomarkers for CRC, namely LPCs, PCs and DAGs. PCs and LPCs
containing polyunsaturated fatty acids were decreased, whereas LPCs
and DAGs containing saturated fatty acids were increased in CRC
patients. To the best of our knowledge, the present study was the
first to use an UPLC-QTOF-MS-based serum metabolite analysis
approach to compare CRC patients at various time-points of
treatment with healthy controls. Although the number of
participants included in the present study was relatively small, a
clear discrimination between CRC patients and healthy volunteers
was observed. However, the absence of significant differences in
metabolite profiles between pre-operative and post-operative groups
may suggest the importance and necessity of post-operative medical
procedures, such as chemotherapy. Once the serum metabonomic
results of the present study are validated in a larger number of
patients, it is anticipated that serum analysis using UPLC-QTOF-MS
may become a standard clinical procedure to effectively diagnose
and monitor CRC patients.
Acknowledgments
The present study was supported by the National
Basic Research Program of China (973 Program; grant no.
2013CB531402). The authors would like to thank the State Key
Laboratory for Diagnosis and Treatment of Infectious Diseases, the
First Affiliated Hospital, Medical College, Zhejiang University
(Hangzhou, China) for their assistance with the operation of the
UPLC-QTOF-MS apparatus.
References
|
1
|
Sideris M and Papagrigoriadis S: Molecular
biomarkers and classification models in the evaluation of the
prognosis of colorectal cancer. Anticancer Res. 34:2061–2068.
2014.PubMed/NCBI
|
|
2
|
Zhang A, Sun H, Yan G, Wang P, Han Y and
Wang X: Metabolomics in diagnosis and biomarker discovery of
colorectal cancer. Cancer Lett. 345:17–20. 2014. View Article : Google Scholar
|
|
3
|
Møller Sørensen N, Vejgaard Sørensen I,
Ørnbjerg Würtz S, Schrohl AS, Dowell B, Davis G, Jarle Christensen
I, Nielsen HJ and Brünner N: Biology and potential clinical
implications of tissue inhibitor of metalloproteinases-1 in
colorectal cancer treatment. Scand J Gastroenterol. 43:774–786.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berkovich L, Shpitz B, Ghinea R, Greemland
I, Kravtsov V, Kidron D, Mishaeli M and Avital S: Evaluation of
peritoneal CEA levels following colorectal cancer surgery. J Surg
Oncol. 110:458–462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goedert JJ, Sampson JN, Moore SC, Xiao Q,
Xiong X, Hayes RB, Ahn J, Shi J and Sinha R: Fecal metabolomics:
Assay performance and association with colorectal cancer.
Carcinogenesis. 35:2089–2096. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Griffin JL and Bollard ME: Metabonomics:
Its potential as a tool in toxicology for safety assessment and
data integration. Curr Drug Metab. 5:389–398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu T, Xie G, Ni Y, Liu T, Yang M, Wei H,
Jia W and Ji G: Serum metabolite signatures of type 2 diabetes
mellitus complications. J Proteome Res. 14:447–456. 2015.
View Article : Google Scholar
|
|
8
|
Chan AW, Gill RS, Schiller D and Sawyer
MB: Potential role of metabolomics in diagnosis and surveillance of
gastric cancer. World J Gastroenterol. 20:12874–12882. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao L, Wan L, Qiu X, Li R, Liu S and Wang
D: A metabonomics profiling study on phlegm syndrome and
blood-stasis syndrome in coronary heart disease patients using
liquid chromatography/quadrupole time-of-flight mass spectrometry.
Evid Based Complement Alternat Med. 2014:3851022014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fanos V, Pintus MC, Lussu M, Atzori L,
Noto A, Stronati M, Guimaraes H, Marcialis MA, Rocha G, Moretti C,
et al: Urinary metabolomics of bronchopulmonary dysplasia (BPD):
Preliminary data at birth suggest it is a congenital disease. J
Matern Fetal Neonatal Med. 27(Suppl 2): 39–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma YL, Qin HL, Liu WJ, Peng JY, Huang L,
Zhao XP and Cheng YY: Ultra-high performance liquid
chromatography-mass spectrometry for the metabolomic analysis of
urine in colorectal cancer. Dig Dis Sci. 54:2655–2662. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wilson ID, Nicholson JK, Castro-Perez J,
Granger JH, Johnson KA, Smith BW and Plumb RS: High resolution
'ultra performance' liquid chromatography coupled to oa-TOF mass
spectrometry as a tool for differential metabolic pathway profiling
in functional genomic studies. J Proteome Res. 4:591–598. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akkoca AN, Yanık S, Ozdemir ZT, Cihan FG,
Sayar S, Cincin TG, Cam A and Ozer C: TNM and modified Dukes
staging along with the demographic characteristics of patients with
colorectal carcinoma. Int J Clin Exp Med. 7:2828–2835.
2014.PubMed/NCBI
|
|
14
|
Fuhrer T and Zamboni N: High-throughput
discovery metabolomics. Curr Opin Biotechnol. 31:73–78. 2015.
View Article : Google Scholar
|
|
15
|
Tsakanova GV, Ayvazyan VA, Boyajyan AS,
Arakelova EA, Grigoryan GS, Guevorkyan AA and Mamikonyan AA: A
comparative study of antioxidant system and intensity of lipid
peroxidation in type 2 diabetes mellitus and ischemic stroke
aggravated and not aggravated by type 2 diabetes mellitus. Bull Exp
Biol Med. 151:564–566. 2011. View Article : Google Scholar
|
|
16
|
Balci H, Genc H, Papila C, Can G, Papila
B, Yanardag H and Uzun H: Serum lipid hydroperoxide levels and
paraoxonase activity in patients with lung, breast and colorectal
cancer. J Clin Lab Anal. 26:155–160. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Navarro-Compán V, Melguizo-Madrid E,
Hernández-Cruz B, Santos-Rey K, Leyva-Prado C, González-Martín C,
Navarro-Sarabia F and González-Rodríguez C: Interaction between
oxidative stress and smoking is associated with an increased risk
of rheumatoid arthritis: A case-control study. Rheumatology
(Oxford). 52:487–493. 2013. View Article : Google Scholar
|
|
18
|
Shindou H, Hishikawa D, Harayama T, Yuki K
and Shimizu T: Recent progress on acyl CoA: Lysophospholipid
acyltransferase research. J Lipid Res. 50(Suppl): S46–S51. 2009.
View Article : Google Scholar :
|
|
19
|
Baltaziak M, Wincewicz A, Kanczuga-Koda L,
Lotowska JM, Koda M, Sulkowska U, Baltaziak M, Podbielski M,
Sobaniec-Lotowska ME and Sulkowski S: The relationships between
hypoxia-dependent markers: HIF-1alpha, EPO and EPOR in colorectal
cancer. Folia Histochem Cytobiol. 51:320–325. 2013. View Article : Google Scholar
|
|
20
|
Zhang J, Cao J, Ma S, Dong R, Meng W, Ying
M, Weng Q, Chen Z, Ma J, Fang Q, et al: Tumor hypoxia enhances
non-small cell lung cancer metastasis by selectively promoting
macrophage M2 polarization through the activation of ERK signaling.
Oncotarget. 5:9664–9677. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu Y, Lin N, Chen Z and Xu R:
Hypoxia-induced secretion of platelet-derived growth factorBB by
hepatocellular carcinoma cells increases activated hepatic stellate
cell proliferation, migration and expression of vascular
endothelial growth factor A. Mol Med Rep. 11:691–697. 2015.
|
|
22
|
Granchi C, Fancelli D and Minutolo F: An
update on therapeutic opportunities offered by cancer glycolytic
metabolism. Bioorg Med Chem Lett. 24:4915–4925. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Catalá A: Lipid peroxidation of membrane
phospholipids generates hydroxy-alkenals and oxidized phospholipids
active in physiological and/or pathological conditions. Chem Phys
Lipids. 157:1–11. 2009. View Article : Google Scholar
|
|
24
|
Sugiyama A and Sun J: Immunochemical
detection of lipid hydroperoxide- and aldehyde-modified proteins in
diseases. Subcell Biochem. 77:115–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoo MH, Gu X, Xu XM, Kim JY, Carlson BA,
Patterson AD, Cai H, Gladyshev VN and Hatfield DL: Delineating the
role of glutathione peroxidase 4 in protecting cells against lipid
hydroperoxide damage and in Alzheimer's disease. Antioxid Redox
Signal. 12:819–827. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ganesan K, Ivanova T, Wu Y, Rajasegaran V,
Wu J, Lee MH, Yu K, Rha SY, Chung HC, Ylstra B, et al: Inhibition
of gastric cancer invasion and metastasis by PLA2G2A, a novel
beta-catenin/TCF target gene. Cancer Res. 68:4277–4286. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fijneman RJ, Peham JR, van de Wiel MA,
Meijer GA, Matise I, Velcich A and Cormier RT: Expression of
Pla2g2a prevents carcinogenesis in Muc2-deficient mice. Cancer Sci.
99:2113–2119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Avoranta T, Sundström J, Korkeila E,
Syrjänen K, Pyrhönen S and Laine J: The expression and distribution
of group IIA phospholipase A2 in human colorectal tumours. Virchows
Arch. 457:659–667. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma J, Yu J, Su X, Zhu C, Yang X, Sun H,
Chen D, Wang Y, Cao H and Lu J: UPLC-MS-based serum metabonomics
for identifying acute liver injury biomarkers in Chinese miniature
pigs. Toxicol Lett. 225:358–366. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nankar SA, Prajapati JS and Pande AH:
Apolipoprotein E derived peptides inhibit the pro-inflammatory
effect of lysophosphatidylcholine. Protein Pept Lett. 21:101–107.
2014. View Article : Google Scholar
|
|
31
|
Bassa BV, Noh JW, Ganji SH, Shin MK, Roh
DD and Kamanna VS: Lysophosphatidylcholine stimulates EGF receptor
activation and mesangial cell proliferation: Regulatory role of Src
and PKC. Biochim Biophys Acta. 1771:1364–1371. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuliszkiewicz-Janus M, Tuz MA and
Baczyński S: Application of 31P MRS to the analysis of phospholipid
changes in plasma of patients with acute leukemia. Biochim Biophys
Acta. 1737:11–15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taylor LA, Arends J, Hodina AK, Unger C
and Massing U: Plasma lyso-phosphatidylcholine concentration is
decreased in cancer patients with weight loss and activated
inflammatory status. Lipids Health Dis. 6:172007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Süllentrop F, Moka D, Neubauer S, Haupt G,
Engelmann U, Hahn J and Schicha H: 31P NMR spectroscopy of blood
plasma: Determination and quantification of phospholipid classes in
patients with renal cell carcinoma. NMR Biomed. 15:60–68. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Umezu-Goto M, Tanyi J, Lahad J, Liu S, Yu
S, Lapushin R, Hasegawa Y, Lu Y, Trost R, Bevers T, et al:
Lysophosphatidic acid production and action: Validated targets in
cancer? J Cell Biochem. 92:1115–1140. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Poli A, Mongiorgi S, Cocco L and Follo MY:
Protein kinase C involvement in cell cycle modulation. Biochem Soc
Trans. 42:1471–1476. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Poli A, Ramazzotti G, Matteucci A, Manzoli
L, Lonetti A, Suh PG, McCubrey JA and Cocco L: A novel
DAG-dependent mechanism links PKCa and Cyclin B1 regulating cell
cycle progression. Oncotarget. 5:11526–11540. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Domart MC, Hobday TM, Peddie CJ, Chung GH,
Wang A, Yeh K, Jethwa N, Zhang Q, Wakelam MJ, Woscholski R, et al:
Acute manipulation of diacylglycerol reveals roles in nuclear
envelope assembly & endoplasmic reticulum morphology. PLoS One.
7:e511502012. View Article : Google Scholar :
|