Introduction
Transfusion-related acute lung injury (TRALI) is a
complex syndrome which is characterized by acute hypoxia and
non-cardiogenic pulmonary edema occurring within six hours of blood
transfusion and is the leading cause of transfusion-associated
morbidity and mortality (1–3).
Platelets have been implicated in the triggering of
neutrophils, which leads to the damage of the vasculature (4–6).
CD62P is a Ca2+-dependent receptor stored in the
alpha-granules of platelets and Weibel-Palade bodies of endothelial
cells (7,8). It translocates to the plasma membrane
and is released into the plasma in soluble form during platelet
activation (9,10), mediating the interaction of
activated endothelial cells or platelets with leukocytes upon
inflammatory and thrombogenic challenges (11–13).
A study by Looney et al (14) found that depletion of either
neutrophils or platelets had protective effects against lung injury
in an immune-mediated mouse model of TRALI. CD62P was identified to
be an important indicator of platelet activation (15,16)
and activated platelets have been implicated in TRALI (17,18).
However, the precise roles of CD62P in the occurrence of TRALI have
remained elusive.
The present study assessed CD62P accumulation during
storage of apheresis platelet concentrates (A-Plts) and established
a murine model of TRALI to further investigate the role of CD62P in
TRALI.
Materials and methods
A-Plts collection
The present study was approved by the Ethics
Committee of The General Hospital of the People's Liberation Army
(Beijing, China). A total of 25 A-Plts samples were collected from
healthy volunteers and stored according to standard procedures
according to the blood donation law of the People's Republic of
China.
Assessment of CD62P
The CD62P concentration in A-Plts was measured using
a Human sP-Selectin/CD62P ELISA kit (cat. no. SBBE6; R&D
Systems, Inc., Minneapolis, MN, USA), following the manufacturer's
instructions.
Establishment of a TRALI model and
anti-CD62P antibody treatment
Male BALB/c mice, aged 8–10 weeks and weighing
250±30 g were purchased from Shanghai Slac Laboratory Animal
Company (Shanghai, China). A total of 40. were used, which were
maintained in individual cages in a room with a controlled
temperature (22–24°C) and light cycle (12 h light/dark), free
access to food and fresh water. The TRALI model was constructed
according to Looney's method (14). The mice were anesthetized with
ketamine (80 mg/kg; Eurovet Animal Health B.V., Bladel, The
Netherlands) and xylazine (12 mg/kg; Bayer AG, Leverkusen, Germany)
intra-peritoneally (i.p.). Subsequently, the mice were placed in a
supine position on a warming blanket and the jugular vein was
isolated. A 30-gauge sterile needle was attached to polyethylene
tubing and venous blood was aspirated from the jugular vein to
verify intravascular placement of the needle. The mice were
injected with lipopolysaccharide (0.1 mg/kg i.p.) 24 h prior to
intravenous injection with 150–250 µl (6 mg/ml) mouse monoclonal
anti-major histocompatibility complex (MHC)-1 immunoglobulin
(Ig)G2a, κ [4.5 mg/kg; cat. no. HB-79; American Type Culture
Collection (ATCC), Manassas, VA, USA], while control mice were
injected with phosphate-buffered saline (PBS; 0.1 mg/kg i.p.) 24 h
prior to injection with isotype-matched mouse monoclonal antibody
(mAb) (IgG2a, κ; 4.5 mg/kg; cat. no. CRL-1908; ATCC). For CD62P
neutralization studies, mice received rat monoclonal anti-CD62P
antibody (25 µg/mouse; IgG1, λ; cat. no. 553743; BD Biosciences,
San Jose, CA, USA) or isotype control antibody 15 min prior to
TRALI. The skin was sutured with prolene 5-0 (Johnson &
Johnson, Inc., St. Stevens-Woluwe, Belgium). The mice were placed
back into their cages following recovery from anaesthesia. The mice
were sacrificed 2 h following the establishment of the TRALI model
via an i.p. injection of pentobarbital (200 mg/kg; Sigma-Aldrich,
St. Louis, MO, USA).
Branch alveolar lavage fluid (BALF) and
blood collection
The right lung was ligated, the left lung was
lavaged with 2 ml 0.9% saline through the tracheal cannula for
three times and the BALF was retrieved. The BALF was centrifuged
for 15 min at 450 × g and the supernatant was stored at lung was
lavaged with 2 ml 0.9% saline through the tracheal cannula for
three times and the BALF was retrieved. The BALF was centrifuged
for 15 min at 450 × g and the supernatant was −80°C for measurement
of protein levels. 2 h following TRALI, blood was collected from
the carotic artery and placed in 3.2% sodium citrate-containing
tubes (Wenzhou Gande Medical Instrument Co., Ltd., Wenzhou, China).
Following centrifugation at 800 × g for 15 min, the plasma was
separated and stored at −80°C.
Determination of total protein, cytokines
and thrombin-anti-thrombin complex (TATc)
The concentration of total protein in the BALF was
measured using a Bradford Protein Assay kit (cat. no. 5000001;
Bio-Rad Laboratories Inc, Hercules, CA, USA) following the
manufacturer's instructions. Briefly, albumin was used to prepare a
protein standard curve. Samples were diluted with buffer, then dye
reagent was added to each sample and incubated for 5 min. The
absorbance at 590 nm was measured using a visible light
spectrophotometer (Alpha 1102; LASPEC Inc., Shanghai, China). The
concentration of each sample was calculated according to the
protein standard curve.
The concentrations of interleukin (IL)-6, macrophage
inflammatory protein 2 (MIP-2) and keratinocyte-derived chemokine
(KC) in the plasma and the BALF was determined using the following
commercial ELISA kits: Mouse IL-6 Quantikine ELISA kit (cat. no.
M6000B), Mouse CXCL2/MIP-2 Quantikine ELISA kit (cat. no. MM200)
and a Mouse CXCL1/KC Quantikine ELISA kit (cat. no. MKC00B, all
obtained from R&D Systems. The detection limit was 1.8, 1.5 and
2 pg/ml, respectively. TATc in the plasma and the BALF was measured
using a Mouse thrombin-antithrombin complex TAT ELISA kit (cat. no.
MU30254; Bio-Swamp Life Science, Wuhan, China), according to the
manufacturer's instructions.
Measurement of lung wet-to-dry (W/D)
weight ratio
The middle lobe of the right lung was removed and
the wet weight was determined. Following drying at 60°C for 72 h,
the dry weight was determined. The lung W/D weight ratio was
calculated as follows: W/D weight ratio = wet weight/dry
weight.
Myeloperoxidase (MPO) assay
The lung tissues were homogenized in PBS containing
0.1% NP40 in a Dounce glass homogenizer (Thomas Scientific,
Swedesboro, NJ, USA) and centrifuged for 10 min at 13,000 xg and
4°C to remove any insoluble material. MPO activity was measured
using an MPO Activity Colorimetric Assay kit (cat. no. K744-100;
BioVision, Milpitas, CA, USA), according to the manufacturer's
instructions.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Student's t test was used to assess differences
between the two groups. Statistical analysis was performed using
SPSS 19.0 statistical software (IBM SPSS, Armonk, NY, USA).
P<0.05 was considered to indicate a significant difference
between values.
Results
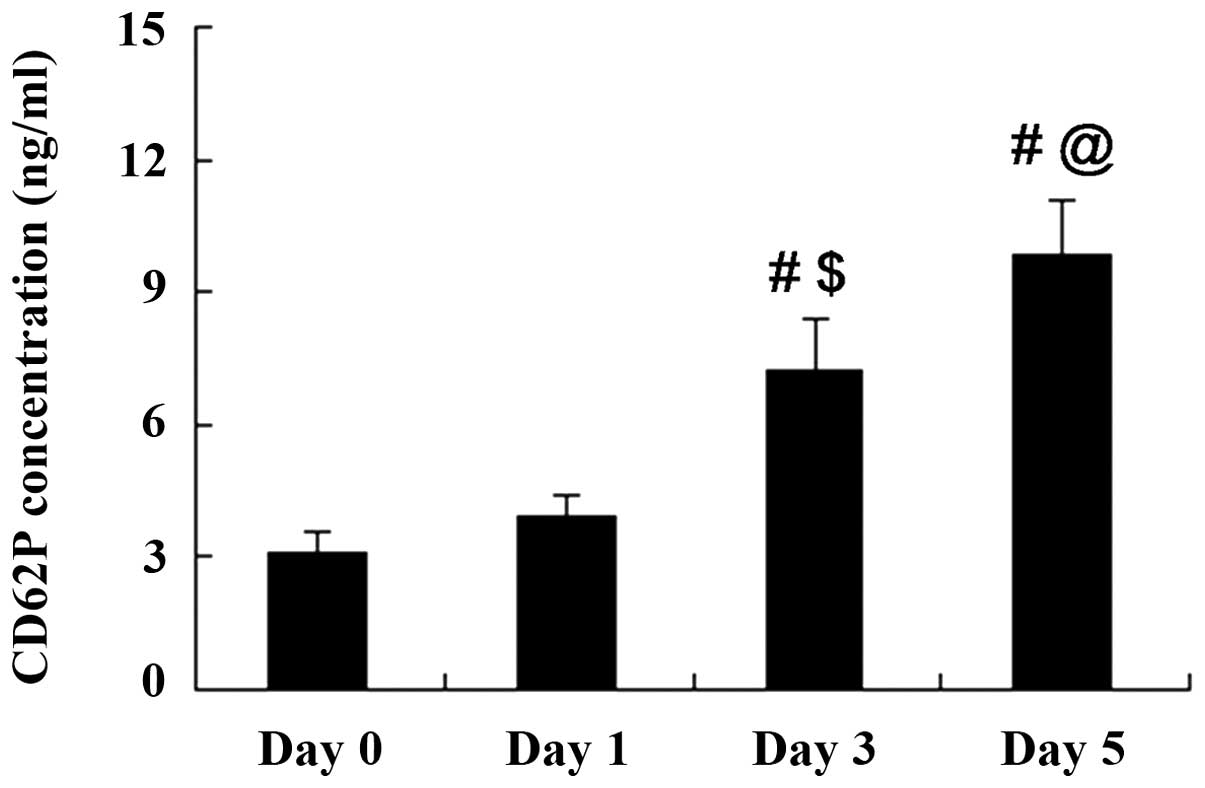
CD62P accumulates in stored A-Plts
CD62P expression in A-Plts with a storage time of 0,
1, 3 and 5 days was detected using a commercial ELISA kit. As shown
in Fig. 1, the CD62P concentration
in A-Plts was increased with the storage time. CD62P concentrations
on days 0 and 1 of storage were not significantly different
(P>0.05). However, CD62P concentrations on days 3 and 5 of
storage were significantly increased compared with those at days 0
and 1.
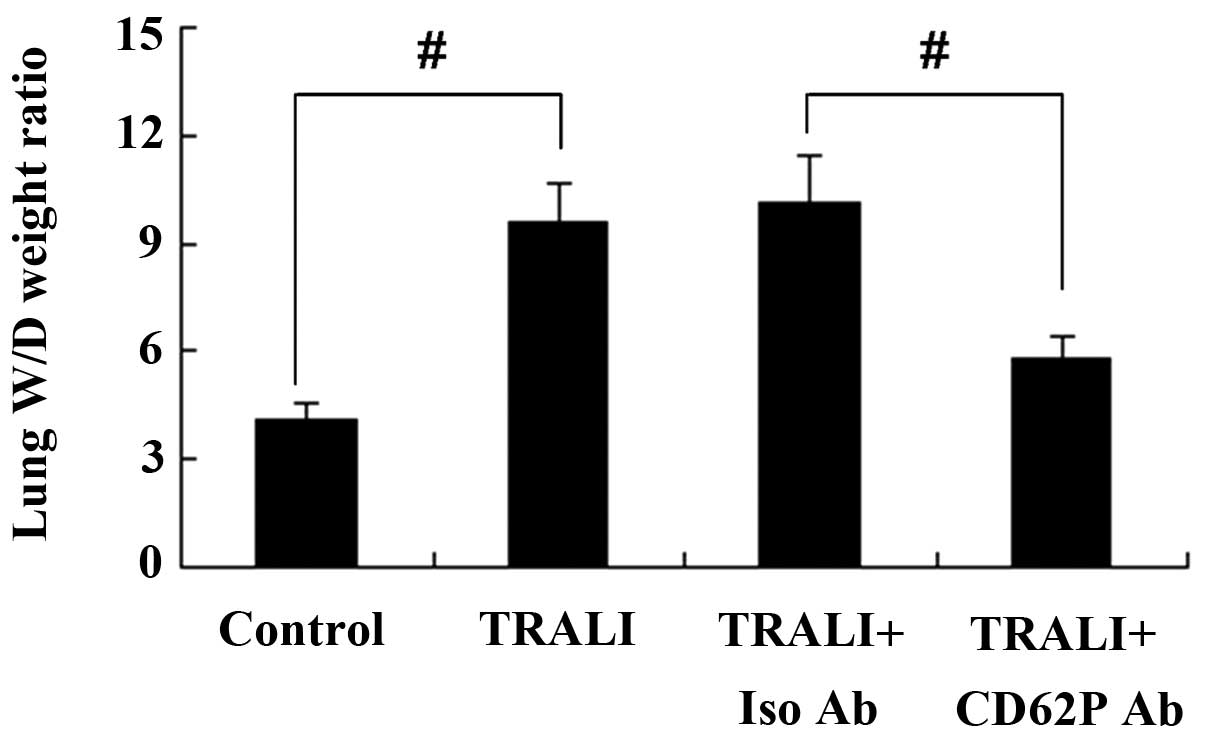
CD62P is involved in the formation of
pulmonary edema in a murine model of TRALI
As a measure of pulmonary edema, the lung W/D weight
ratio of mice was determined following TRALI. As shown in Fig. 2, compared with the control group, a
significant increase of the W/D weight ratio was observed in the
TRALI group (P<0.01). To investigate the effect of CD62P
knockdown on TRALI, mice were treated with anti-CD62P antibody. The
results showed that knockdown of CD62P resulted in a decreased lung
wet-to-dry weight ratio of mice with TRALI.
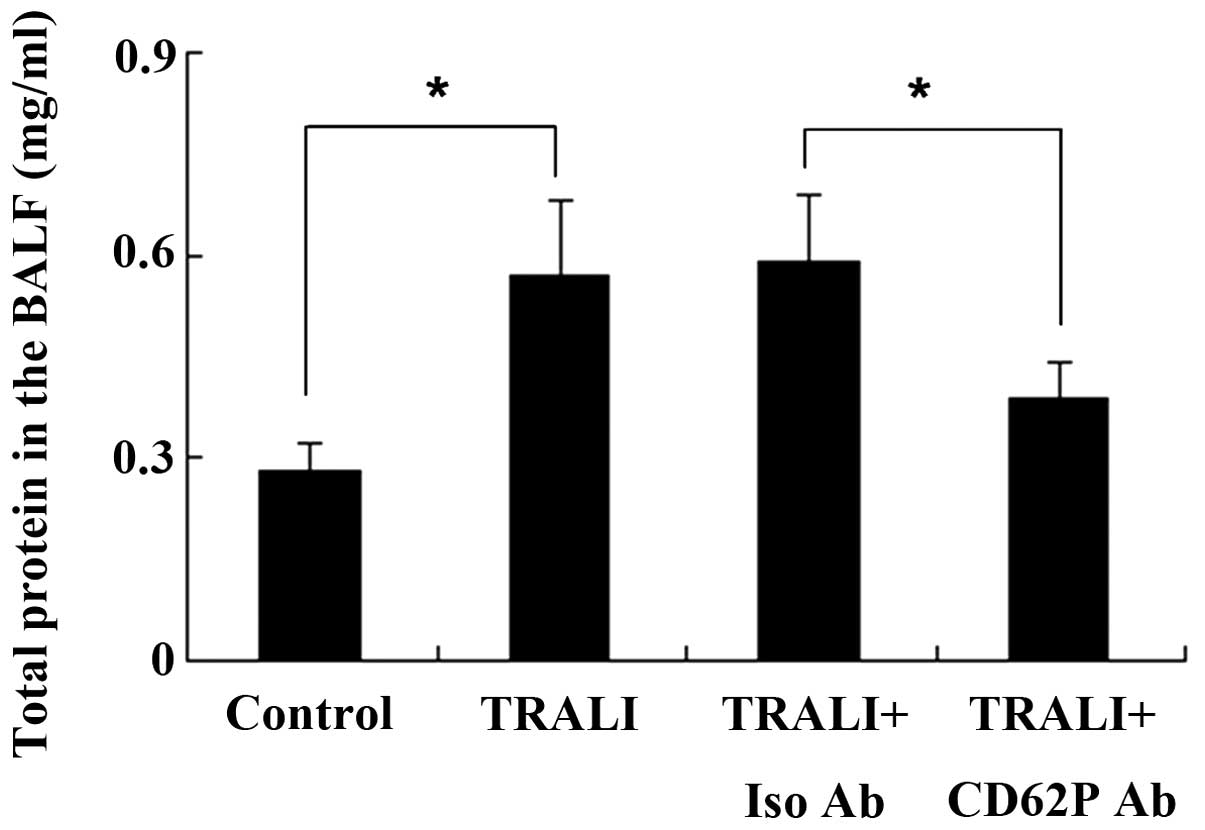
CD62P is associated with increases in
total protein in the BALF following TRALI
Total protein in the BALF was measured using the
Bradford method. The total protein concentration in the BALF in the
control group was 0.28±0.04 mg/ml; however, the total protein
concentration was increased to 0.57±0.11 mg/ml following induction
of TRALI (P<0.05) (Fig. 3).
Total protein in the BALF was significantly different between the
TRALI + isotype control antibody group and the TRALI + anti-CD62P
antibody group, and knockdown of CD62P reduced total protein in the
BALF.
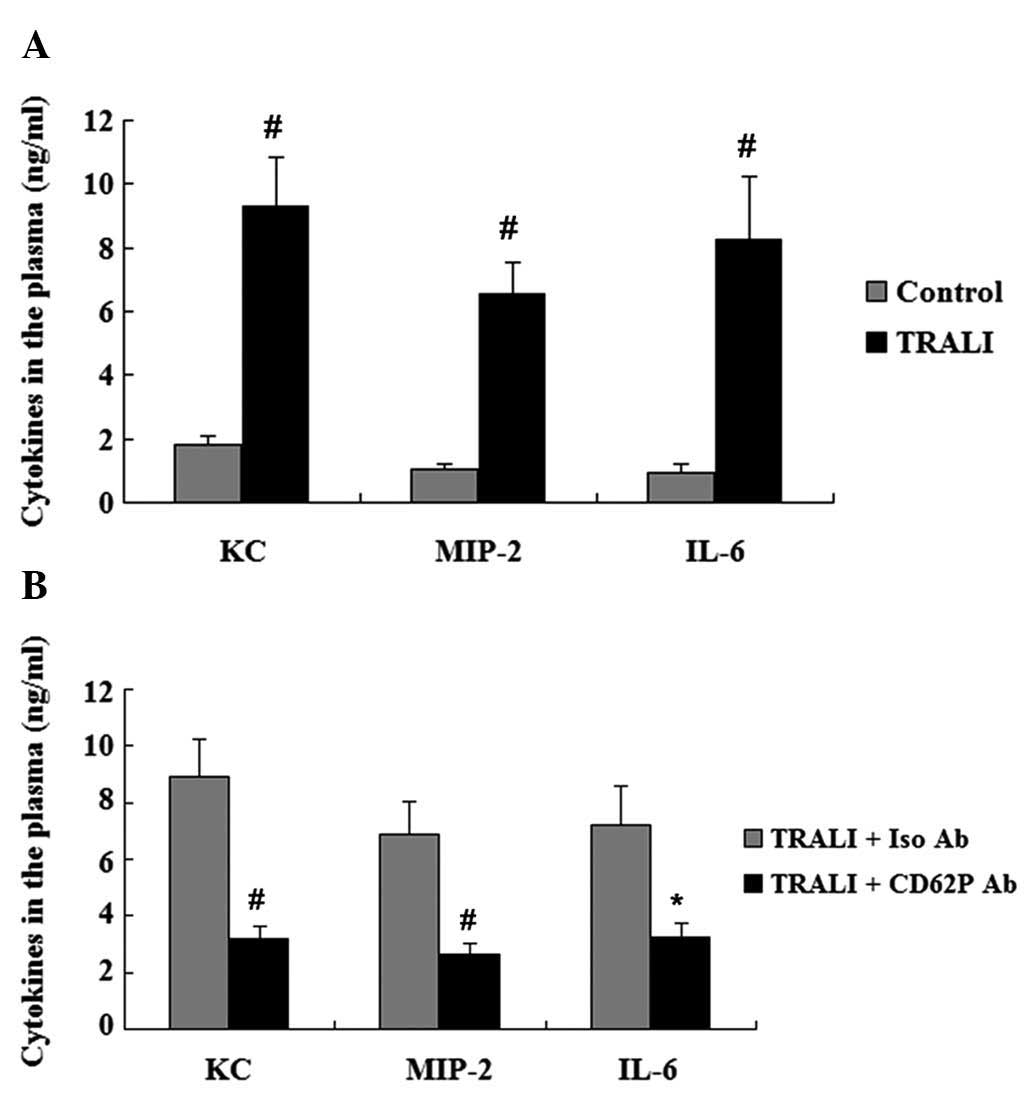
CD62P is associated with increases in
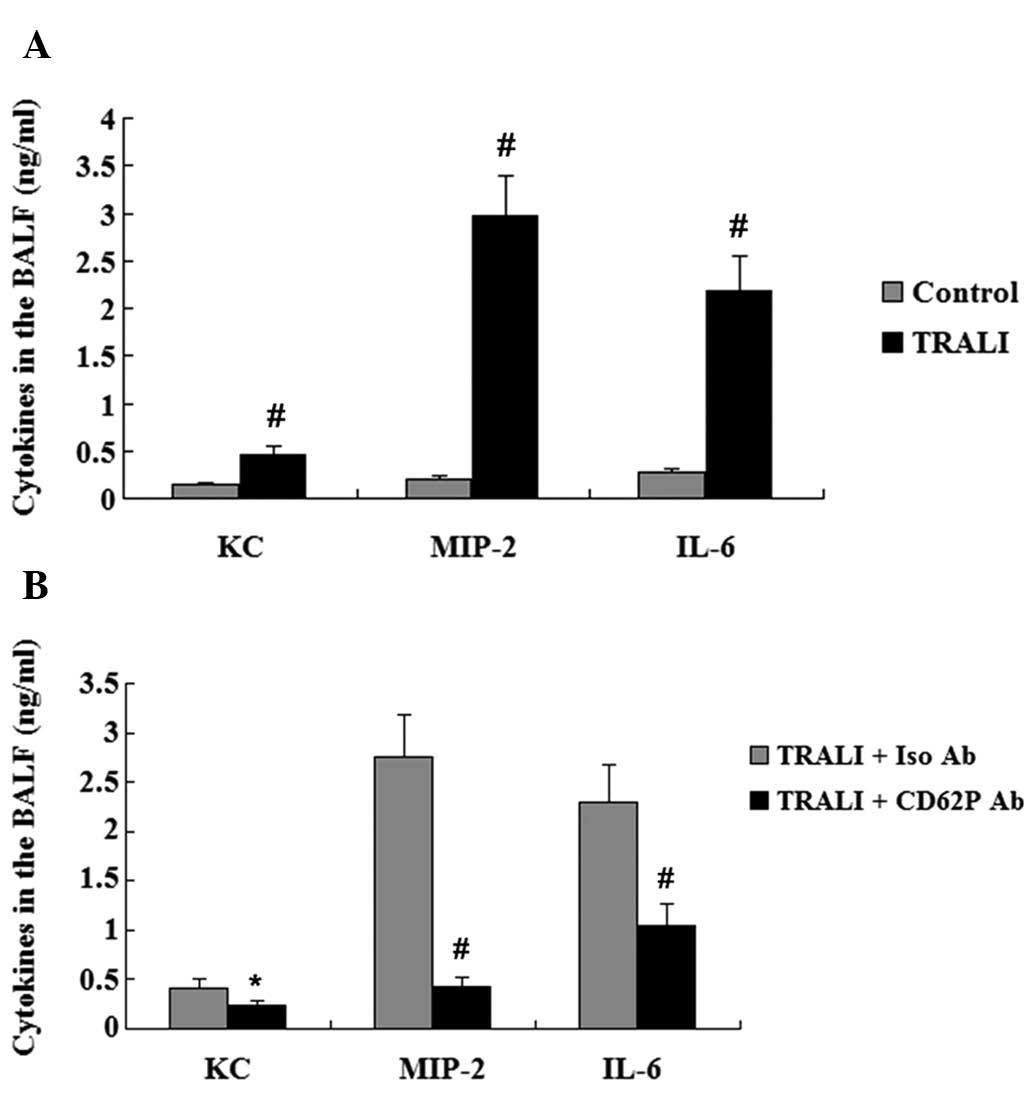
cytokines in the plasma and BALF following TRALI
The concentration of KC, MIP-2 and IL-6 in the
plasma and the BALF was assessed by ELISA. Elevated plasma levels
of KC, MIP-2 and IL-6 were observed in the TRALI group compared
with those in the control group (P<0.01) (Fig. 4A). In addition, the concentrations
of KC, MIP-2 and IL-6 in the BALF were also increased in the TRALI
group compared with those in the control group (P<0.01)
(Fig. 5A). However, following
treatment with anti-CD62P antibody, the elevation of KC, MIP-2 and
IL-6 in the BALF and the plasma of mice with TRALI was markedly
attenuated (Figs. 4B and 5B).
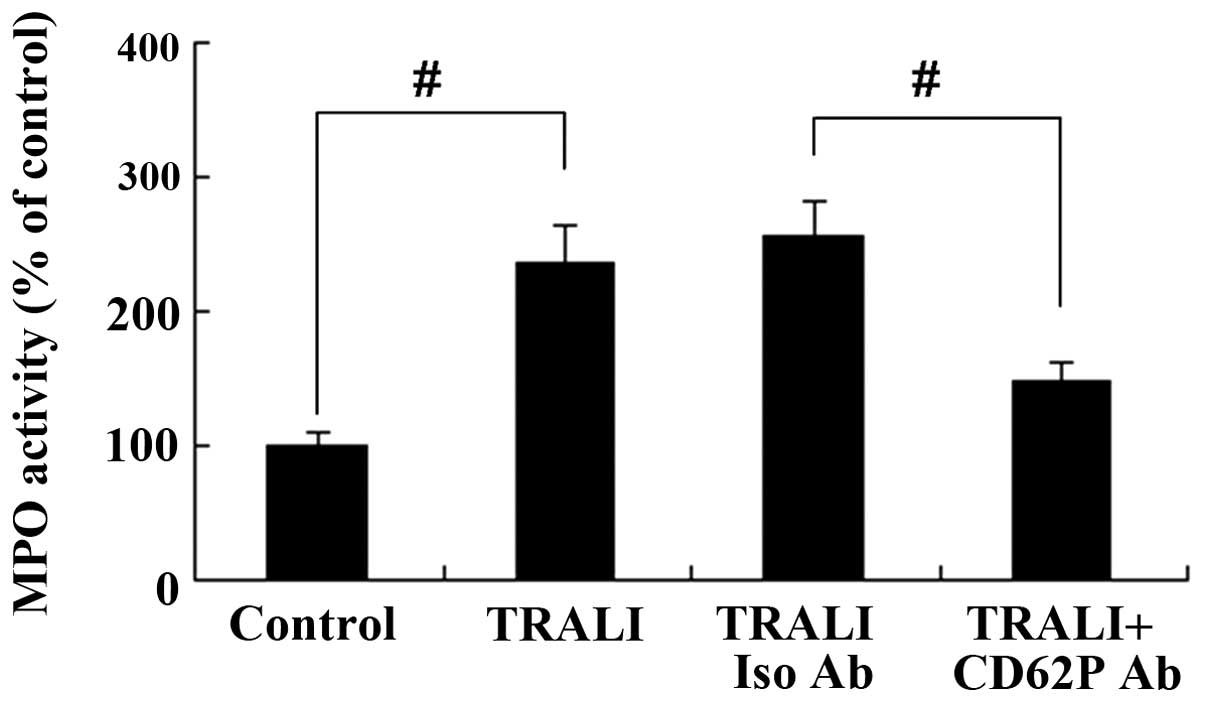
CD62P is associated with increases in MPO
activity in the lung following TRALI
MPO activity in the lung tissue was assessed using a
commercially available ELISA activity assay kit. As shown in
Fig. 6, there was a significant
increase of MPO activity in the TRALI group in comparison to that
in the control group (P<0.01). However, knockdown of CD62P
caused a decrease of MPO activity in the lung tissue of mice with
TRALI (P<0.01).
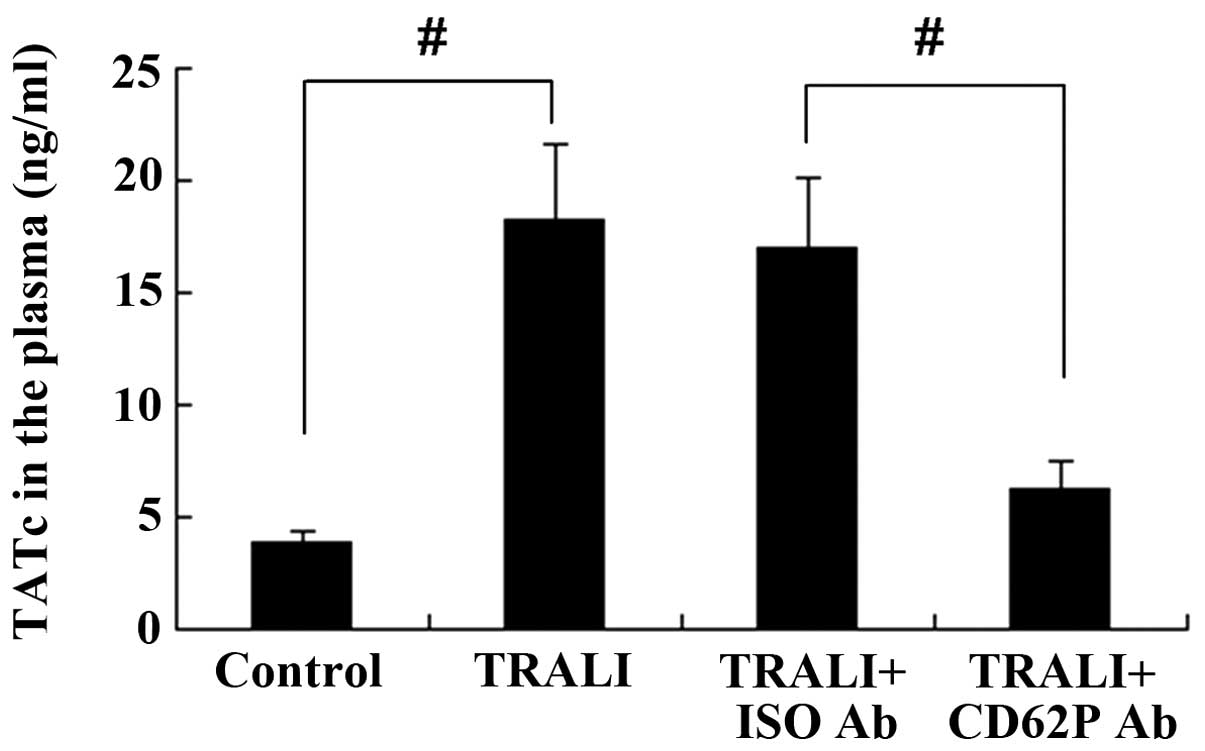
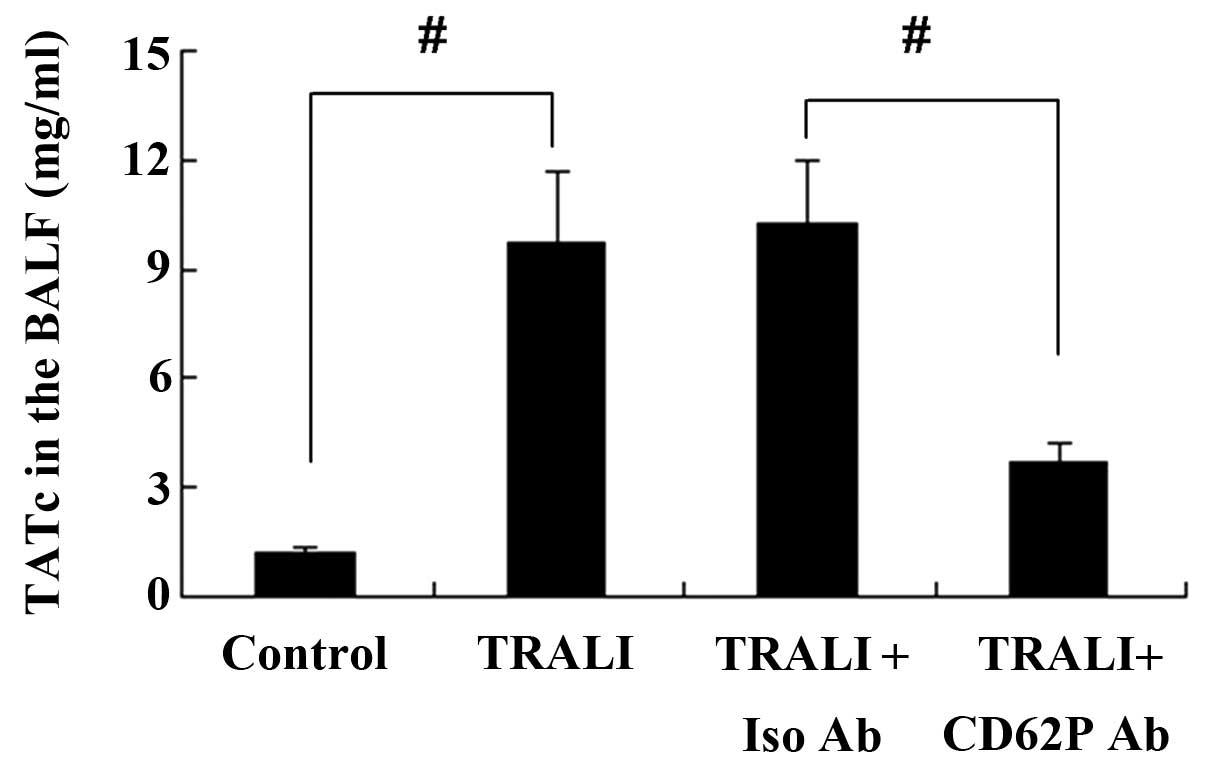
CD62P is associated with increases in
TATc in the plasma and BALF following TRALI
The concentration of TATc in the BALF and the plasma
was determined using an ELISA kit. TATc levels in the plasma were
increased in the TRALI group compared with those in the control
group (P<0.01) (Fig. 7).
Similar results were observed for TATc levels in the BALF (Fig. 8). However, knockdown of CD62P
attenuated the increases of TATc in the BALF and the plasma of mice
with TRALI, and the levels of TATc were significantly lower in the
TRALI + anti-CD62P antibody group compared with those in the TRALI
+ Iso CD62P antibody group (P<0.01).
Discussion
Certain biomolecules have been demonstrated to be
linked with TRAIL, including soluble CD40 ligand, antileukocyte
antibodies and lipids (19–21).
Clinical studies have indicated that the age of the transfused
blood may pre-dispose patients to TRALI (22) and older platelet concentrates are
associated with an increased prevalence and severity of
transfusion-associated complications (23–25).
As CD62P accumulation during storage of A-Plts may be implicated in
TRALI (15–18), the present study investigated the
precise roles of CD62P in TRALI.
The presence of MHC class I/II antibodies in the
transfusion recipient has been indicated to cause TRALI (26–28)
and several studies have used animal models of TRALI induced by
infusion with antibodies against MHC antigens (14,18,29).
In the present study, mice were treated with monoclonal MHC-1
antibody to induce TRALI according to Looney's method (14).
The lung W/D weight ratio is an indicator of
pulmonary edema, and the present study showed that the lung W/D
weight ratio was increased in the TRALI group compared with that in
the control group. Looney et al (18) reported that infusion with MHC-1
antibody caused a decrease in BALF clearance and an increased
vascular permeability. The present study we found that infusion
with MHC-1 antibody resulted in significantly elevated levels of
total protein as well as cytokines in the BALF, including KC, MIP-2
and IL-6. These results indicated a decreased BALF clearance and an
increased vascular permeability in mice with TRALI. Infusion with
MHC-1 antibody not only resulted in pulmonary inflammation, but
also in systemic inflammation, as indicated by elevated levels of
KC, MIP-2 and IL-6 in the plasma. To further determine the role of
CD62P in TRALI, the present study investigated whether in
vivo knockdown of CD62P was able to affect the severity of
TRALI and its associated effects.
The increases of the lung W/D weight ratio, total
protein in the BALF as well as cytokines in the BALF and the plasma
were significantly attenuated following anti-CD62P antibody
treatment. These results suggested that CD62P was associated with
the induction of pulmonary edema, inhibition of BALF clearance, as
well as with pulmonary and systemic inflammation in TRALI.
The lung MPO activity, which is an indicator of the
total neutrophil content in the lung, was significantly increased
in the TRALI group compared with that in the control group.
However, anti-CD62P antibody treatment markedly inhibited the MPO
activity in the lung. This result suggested that CD62P was
associated with neutrophil accumulation in TRALI.
TRALI is the result of endothelial activation, and
the endothelium initiates and regulates coagulation (30). It was reported that coagulopathy
has important roles in TRALI (31). In the present study, infusion with
MHC-1 antibody resulted in increased pulmonary and systemic
coagulation, with increased thrombin generation as reflected by the
TATc levels. Anti-CD62P antibody treatment attenuated the increase
of pulmonary and systemic coagulation in TRALI, as evidenced by
decreased TATc levels in the TRALI + anti-CD62P antibody group
compared with those in the TRALI group. These results indicated
that CD62P was involved in pulmonary and systemic coagulation in
TRALI.
In conclusion, the present study established a mouse
model of TRALI as evidenced by the presence of pulmonary edema,
accompanied by decreased BALF clearance, increased pulmonary and
systemic inflammation, elevated lung MPO activity, increased
pulmonary as well as systemic coagulation in the MHC-1 antibody
infusion group compared with that in the control group. Treatment
with anti-CD62P antibody, which silences the interactions involving
CD62P, attenuated TRALI in the experimental mice. The present study
therefore supported the notion that CD62P is involved in mediating
TRALI.
As CD62P accumulates during storage of A-Plts and
CD62P is implicated in TRALI, the present study may provide a
molecular basis for enhancing the clinical safety and effectiveness
of platelet transfusion.
References
|
1
|
Gajic O and Moore SB: Transfusion-related
acute lung injury. Mayo Clin Proc. 80:766–770. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Toy P, Popovsky MA, Abraham E, Ambruso DR,
Holness LG, Kopko PM, McFarland JG, Nathens AB, Silliman CC and
Stroncek D; National Heart, Lung and Blood Institute Working Group
on TRALI: Transfusion-related acute lung injury: Definition and
review. Crit Care Med. 33:721–726. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Holness L, Knippen MA, Simmons L and
Lachenbruch PA: Fatalities caused by TRALI. Transfus Med Rev.
18:184–188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weyrich AS and Zimmerman GA: Platelets:
Signaling cells in the immune continuum. Trends Immunol.
25:489–495. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Semple JW, Italiano JE Jr and Freedman J:
Platelets and the immune continuum. Nat Rev Immunol. 11:264–274.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vieira-de-Abreu A, Campbell RA, Weyrich AS
and Zimmerman GA: Platelets: Versatile effector cells in
hemostasis, inflammation and the immune continuum. Semin
Immunopathol. 34:5–30. 2012. View Article : Google Scholar
|
|
7
|
Furie B, Furie BC and Flaumenhaft R: A
journey with platelet P-selectin: The molecular basis of granule
secretion, signalling and cell adhesion. Thromb Haemost.
86:214–221. 2001.PubMed/NCBI
|
|
8
|
McEver RP: Selectins: Lectins that
initiate cell adhesion under flow. Curr Opin Cell Biol. 14:581–586.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamburger SA and McEver RP: GMP-140
mediates adhesion of stimulated platelets to neutrophils. Blood.
75:550–554. 1990.PubMed/NCBI
|
|
10
|
Larsen E, Celi A, Gilbert GE, Furie BC,
Erban JK, Bonfanti R, Wagner DD and Furie B: PADGEM protein: A
receptor that mediates the interaction of activated platelets with
neutrophils and monocytes. Cell. 59:305–312. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Geng JG, Bevilacqua MP, Moore KL, McIntyre
TM, Prescott SM, Kim JM, Bliss GA, Zimmerman GA and McEver RP:
Rapid neutrophil adhesion to activated endothelium mediated by
GMP-140. Nature. 343:757–760. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma L, Raycroft L, Asa D, Anderson DC and
Geng JG: A sialo-glycoprotein from human leukocytes functions as a
ligand for P-selectin. J Biol Chem. 269:27739–27746.
1994.PubMed/NCBI
|
|
13
|
Lorant DE, Patel KD, Mclntyre TM, McEver
RP, Prescott SM and Zimmerman GA: Coexpression of GMP-140 and PAF
by endothelium stimulated by histamine or thrombin: A juxtacrine
system for adhesion and activation of neutrophils. J Cell Biol.
115:223–234. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Looney MR, Nguyen JX, Hu Y, Van Ziffle JA,
Lowell CA and Matthay MA: Platelet depletion and aspirin treatment
protect mice in a two-event model of transfusion-related acute lung
injury. J Clin Invest. 119:3450–3461. 2009.PubMed/NCBI
|
|
15
|
Wachowicz B, Olas B, Zbikowska HM and
Buczyński A: Generation of reactive oxygen species in blond
platelets. Platelets. 13:175–182. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JH, Kim JT and Cho YG: Effect of
nitric oxide on the cryopreservation of platelets. Korean J Lab
Med. 28:136–143. 2008.In Korean. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hidalgo A, Chang J, Jang JE, Peired AJ,
Chiang EY and Frenette PS: Heterotypic interactions enabled by
polarized neutrophil microdomains mediate thromboinflammatory
injury. Nat Med. 15:384–391. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Looney MR, Su X, Van Ziffle JA, Lowell CA
and Matthay MA: Neutrophils and their Fc gamma receptors are
essential in a mouse model of transfusion-related acute lung
injury. J Clin Invest. 116:1615–1623. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thomas GM, Carbo C, Curtis BR, Martinod K,
Mazo IB, Schatzberg D, Cifuni SM, Fuchs TA, von Andrian UH,
Hartwig, et al: Extracellular DNA traps are associated with the
pathogenesis of TRALI in humans and mice. Blood. 119:6335–6343.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khan SY, Kelher MR, Heal JM, Blumberg N,
Boshkov LK, Phipps R, Gettings KF, McLaughlin NJ and Silliman CC:
Soluble CD40 ligand accumulates in stored blood components, primes
neutrophils through CD40, and is a potential cofactor in the
development of transfusion-related acute lung injury. Blood.
108:2455–2462. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sachs UJ, Hattar K, Weissmann N, Bohle RM,
Weiss T, Sibelius U and Bux J: Antibody-induced neutrophil
activation as a trigger for transfusion-related acute lung injury
in an ex vivo rat lung model. Blood. 107:1217–1219. 2006.
View Article : Google Scholar
|
|
22
|
Silliman CC, Boshkov LK, Mehdizadehkashi
Z, Elzi DJ, Dickey WO, Podlosky L, Clarke G and Ambruso DR:
Transfusion-related acute lung injury: Epidemiology and a
prospective analysis of etiologic factors. Blood. 101:454–462.
2003. View Article : Google Scholar
|
|
23
|
Heddle NM, Klama L, Singer J, Richards C,
Fedak P, Walker I and Kelton JG: The role of the plasma from
platelet concentrates in transfusion reactions. N Engl J Med.
331:625–628. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Muylle L, Wouters E, De Bock R and
Peetermans ME: Reactions to platelet transfusion: The effect of the
storage time of the concentrate. Transfus Med. 2:289–293. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sarkodee-Adoo CB, Kendall JM, Sridhara R,
Lee EJ and Schiffer CA: The relationship between the duration of
platelet storage and the development of transfusion reactions.
Transfusion. 38:229–235. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Popovsky MA, Chaplin HC Jr and Moore SB:
Transfusion-related acute lung injury: A neglected, serious
complication of hemotherapy. Transfusion. 32:589–592. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Flesch BK and Neppert J:
Transfusion-related acute lung injury caused by human leucocyte
antigen class II antibody. Br J Haematol. 116:673–676. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kopko PM, Popovsky MA, MacKenzie MR,
Paglieroni TG, Muto KN and Holland PV: HLA class II antibodies in
transfusion-related acute lung injury. Transfusion. 41:1244–1248.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Strait RT, Hicks W, Barasa N, Mahler A,
Khodoun M, Köhl J, Stringer K, Witte D, Van Rooijen N, Susskind BM
and Finkelman FD: MHC class I-specific antibody binding to
nonhematopoietic cells drives complement activation to induce
transfusion-related acute lung injury in mice. J Exp Med.
208:2525–2544. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mackie IJ and Bull HA: Normal haemostasis
and its regulation. Blood Rev. 3:237–250. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vlaar AP, Hofstra JJ, Levi M, Kulik W,
Nieuwland R, Tool AT, Schultz MJ, de Korte D and Juffermans NP:
Supernatant of aged erythrocytes causes lung inflammation and
coagulopathy in a 'two-hit' in vivo syngeneic transfusion model.
Anesthesiology. 113:92–103. 2010. View Article : Google Scholar : PubMed/NCBI
|