Introduction
Diabetes mellitus (DM) is a heterogeneous group of
metabolic disorders characterized by hyperglycemia with impaired
metabolism of carbohydrate, fat and proteins as a result of defects
in insulin secretion and/or insulin action (1,2). The
incidence of diabetes has been steadily increasing and is expected
to rise to 439 million adults in 2030 worldwide (3). Type 1 diabetes (T1D) is a T
cell-mediated autoimmune disorder caused by decreased insulin
production due to destruction of insulin-secreting β-cells in
pancreatic islets (4–6). Although transplantation of islets of
Langerhans has recently been suggested to be an efficient
cell-based therapy for T1D, the outcome has remained poor due to
the risk of immunological rejection as well as rareness of donors
(7–9). Therefore, it is required to identify
novel renewable sources for expanding pancreatic islets.
A growing number of stem cell biological studies
have suggested that somatic stem cells, i.e. mesenchymal stem cells
(MSCs), including bone marrow and adipose stromal progenitor cells,
have potential therapeutic value for DM (10,11).
Since MSCs, particularly MSCs from bone marrow (BMSCs), have the
potential for multiple passages in culture and differentiation into
various cell types, including endocrine cells of the pancreas
(12), they are particularly
promising for use in the treatment DM. BMSCs have been evidenced to
have therapeutic value in the treatment of T1D due to their
potential for differentiation into insulin-secreting cells and
their immunomodulatory properties (6,13,14).
Although adipose-derived stromal cells (AdSCs) share a number of
characteristics with BMSCs, which have the ability to proliferate
and differentiate into a variety of cell types (15), it has largely remained elusive
whether AdSCs can differentiate into insulin-producing cells
(IPCs). Thus, the present study aimed to investigate the ability of
the rabbit (r)AdSCs to differentiate into IPCs, which may offer a
potentially effective therapeutic approach in cell therapy of
DM.
Materials and methods
Isolation and culture of AdSCs
In total, three male New Zealand White rabbits
(weight, 2–3 kg) were purchased from the Experimental Animal Center
of Changchun Biological Institute (Changchun, China) and maintained
in specific pathogen-free conditions. Rabbits were sacrificed by
cervical dislocation. Subcutaneous adipose tissues of the inguinal
region were harvested using scissors under sterile conditions. To
remove red blood cells and tissue debris, adipose tissue was washed
with an equal volume of sterile phosphate-buffered saline (PBS)
containing 1% 100 U/ml penicillin, or 100 mg/ml streptomycin (Gibco
Life Technologies, Carlsbad, CA, USA). Washed tissue fragments were
digested with 0.075% collagenase II (Sigma-Aldrich, St. Louis, MO,
USA). The cell density was adjusted to 1×105/ml and
cells were plated in 100-cm2 tissue culture flasks in
low-glucose Dulbecco's Modified Eagle Medium (LG-DMEM; Gibco-BRL,
Invitrogen Life Technologies, Inc. Carlsbad, CA, USA) supplemented
with 10% fetal bovine serum (FBS; Sigma-Aldrich). Cells were
cultured in an incubator at 37°C in a humidified atmosphere
containing 5% CO2 and medium was replaced every 3–4
days. Adherent cells were sub-cultured by detachment with 0.25%
trypsin (containing 0.02% EDTA; Sigma-Aldrich) when they were
80–90% confluent. Cells of passage three were used in the
subsequent experiments. The study was approved by the ethics
committee of Jilin University (Changchun, China).
Induction of differentiation into
insulin-secreting cells
rAdSCs of the third passage and of 80% confluency
were used for induction of differentiation. The cells were seeded
into 24-well plates at a density of 1×105/well and were
divided into an induction group and a control group. The induction
group was cultured in DMEM containing 20 ng/ml epidermal growth
factor (Invitrogen Life Technologies, Inc.) for 24 h at 37°C in a
humidified atmosphere containing 5% CO2 and then
supplemented with serum-free DMEM containing 10 mM niacinamide
(Sigma-Aldrich) and l00 nM glucagon-like peptide (GLP-1;
Sigma-Aldrich) followed by 21 days of culture. The media were
replaced every three days. The control group was cultured in
serum-free DMEM without niacinamide or GLP-1.
Dithizone (DTZ) staining
DTZ (ADL) stock solution was prepared by dissolving
10 mg DTZ in 1 ml of dimethyl sulfoxide (Sigma-Aldrich). The
staining solution was filtered through a 0.2-mm filter, and for
in vitro DTZ staining, 10 µl stock solution was added
to 1 ml culture medium. The cells of the induction and control
groups were incubated at 37°C for 25 min in the DTZ solution. After
the cells were rinsed three times with PBS, crimson red-stained
clusters were observed under an X51 inverted light microscope
(Olympus, Tokyo, Japan).
As positive control cells, pancreatic matrix cells
of rabbits were used. Briefly, rabbit pancreatic tissue was
isolated from an adult New-Zealand rabbit. The tissue was washed
twice with an equal volume of sterile D-Hanks solution
(Sigma-Aldrich) after excision. Washed tissue fragments were
digested with 0.075% collagenase II (Sigma-Aldrich) for 30 min at
37°C and then centrifuged at 1,200 ×g for 10 min. Subsequently, the
collected cells were filtered through a 100-µm cell strainer
(Greiner Bio-One, Kremsmünster, Austria) and incubated at 37°C for
25 min in the DTZ solution as a positive staining control. After
the cells were rinsed three times with PBS, crimson red-stained
clusters were observed under an X51 inverted light microscope
(Olympus, Tokyo, Japan).
RNA extraction and reverse transcription
polymerase chain reaction (RT-PCR) analysis
Total RNA was isolated from undifferentiated rAdSCs
(negative control), human beta-cells (positive control; Shanghai
Institute for Biological Sciences, Shanghai, China) and
differentiated rAdSCs at the final stage of induction using TRIzol
reagent (Invitrogen Life Technologies). RT was performed with 5
µg of total RNA purified after DNAse I treatment using a
commercially available RT-PCR kit (Takara Bio Inc., Otsu, Japan)
according to the manufacturer's instructions. According to the
GenBank database (http://www.ncbi.nlm.nih.gov/genbank) entries for the
cDNA sequences of insulin, PDX1 and GLUT2,
corresponding primers were designed and synthesized by Shanghai
Biological Engineering Company (Shanghai, China). For insulin, the
forward primer was 5′-ATCAAGCAGATCACTGTCCTTCT-3′ and the reverse
primer was 5′-GAGAGCTTCCACCAGGTGTG-3′. The PCR mixture (Takara
Biotechnology Co., Ltd., Dalian, China) contained cDNA (4
µl), 25 mM desoxyribonucleo-tide triphosphates (1
µl), 10X PCR buffer (5 µl), forward and reverse
primer (50 pmol; 2 µl), Taq enzyme (1 U; 2.0 µl) and
double-distilled H2O (34 µl). The conditions for
PCR were as follows: 94°C for 3 min; 30 cycles of 94°C for 20 sec,
62°C for 20 sec, 72°C for 30 sec, and a final elongation at 72°C
for 5 min. PDX1 primers were as follows: Forward,
5′-TCCCATGGATGAAGTCTACC-3′ and reverse, 5′-TGTCCTCCTCCTTTTTCCAC-3′.
The components of the PCR mixture were the same as those above. The
PCR conditions were as follows: 30 cycles of 94°C for 30 sec, 60°C
for 30 sec and 72°C for 30 sec, and a final elongation at 72°C for
5 min. GLUT2 primers were as follows: Forward,
5′-AGTACAATGACAGAAGATAAGGTC-3′ and reverse,
5′-AGCTCCAACTAATGACAGAATG-3′. The components of the PCR mixture
were the same to those above. The PCR conditions were as follows:
32 cycles of 94°C for 30 sec, 56°C for 30 sec and 72°C for 1 min,
and a final elongation at 72°C for 5 min. The PCR products were
subjected to 1% agarose gel electrophoresis. GAPDH served as an
internal reference to normalize the expression of the respective
target genes. The GAPDH primers were as follows: Forward,
5′-GAAGGTGAAGGTCGGAGTC-3′, and reverse, 5′-GAAGATGGTGATGGGATTTC-3′.
The PCR conditions were as follows: 32 cycles of 94°C for 30 sec,
56°C for 30 sec and 72°C for 1 min, and a final elongation at 72°C
for 5 min. Gel images were analyzed using the UVI band map program
(Uvitec, Cambridge, UK). Gel images were observed under a AB1
Veriti Gel Imaging Analysis system (Applied Biosystems Life
Technologies, Foster City, CA, USA) and were analyzed using an UVI
Band Map program (Uvitec, Cambridge, UK). The experiments were
repeated thrice and a t-test was employed for analysis.
Western blot analysis
Total protein was extracted from undifferentiated
AdSCs (negative control), human beta-cells (positive control) and
differentiated AdSCs at the final stage of induction on day 21 were
lysed in radioimmunoprecipitation assay buffer (Sigma-Aldrich)
containing 10 mM Tris-HCl (pH 8.0), 1% NP-40, 10% glycerol, 0.1%
SDS, 1 mM EDTA and 100 mM NaCl with protease inhibitor cocktail
(Roche Diagnostics, Basel, Switzerland). The protein concentration
was determined using the bicinchoninic acid protein assay kit
(KeyGEN Biotech, Nanjing, China). Equal amounts of protein (30
µg/lane) from the cell lysates were separated by 10%
SDS-PAGE and transferred onto nitrocellulose membranes (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). The membrane was incubated
for 2 h in PBS containing 0.1% Tween-20 (Sigma-Aldrich) and 5%
skimmed milk (Sigma-Aldrich) to block non-specific binding. The
membranes were then incubated overnight at 4°C with mouse
polyclonal anti-rabbit insulin (1:1,000; cat. no. sc-9168; Santa
Cruz Biotechnology, Inc.). Mouse polyclonal anti-human GAPDH
(1:10,000; cat. no. sc-25778; Santa Cruz Biotechnology, Inc.) was
used as a loading control. The membranes were incubated with
polyclonal goat anti-mouse horseradish peroxidase-conjugated
secondary antibody (1:10,000; cat. no. sc-2004; Santa Cruz
Biotechnology, Inc.) at room temperature for 2 h, and proteins were
detected by enhanced chemiluminescence western blotting detection
system (Thermo Fisher Scientific, Waltham, MA, USA), and were
analyzed using image analysis software ImageJ 3.1 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Glucose challenge assay
The cell density of differentiated rAdSCs was
adjusted to 1×105/ml, cells were seeded into a 24-well
plate (100 µl/well) and wells were divided into LG group and
high-glucose (HG) group (24 wells per group). Undifferentiated
rAdSCs were used as a control group. Following differentiation of
the cells for 7, 14 and 21 days, the LG group and HG group were
established by adding DMEM containing 10.0 mmol/l glucose and DMEM
containing 30.0 mmol/l glucose, respectively, followed by 24 h of
incubation. Then insulin production was measured in the cell
supernatants using rabbit insulin ELISA kits (Invitrogen Life
Technologies, Inc.).
Statistical analysis
Values are expressed as the mean ± standard
deviation of data from at least three independent experiments.
Student's t-test and one-way analysis of variance were used
with the level of significance set as P<0.05. All data were
analyzed using GraphPad Prism version 5.01 for Windows®
(GraphPad Software, Inc., La Jolla, CA, USA).
Results
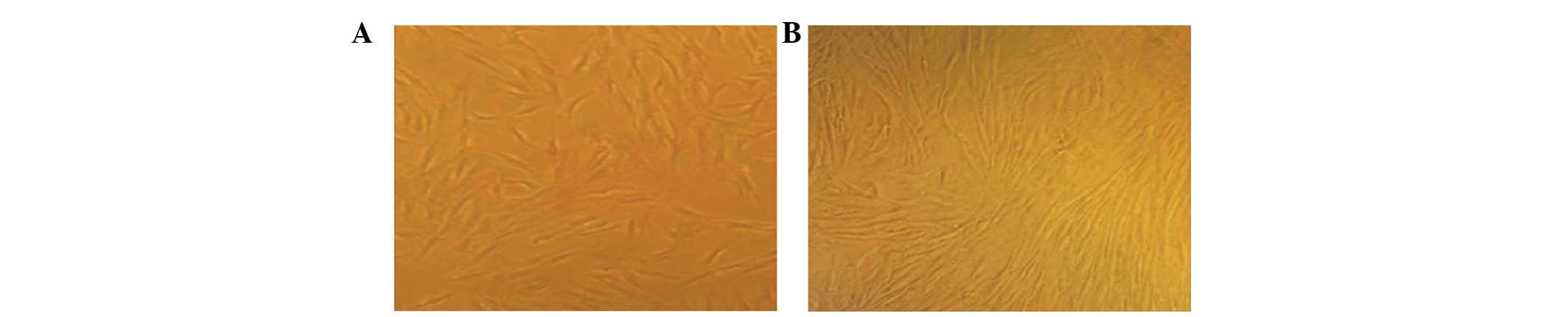
rAdSCs undergo morphological changes
during differentiation
During differentiation, morphological changes of
rAdSCs were observed under a phase contrast inverted microscope.
Prior to differentiation, the rAdSCs exhibited a typical stromal
cell-like morphology (Fig. 1A). Of
note, upon differentiation, the cells began to contract, the
ecphyma became shorter, and the morphology changed from
spindle-like to a round shape (Fig.
1B).
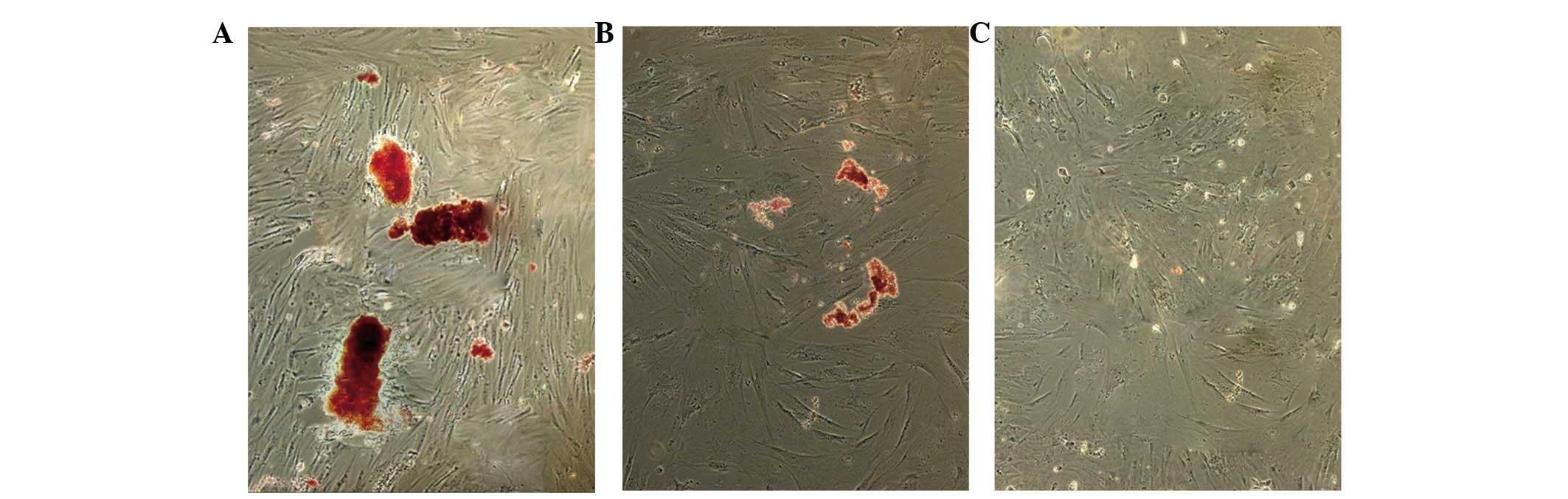
rAdSCs differentiate into
insulin-producing, cluster-forming cells
The present study identified IPCs by DTZ staining.
Regions of pancreatic endocrine cells in culture can be
specifically labeled, since beta-cells in the islet contain a large
amount of zinc, which can be specifically labeled with the
zinc-chelating dye DTZ (16–18).
As shown in Fig. 2A, pancreatic
endocrine cells (positive control) were distinctly stained crimson
red by DTZ. Furthermore, rAdSCs in the induction group were
distinctly stained crimson red by DTZ at the final step of
differentiation on day 21 (Fig.
2B), while undifferentiated cells (negative control) were
negative for DTZ (Fig. 2C).
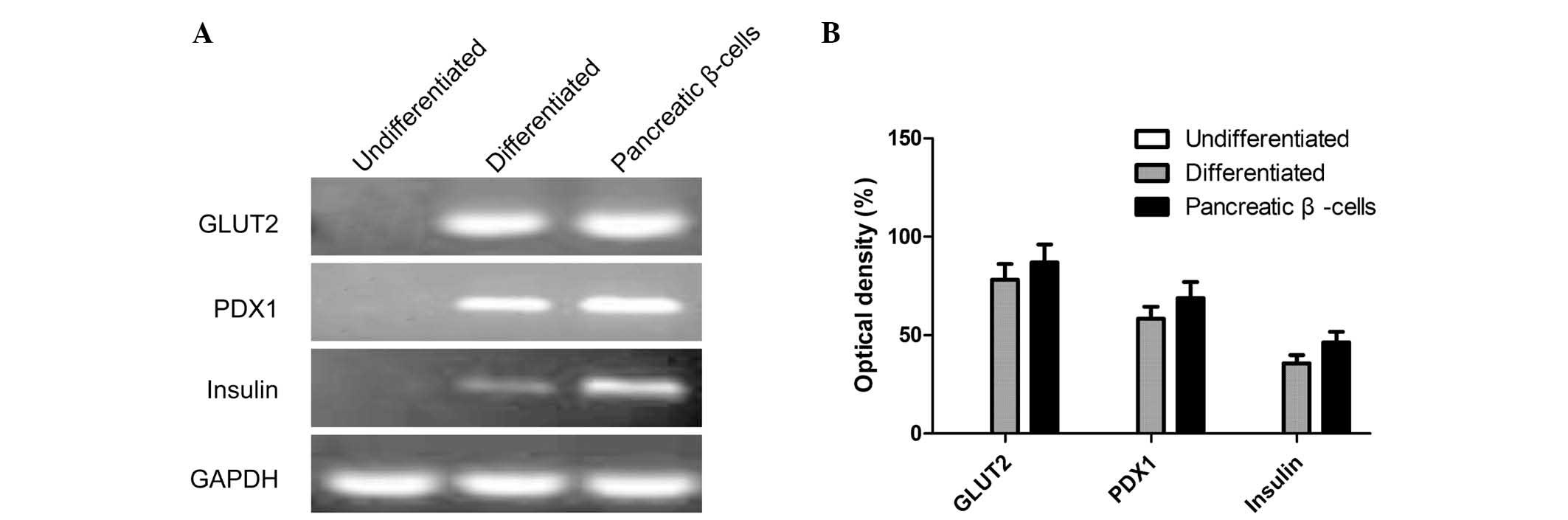
rAdSCs are able to differentiate into
insulin-producing pancreatic endocrine cells
To determine whether the rAdSCs had differentiated
into pancreatic endocrine cells, the expression of genes associated
with pancreatic endocrine-cell development and function, including
the mRNA expression of insulin, PDX1 and GLUT2, were
examined by RT-PCR analysis. The results showed that
insulin, PDX1 and GLUT2 were expressed in
human beta-cells (positive control) as well as in differentiated
AdSCs at the final stage of induction, while negative expression
was observed in undifferentiated cells (negative control) (Fig. 3A and 3B).
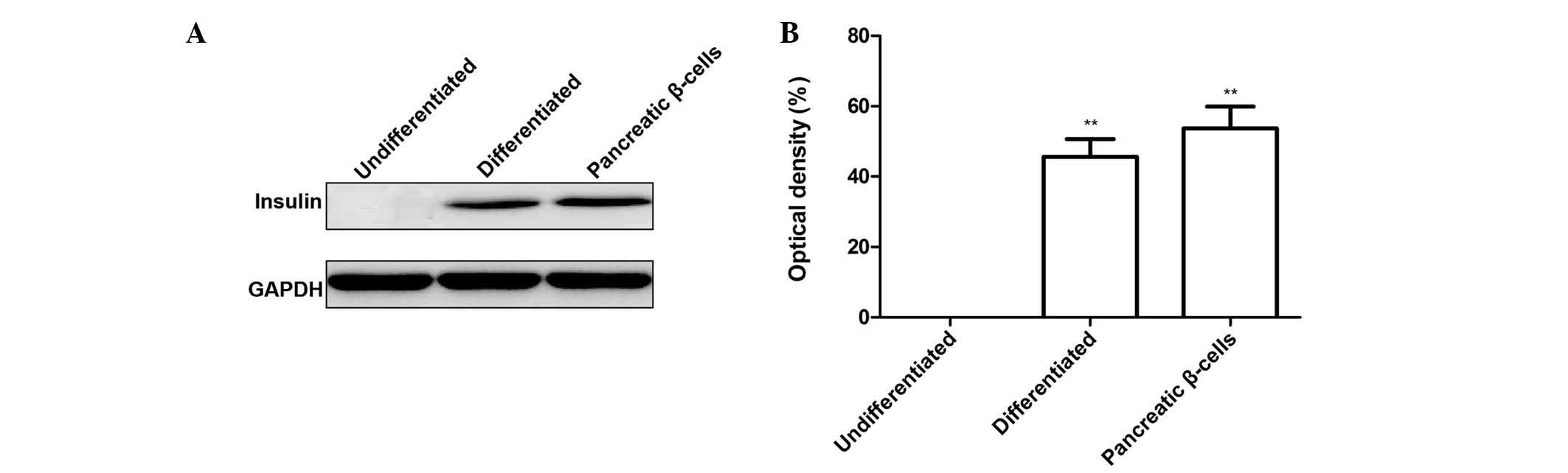
To confirm the insulin expression of the IPCs at the
protein level, western blot analysis was performed. It was found
that insulin protein was expressed in human beta-cells (positive
control) as well as in differentiated AdSCs, while undifferentiated
AdSCs did not express any insulin (Fig. 4A and 4B). In addition, insulin protein in
beta-cells was markedly higher than that of differentiated AdSCs
(Fig. 4B). All of the above
results indicated that AdSCs possess the potential to differentiate
into functional IPCs.
Measurement of insulin production of
glucose challenge assay
To quantify the insulin production of AdSC-derived
IPCs following glucose challenge, an ELISA assay was performed.
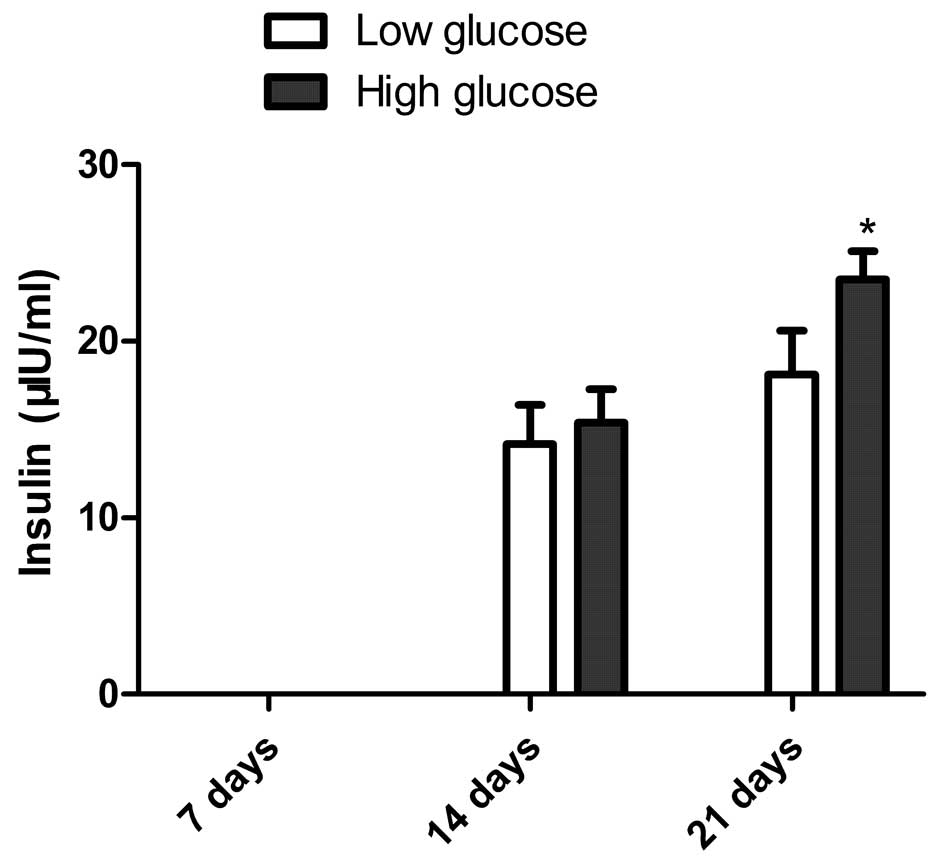
Insulin expression was negative in the HG and LG groups after 7
days of differentiation. While insulin was detected in the culture
supernatant of AdSCs induced to differentiate for 14 days, there
was no significant difference in insulin production between the LG
group (14.2±2.2 µIU/ml) and the HG group (15.4±1.9
µIU/ml) (P>0.05; Fig.
5). Of note, insulin production was increased in the two groups
after 21 days of differentiation, with insulin production in the HG
group (23.5±1.6 µIU/ml) being significantly higher than that
in the LG group (18.1±2.5 µIU/ml) (P<0.05, Fig. 5). The control cells did not exhibit
any insulin production at the indicated time-points (results not
shown).
Discussion
A number of previous studies have demonstrated the
potential of MSCs obtained from various sources in the treatment of
DM (19,20). AdSCs share a number of
characteristics with BMSCs (12);
therefore, AdSCs have become another hot-spot in the field of stem
cell research besides BMSCs. Zuk et al (21) verified that AdSCs can be isolated
from human lipoaspirates and differentiate toward osteogenic,
adipogenic, myogenic and chondrogenic lineages. A recent study
showed that adipose-derived MSCs were able to differentiate into
IPCs in vitro by induction with β-mercaptoethanol and
nicotinamide (22). Consistent
with this result, the present study showed that rAdSCs
differentiated into IPCs following induction with GLP-1 and
nicotinamide.
Numerous studies have provided protocols for the
efficient transdifferentiation of stem cells into IPCs, with
varying degrees of successful differentiation (12,22).
In order to successfully achieve differentiation of stem cells into
IPCs the key point is to select suitable inductive agents and to
optimize the hemopoietic inductive in vitro
microenvironment, which should resemble the conditions in the body.
GLP-1 is a peptide hormone, which is produced post-translationally
from pro-glucagon genes; GLP-1 stimulates insulin gene translation
and insulin biosynthesis, promotes the differentiation and
generation of insulin-excreting precursor cells (23), stimulates the generation and
differentiation of β cells and reduces their apoptosis (24). PDX-1 is the main controlling gene
in growth of pancreatic endocrine cells; it promotes early
development of pancreatic cells and the advanced differentiation of
β cells (25). GLP-1 is able to
regulate gene expression via multiple signaling pathways, promote
the synthesis of PDX-1 and increase the binding activity of PDX-1
to promoters (26). It has been
shown that GLP-1 is able to induce stem-cell differentiation into
islets cells with a high differentiation induction rate (26). The results of the present study
showed that AdSCs were able to differentiate into IPCs by induction
with DMEM supplemented with GLP-1 and nicotinamide.
Niacinamide is an inhibitor of adenosine diphosphate
ribose synthesis, which can promote the differentiation of
pancreatic cells in human fetuses, as well as maintain normal
responses of islet cells to glucose stimulation in a HG environment
over a long time period (27).
Therefore, niacinamide is widely used as a constitutive component
of induction media for the differentiation of stem cells into IPCs
(28). The results of the present
study showed that GLP-1 in combination with niacinamide
successfully induced the differentiation of AdSCs into IPCs.
Although the present study showed that rAdSCs were
able to differentiate into IPCs, their insulin secretion was far
lower than that of normal human pancreatic islet cells, which may
have been due to the fact that the rAdSCs had not been selected
thoroughly enough. Lower insulin production may also have been due
to incomplete differentiation, and further studies should further
optimize the differentiation conditions Molecular markers of islet
stem cells are of high significance for the identification and
purification from the mass of other cells. While no specific marker
is currently available, purified clones from insulin-secreting stem
cells may be employed, which should be developed in future
studies.
Acknowledgments
The authors gratefully acknowledge the financial
support provided by the Science and Technology Research and
Innovation Team funded by Jilin province (no. JL2012058).
References
|
1
|
Gao D, Xie J, Zhang J, Feng C, Yao B, Ma
K, Li J, Wu X, Huang S and Fu X: MSC attenuate diabetes-induced
functional impairment in adipocytes via secretion of insulin-like
growth factor-1. Biochem Biophys Res Commun. 452:99–105. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
American Diabetes A: Diagnosis and
classification of diabetes mellitus. Diabetes Care. 36(Suppl 1):
S67–S74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shaw JE, Sicree RA and Zimmet PZ: Global
estimates of the prevalence of diabetes for 2010 and 2030. Diabetes
Res Clin Pract. 87:4–14. 2010. View Article : Google Scholar
|
|
4
|
Chen LB, Jiang XB and Yang L:
Differentiation of rat marrow mesenchymal stem cells into
pancreatic islet beta-cells. World J Gastroenterol. 10:3016–3020.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oh SH, Muzzonigro TM, Bae SH, LaPlante JM,
Hatch HM and Petersen BE: Adult bone marrow-derived cells
trans-differentiating into insulin-producing cells for the
treatment of type I diabetes. Lab Invest. 84:607–617. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vija L, Farge D, Gautier JF, Vexiau P,
Dumitrache C, Bourgarit A, Verrecchia F and Larghero J: Mesenchymal
stem cells: Stem cell therapy perspectives for type 1 diabetes.
Diabetes Metab. 35:85–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu XH, Liu CP, Xu KF, Mao XD, Zhu J, Jiang
JJ, Cui D, Zhang M, Xu Y and Liu C: Reversal of hyperglycemia in
diabetic rats by portal vein transplantation of islet-like cells
generated from bone marrow mesenchymal stem cells. World J
Gastroenterol. 13:3342–3349. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nejad-Dehbashi F, Hashemitabar M,
Orazizadeh M, Bahramzadeh S, Shahhosseini Pourshoushtary E and
Khorsandi L: The effects of exendine-4 on insulin producing cell
differentiation from rat bone marrow-derived mesenchymal stem
cells. Cell J. 16:187–194. 2014.PubMed/NCBI
|
|
9
|
Zhang YH, Wang HF, Liu W, Wei B, Bing LJ
and Gao YM: Insulin-producing cells derived from rat bone marrow
and their autologous transplantation in the duodenal wall for
treating diabetes. Anat Rec (Hoboken). 292:728–735. 2009.
View Article : Google Scholar
|
|
10
|
Gimble JM, Katz AJ and Bunnell BA:
Adipose-derived stem cells for regenerative medicine. Circ Res.
100:1249–1260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Noël D, Caton D, Roche S, Bony C, Lehmann
S, Casteilla L, Jorgensen C and Cousin B: Cell specific differences
between human adipose-derived and mesenchymalstromal cells despite
similar differentiation potentials. Exp Cell Res. 314:1575–1584.
2008. View Article : Google Scholar
|
|
13
|
Tyndall A, Walker UA, Cope A, Dazzi F, De
Bari C, Fibbe W, Guiducci S, Jones S, Jorgensen C, Le Blanc K, et
al: Immunomodulatory properties of mesenchymal stem cells: A review
based on an interdisciplinary meeting held at the Kennedy Institute
of Rheumatology Division, London, UK, 31 October 2005. Arthritis
Res Ther. 9:3012007. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krampera M, Pasini A, Pizzolo G, Cosmi L,
Romagnani S and Annunziato F: Regenerative and immunomodulatory
potential of mesenchymal stem cells. Curr Opin Pharmacol.
6:435–441. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takahara K, Ii M, Inamoto T, Komura K,
Ibuki N, Minami K, Uehara H, Hirano H, Nomi H, Kiyama S, et al:
Adipose-derived stromal cells inhibit prostate cancer cell
proliferation inducing apoptosis. Biochem Biophys Res Commun.
446:1102–1107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li L, Li F, Qi H, Feng G, Yuan K, Deng H
and Zhou H: Coexpression of Pdx1 and betacellulin in mesenchymal
stem cells could promote the differentiation of nestin-positive
epithelium-like progenitors and pancreatic islet-like spheroids.
Stem Cells and Dev. 17:815–823. 2008. View Article : Google Scholar
|
|
17
|
Bruin JE, Erener S, Vela J, Hu X, Johnson
JD, Kurata HT, Lynn FC, Piret JM, Asadi A, Rezania A and Kieffer
TJ: Characterization of polyhormonal insulin-producing cells
derived in vitro from human embryonic stem cells. Stem Cell Res.
12:194–208. 2014. View Article : Google Scholar
|
|
18
|
Wei R, Yang J, Hou W, Liu G, Gao M, Zhang
L, Wang H, Mao G, Gao H, Chen G and Hong T: Insulin-producing cells
derived from human embryonic stem cells: Comparison of definitive
endoderm- and nestin-positive progenitor-based differentiation
strategies. PloS one. 8:e725132013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao F, Wu DQ, Hu YH, Jin GX, Li GD, Sun TW
and Li FJ: In vitro cultivation of islet-like cell clusters from
human umbilical cord blood-derived mesenchymal stem cells. Transl
Res. 151:293–302. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chandra V, Swetha G, Muthyala S, Jaiswal
AK, Bellare JR, Nair PD and Bhonde RR: Islet-like cell aggregates
generated from human adipose tissue derived stem cells ameliorate
experimental diabetes in mice. PloS one. 6:e206152011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zuk PA, Zhu M, Ashjian P, De Ugarte DA,
Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P and Hedrick
MH: Human adipose tissue is a source of multipotent stem cells. Mol
Biol Cell. 13:4279–4295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moshtagh PR, Emami SH and Sharifi AM:
Differentiation of human adipose-derived mesenchymal stem cell into
insulin-producing cells: An in vitro study. J Physiol Biochem.
69:451–458. 2013. View Article : Google Scholar
|
|
23
|
Bregenholt S, Møldrup A, Blume N, Karlsen
AE, Nissen Friedrichsen B, Tornhave D, Knudsen LB and Petersen JS:
The long-acting glucagon-like peptide-1 analogue, liraglutide,
inhibits beta-cell apoptosis in vitro. Biochem Biophys Res Commun.
330:577–584. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hui H, Nourparvar A, Zhao X and Perfetti
R: Glucagon-like peptide-1 inhibits apoptosis of insulin-secreting
cells via a cyclic 5′-adenosine monophosphate-dependent protein
kinase A- and a phosphatidylinositol 3-kinase-dependent pathway.
Endocrinology. 144:1444–1455. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li LX, MacDonald PE, Ahn DS, Oudit GY,
Backx PH and Brubaker PL: Role of phosphatidylinositol
3-kinasegamma in the beta-cell: Interactions with glucagon-like
peptide-1. Endocrinology. 147:3318–3325. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X, Zhou J, Doyle ME and Egan JM:
Glucagon-like peptide-1 causes pancreatic duodenal homeobox-1
protein translocation from the cytoplasm to the nucleus of
pancreatic beta-cells by a cyclic adenosine monophosphate/protein
kinase A-dependent mechanism. Endocrinology. 142:1820–1827.
2001.PubMed/NCBI
|
|
27
|
Ji D, Li GY and Osborne NN: Nicotinamide
attenuates retinal ischemia and light insults to neurones.
Neurochem Int. 52:786–798. 2008. View Article : Google Scholar
|
|
28
|
Yang SJ, Choi JM, Kim L, Park SE, Rhee EJ,
Lee WY, Oh KW, Park SW and Park CY: Nicotinamide improves glucose
metabolism and affects the hepatic NAD-sirtuin pathway in a rodent
model of obesity and type 2 diabetes. J Nutr Biochem. 25:66–72.
2014. View Article : Google Scholar
|