Introduction
Lung cancer is the most common type of cancer and it
is the leading cause of cancer-related mortality worldwide
(1). The prognosis for patients
with lung cancer is generally poor, even following complete
surgical resection (2), with
recurrence rates of 15–30% and 5-year survival rates of 60–70%
(3). There is an increasingly
broad range of therapeutic options for recurrent or unresectable
lung adenocarcinoma, for example, customized chemotherapy (4,5) and
molecular-targeted therapies, including bevacizumab (6,7),
erlotinib (4,8) and gefitinib (4). By contrast, there are few therapeutic
options for recurrent lung SqCC. Therefore, accurate prognostic
indicators for patients with lung SqCC are required.
Recent studies have demonstrated immunohistochemical
expression patterns of cell-cycle-related molecules in lung cancer,
such as p53, retinoblastoma protein, cyclin D1, p27 and Ki-67
(9–13). Ki-67 is a nuclear protein that is
expressed during the G1, S, G2 and M phases
of the mitotic cell cycle, although it is not expressed during the
non-mitotic G0 phase (14–17).
The genetic locus of Ki-67 has not yet been characterized. However,
it has been assigned to chromosome 10. A number of studies have
demonstrated that cell proliferative activity, as indicated by the
Ki-67 labeling index (Ki-67 LI), correlates with cell growth
(14–17). However, to the best of our
knowledge there have been no investigations into the association
between Ki-67 LI and lung cancer tumor growth patterns.
Using the general criteria for esophageal and
gastric cancer studies (18–25),
the tumor infiltration patterns (INF) of lung SqCC have been
classified into two groups: Lung tissue specimens with and without
clear boundaries between tumor tissue and healthy surrounding
tissue, which are termed INFc(−) and INFc(+) respectively (24). Masuda et al (24) demonstrated that INFc(+) was
significantly associated with venous invasion, the scirrhous
stromal tumor type and a lower postoperative survival in patients
with lung SqCC. Therefore, INFc(+) may be a useful indicator for
the level of local aggressiveness and invasiveness of lung
SqCC.
In the present study, the association between INF
components and immunohistochemical Ki-67 LI was analyzed. The
present study also investigated the clinicopathological
significance of cell proliferation and tumor invasiveness at the
invasive front of lung SqCCs.
Materials and methods
Lung cancer specimens
Cancer tissue specimens were obtained from
surgically resected lung SqCC tissue following obtaining informed
consent from 89 patients (85 males and four females; age range,
43–85 years; mean age, 67.2 ± 0.9 years). The study was approved by
the ethics committee of the Institutional Review Board of Tokai
University Hospital (Isehara, Japan). All patients had undergone
radical surgery (lobectomy and mediastinal lymphadenectomy) at
Tokai University Hospital between January 2001 and December 2006.
Tumor stages were defined according to the TNM classification of
the International Union Against Cancer (UICC; 5), and the
histological types were defined according to the World Health
Organization classification (26).
The median postoperative follow-up duration was 1,572 days (range
41–3,837 days).
Histological examination
Lung tissue specimens were immediately fixed with
10% buffered formalin for 24–48 h and embedded in paraffin (Wako
Pure Chemical. Industries Ltd., Osaka, Japan). Tissue samples were
cut at 5–10 mm intervals. Tumor invasion and lymphatic invasion
were examined in 4-µm sections, that were stained with
hematoxylin and eosin. The extent of lymphatic invasion in the
tissue specimens was classified as follows: ly0, no lymphatic
invasion; ly1+, mild lymphatic invasion; ly2+, moderate lymphatic
invasion; and ly3+, severe lymphatic invasion. Vascular and pleural
invasion was evaluated using Elastica van Gieson staining for
detection of elastic fibres. The degree of venous invasion in the
tissue specimens was classified as follows: v0, no venous invasion;
v1+, minimal venous invasion (one or two foci of venous invasion in
one histological section); v2+, moderate venous invasion (three or
four foci); or v3+, severe venous invasion (five or more foci).
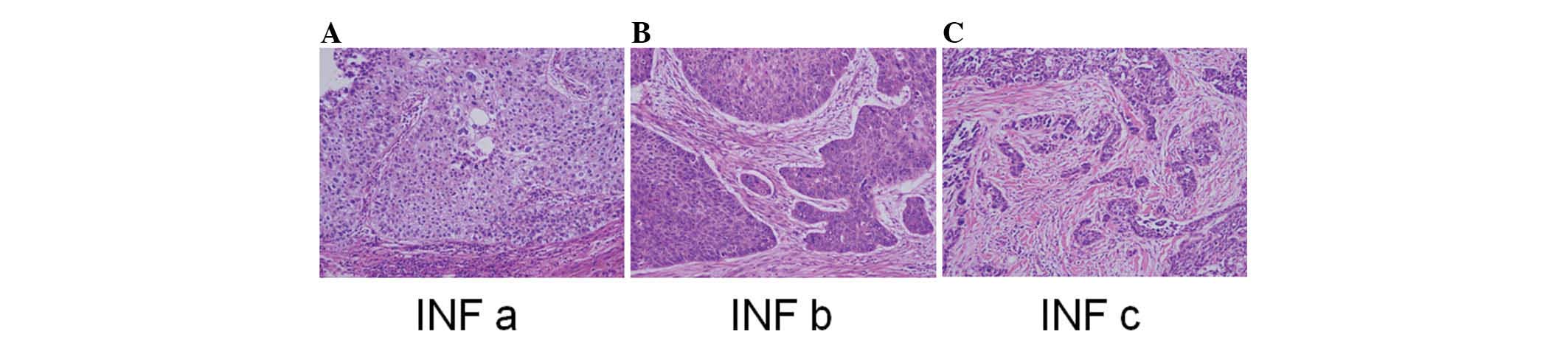
INF at the invasive front of the SqCC was classified
into three groups according to the general criteria for gastric
cancer studies (18–20,24):
INFa, cancer nests exhibit expanding growth and a distinct boundary
with the surrounding tissue; INFb, the manner of growth and
invasive pattern is intermediate between those of INFa and INFc;
and INFc, cancer nests exhibit infiltrative growth without a clear
boundary between the tumor tissue and surrounding healthy tissue.
However, a number of samples exhibited intermediate
characteristics, for example, INFa>b (5,20,27).
Therefore, SqCC tissue specimens were further classified into seven
categories: INFa, INFa>b, INFa<b, INFb, INFb>c, INFb<c
and INFc. These seven categories were allocated into two broader
groups: Those cases with an INFc component [INFc(+); comprising
INFb>c, INFb<c and INFc], and those without an INFc component
[INFc (−); comprising INFa, INFa>b, INFa<b and INFb].
The stromal types, that is, cancer cells/stroma
(c/s) ratio in the cancerous lesion were also classified into three
groups: Medullary type, the stroma is limited (high c/s ratios);
intermediate type, the quantity of stroma is intermediate between
those of the scirrhous and medullary types (intermediate c/s
ratios); and scirrhous type, the stroma is abundant (low c/s
ratios) t (19).
Immunohistochemical analysis
Deparaffinized and dehydrated 4-µm paraffin
sections were immersed in 0.3% hydrogen peroxide
(H2O2) in methanol (Wako Pure Chemical.
Industries Ltd.) for 30 min in order to abolish endogenous
peroxidase activity. Subsequently, the sections were mounted on
aminoacyl silane-coated glass slides and used for
immunohistochemical analysis of Ki-67 expression (Ki-67; rabbit
monoclonal; cat. no. 418071; Nichirei Bioscience, Tokyo, Japan). In
order to facilitate Ki-67 antigen retrieval, the sections were
penetrated by autoclave heating (ES-215, High-pressure steam
sterilizer; Tomy Seiko Co., LTD, Tokyo, Japan) at 121°C for 4 min.
Non-specific binding was abolished using diluted normal sheep serum
(Cosmo Bio Co., Ltd, Tokyo, Japan). Subsequently, a primary
monoclonal antibody, diluted 1:100 in 1% bovine serum albumin (Wako
Pure Chemical. Industries Ltd.) and phosphate-buffered saline
(PBS), was added and incubated overnight at 4°C in a moist chamber.
Following a wash phase using PBS, a secondary anti-rabbit IgG
peroxidase-linked antibody (cat. no. NA934) at 1:100 dilution
(Amersham International plc., Little Chalfont, UK) was applied for
60 min at room temperature. The sections were then treated with
streptavidin-conjugated horseradish peroxidase for 30 min at room
temperature (Funakoshi Co., Ltd., Tokyo, Japan). The reaction
products were visualized using diaminobenzidine tetrahydrochloride
(Muto Pure Chemicals Co., Ltd., Tokyo, Japan) for 4 min in Tris
buffer.
Evaluation of Ki-67 LI
Cells were observed using a 40x objective microscope
(BX50; Olympus, Tokyo, Japan). In each section, ≤1,000 cells were
randomly selected and the positive cells were counted. The cut-off
point for positivity was considered when ≥30% positive cells were
observed. Samples were classified into two groups: high-grade cell
proliferation (Ki-67 LI ≥30%) and low-grade cell proliferation
(Ki-67 LI <30%).
Statistical analysis
Univariate analyses (chi-square tests) were
primarily used for identifying all variables that exhibited
statistically significant differences. P<0.05 was considered to
indicate a statistically significant difference. Cox proportional
hazards regression analysis was conducted in order to determine the
effect of each predictor variable, using univariate analyses.
Univariate and multivariate analyses were conducted in order to
investigate the association between Ki-67 LI and SqCC tumor
invasion. Propensity scores were calculated in the multivariate
analysis, in order to measure the effect of the following
covariates on INFc(+): Age at surgery, gender, tumor size, lymph
node metastasis, lymphatic invasion, histological differentiation
and stromal type. Hazard ratios (HR) and 95% confidence intervals
(CI) were used to assess the independent contributions of
significant factors. In all cases P<0.05 was considered to
indicate a statistically significant difference.
The patient survival time was measured from the date
of surgery to mortality, related to any cause (without
discrimination between mortalities resulting from lung carcinoma
and other causes). Survival curves were created using the
Kaplan-Meier method and compared using the log-rank test. All
analyses were performed using the SPSS II statistical software
package (version 19.0; SPSS, Inc., Chicago, IL, USA).
Results
Lung SqCC cell proliferation
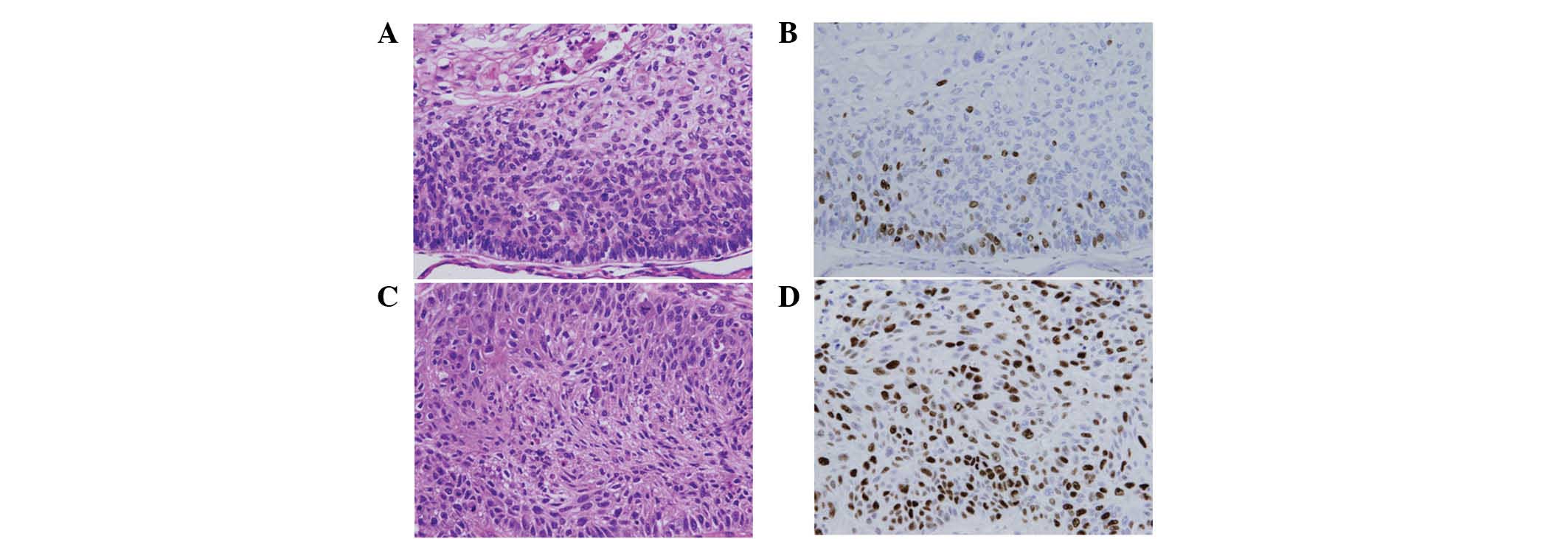
High-grade cell proliferation (≥30% Ki-67 LI) and
low-grade cell proliferation (<30% Ki-67 LI) was observed in
16.9% (15/89) and 83.1% (74/89) of SqCC lung cancer specimens,
respectively (Fig. 1).
Associations between Ki-67 LI and clinicopathological features are
summarized in Table I. INFc(+) was
most common in SqCC lung cancer specimens with high-grade cell
proliferation (≥30% Ki-67 LI) compared with those with low-grade
cell proliferation (<30% Ki-67 LI; P=0.03). However, no
significant difference was detected in prognosis between the high
and low Ki-67 LI groups.
 | Table IKi-67 LI and clinicopathological
features of lung squamous cell carcinoma. |
Table I
Ki-67 LI and clinicopathological
features of lung squamous cell carcinoma.
| Variable | No. patients (%) | Ki-67 LI
| P-value |
|---|
| <30% | ≥30% |
|---|
| Age at surgery
(years) |
| <68 | 45 (50.6) | 6 (13.3) | 39 (86.7) | 0.370 |
| ≥68 | 44 (49.4) | 9 (20.5) | 35 (79.5) | |
| Gender |
| Male | 84 (94.4) | 12 (14.3) | 72 (85.7) | 0.032 |
| Female | 5 (5.6) | 3 (60.0) | 2 (40.0) | |
| Tumor size (mm) |
| ≤30 | 34 (38.2) | 8 (23.5) | 26 (76.5) | 0.186 |
| >30 | 55 (61.8) | 7 (12.7) | 48 (87.3) | |
| Lymph node
metastasis |
| n (−) | 62 (69.7) | 12 (19.4) | 50 (80.6) | 0.539 |
| n (+) | 27 (30.3) | 3 (11.1) | 24 (88.9) | |
| Lymphatic
invasion |
| ly (0, 1) | 75 (84.3) | 11 (14.7) | 64 (85.3) | 0.243 |
| ly (2, 3) | 14 (15.7) | 4 (28.6) | 10 (71.4) | |
| Venous
invasion |
| v (−) | 47 (52.8) | 7 (14.9) | 40 (85.1) | 0.601 |
| v (+) | 42 (47.2) | 8 (19.0) | 34 (81.0) | |
| Histological
differentiation |
| Well, Mod | 81 (91.0) | 14 (17.3) | 67 (82.7) | 1.000 |
| Poor | 8 (9.0) | 1 (12.5) | 7 (87.5) | |
| Stromal type |
| Medullary,
intermediate | 57 (64.0) | 11 (19.3) | 46 (80.7) | 0.411 |
| Scirrhous | 32 (36.0) | 4 (12.5) | 28 (87.5) | |
| Infiltration
pattern |
| INFc (−) | 55 (61.8) | 13 (23.6) | 42 (76.4) | 0.030 |
| INFc(+) | 34 (38.2) | 2 (5.9) | 32 (94.1) | |
Tumor growth patterns of lung SqCC
Lung tissue specimens were initially categorized
into three INF groups (Fig. 2).
They were further classified into seven groups based on the INF of
each specimen: INFa (10, 11.2%), INFa>b (8, 9.0%), INFa<b (0,
0%), INFb (37, 41.6%), INFb>c (27, 30.3%), INFb<c (3, 3.4%)
and INFc (4, 4.5%). The patients with INFc(+) (INFb>c, b<c,
c) had a poor outcome, compared with the patients with INFc(−)
(INFa,a>b, a<b, b) (Table
II). The associations between INFc(+) and clinicopathological
features of patients with lung SqCC according to a univariate
analysis, are summarized in Table
III. INFc(+) was significantly associated with venous invasion
(P=0.032; HR, 2.615; 95% CI, 1.085–6.305), stromal type
(P<0.001; HR, 6.462; 95% CI, 2.483–16.817) and Ki-67 LI
(P=0.044; HR, 4.952; 95% CI, 1.043–23.523) of lung SqCC. INFc(+)
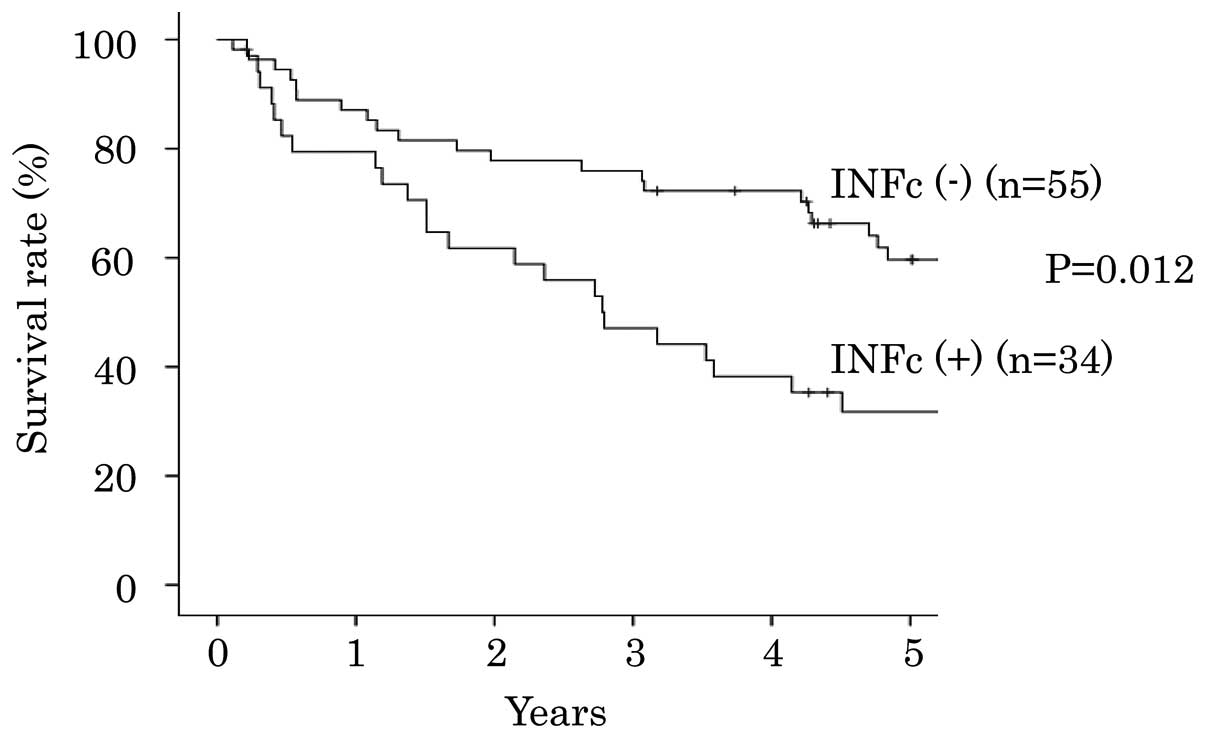
cases exhibited a significantly poorer prognosis compared with INFc
(−) cases (P=0.012; Fig. 3).
 | Table IIINF in lung squamous cell carcinoma
patients. |
Table II
INF in lung squamous cell carcinoma
patients.
| Variable | Number of patients
(%) | Hazard ratio | 95% Confidence
interval | P-value |
|---|
| INF a | 10 (11.2) | 0.991 | 0.392–2.507 | 0.985 |
| INF a>b, a<b,
b, b>c, b<c, c | 79 (88.8) | | | |
| INF a, a>b | 18 (20.2) | 1.155 | 0.556–2.399 | 0.699 |
| INF a<b, b,
b>c, b<c, c | 71 (79.8) | | | |
| INF a, a>b,
a<b | 18 (20.2) | 1.155 | 0.556–2.399 | 0.699 |
| INF b, b>c,
b<c, c | 71 (79.8) | | | |
| INF a, a>b,
a<b, b | 55 (61.8) | 2.069 | 1.163–3.683 | 0.013 |
| INF b>c, b<c,
c | 34 (38.2) | | | |
| INF a>b, a<b,
b, b>c | 82 (92.1) | 1.440 | 0.515–4.027 | 0.487 |
| INF b<c, c | 7 (7.9) | | | |
| INF a>b, a<b,
b, b>c, b<c | 85 (95.5) | 1.171 | 0.283–4.841 | 0.828 |
| INF c | 4 (4.5) | | | |
 | Table IIIAssociation between INFc(+) and
clinicopathological factors (univariate analysis). |
Table III
Association between INFc(+) and
clinicopathological factors (univariate analysis).
| Variable | No. of patients
(%) | Odds ratio | 95% Confidence
interval | P-value |
|---|
| Age at surgery
(years) |
| <68 | 45 (50.6) | 1.845 | 0.776–4.388 | 0.166 |
| ≥68 | 44 (49.4) | | | |
| Gender |
| Male | 84 (94.4) | 1.083 | 0.172–6.839 | 0.932 |
| Female | 5 (5.6) | | | |
| Tumor size
(mm) |
| ≤30 | 34 (38.2) | 0.816 | 0.340–1.961 | 0.650 |
| >30 | 55 (61.8) | | | |
| Lymph node
metastasis |
| n (−) | 62 (69.7) | 1.166 | 0.462–2.939 | 0.745 |
| n (+) | 27 (30.3) | | | |
| Lymphatic
invasion |
| ly (0, 1) | 75 (84.3) | 1.259 | 0.396–4.005 | 0.697 |
| ly (2, 3) | 14 (15.7) | | | |
| Venous
invasion |
| v (−) | 47 (52.8) | 2.615 | 1.085–6.305 | 0.032 |
| v (+) | 42 (47.2) | | | |
| Histological
differentiation |
| Well, Mod | 81 (91.0) | 0.000 | 0.000 | 0.999 |
| Poorly | 8 (9.0) | | | |
| Stromal type |
| Medullary,
intermediate | 57 (64.0) | 6.462 | 2.483–16.817 | <0.001 |
| Scirrhous | 32 (36.0) | | | |
| Ki-67 LI |
| <30% | 15 (16.9) | 4.952 | 1.043–23.523 | 0.044 |
| ≥30% | 74 (83.1) | | | |
Multivariate analyses for prediction of
INFc
Propensity scores were calculated by measuring the
effect of the following covariates on INFc(+): Age at surgery,
gender, tumor size, lymph node metastasis, lymphatic invasion,
histological differentiation and stromal type. Multivariate
logistic regression analysis demonstrated that INFc(+) was
significantly associated with Ki-67 LI (odds ratio, 12.5; 95% CI,
1.5–102.8; P=0.018) and stromal type (odds ratio, 8.4; 95%, CI,
2.9–24.1; P<0.001) following adjustment for the propensity score
(Table IV).
 | Table IVAssociation between INFc(+) and
clinicopathological factors (multivariate analysis). |
Table IV
Association between INFc(+) and
clinicopathological factors (multivariate analysis).
| Variable | Odds ratio | 95% Confidence
interval | P-value |
|---|
| Ki-67 LI |
| <30% | 12.543 | 1.531–102.777 | 0.018 |
| ≥30% | | | |
| Stromal type |
| Medullary,
intermediate Scirrhous | 8.402 | 2.923–24.147 | <0.001 |
| Propensity
score | 0.025 | 0.000–1.349 | 0.070 |
Discussion
As a result of the advances in imaging, diagnostic
techniques and operative procedures, the number of patients with
lung SqCC undergoing surgical resection has increased for the last
three decades. In the present study, 89 surgically resected
specimens of lung tissue from patients with lung SqCC were analyzed
in order to investigate tumor aggressiveness. INF and local tumor
proliferation in lung SqCC were analyzed by measuring cell
proliferation, using Ki-67 expression as a proxy. INFc(+) was most
common in the lung tissue samples from cases with high-grade cell
proliferation (Ki-67 LI ≥30%) compared with those from cases with
low grade cell proliferation (Ki-67 LI<30%). To the best of our
knowledge, this is the first report of an association between INF
and lung SqCC cell proliferation.
A previous study demonstrated a correlation between
the survival rate of patients with lung SqCC, and tumor budding and
histological aggressiveness (24).
In the present study, a significantly greater number of INFc(+)
lung SqCC specimens exhibited high-grade than low-grade cell
proliferation. These results suggest that high-grade cell
proliferation may affect the infiltrative growth of cancer nests.
Furthermore, INFc(+) was significantly associated with the
scirrhous stromal type and positive venous invasion (Table III).
A number of meta-analyses have addressed the
prognostic value of Ki-67 in lung cancer. However its
clinicopathological role remains to be elucidated (28–34).
Ciancio et al (9)
demonstrated that Ki-67 immunostaining of lung tissue specimens
obtained from patients with non-small cell lung cancer (NSCLC)
using fiber-optic bronchoscopy, may be useful for making prognostic
predictions for patients with lung SqCC. Ciancio et al
(9) demonstrated that 42.1% of the
lung cancer cases exhibited high-grade cell proliferation (Ki-67 LI
> 25%). By contrast, the present study demonstrated that 83.1%
of SqCC lung cancer specimens exhibited high-grade cell
proliferation (Ki-67 LI > 30%). The present study analyzed lung
cancer tissues taken from surgical resection, and therefore
examined entire tumor, whereas the investigation of Ciancio et
al (9) used lung cancer
specimens obtained by biopsy. It is hypothesized that the different
procedure used for obtaining the specimens (biopsy vs. surgical
resection) between Ciancio et al (9) and the present study, may explain the
contrasting results in the percentage of lung cancer specimens
exhibiting high-grade cell proliferation. Furthermore, Ciancio
et al (9) examined Ki-67
overexpression in NSCLC cells and the clinical outcomes for
patients with NSCLC, which included SqCC, adenocarcinoma and other
histological types. By contrast, the present study focussed on SqCC
lung tissue. In terms of patient survival, the results of the
present study are in accordance with the conclusions of Ciancio
et al (9). The present
study demonstrated that INFc(+) may be a prognostic factor in SqCC.
However, further investigations are required in order to examine
the molecular and histological associations between tumor
invasiveness and high-grade cell proliferation in lung SqCC.
In conclusion, high-grade cell proliferation, as
measured by Ki-67 LI, significantly correlated with an INF that
indicated a more aggressive lung SqCC. Ki-67 LI may therefore be
used as an indicator of INFc(+) and is a potential prognostic
factor for lung SqCC.
Acknowledgments
The authors would like to thank Professor Hiroyuki
Kobayashi (Department of Clinical Pharmacology, Tokai University
School of Medicine, Isehara, Kanagawa) for help with the
statistical analysis.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Beadsmoore CJ and Screaton NJ:
Classification, staging and prognosis of lung cancer. Eur J Radiol.
45:8–17. 2003. View Article : Google Scholar
|
|
3
|
Goldstraw P, Crowley J, Chansky K, et al:
The IASLC Lung Cancer Staging Project: proposals for the revision
of the TNM stage groupings in the forthcoming (seventh) edition of
the TNM classification of malignant tumours. J Thorac Oncol.
2:706–714. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hong J, Kyung SY, Lee SP, et al:
Pemetrexed versus gefitinib versus erlotinib in previously treated
patients with non-small cell lung cancer. Korean J Intern Med.
25:294–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rossi A, Ricciardi S, Maione P, de Marinis
F and Gridelli C: Pemetrexed in the treatment of advanced
non-squamous lung cancer. Lung Cancer. 66:141–149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sandler A, Gray R, Perry MC, et al:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reck M, von Pawel J, Zatloukal P, et al:
Phase III trial of cisplatin plus gemcitabine with either placebo
or bevacizumab as first-line therapy for nonsquamous non-small-cell
lung cancer: AVAil. J Clin Oncol. 27:1227–1234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosell R, Perez-Roca L, Sanchez JJ, et al:
Customized treatment in non-small-cell lung cancer based on EGFR
mutations and BRCA1 mRNA expression. PLoS One. 4:e51332009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ciancio N, Galasso MG, Campisi R, Bivona
L, Migliore M and Di Maria GU: Prognostic value of p53 and Ki67
expression in fiberoptic bronchial biopsies of patients with non
small cell lung cancer. Multidiscip Respir Med. 7:292012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kosacka M, Piesiak P, Kowal A, Gołecki M
and Jankowska R: Galectin-3 and cyclin D1 expression in non-small
cell lung cancer. J Exp Clin Cancer Res. 30:1012011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sterlacci W, Fiegl M, Hilbe W, et al:
Deregulation of p27 and cyclin D1/D3 control over mitosis is
associated with unfavorable prognosis in non-small cell lung
cancer, as determined in 405 operated patients. J Thorac Oncol.
5:1325–1336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lei B, Liu S, Qi W, et al: PBK/TOPK
expression in non-small-cell lung cancer: its correlation and
prognostic significance with Ki67 and p53 expression.
Histopathology. 63:696–703. 2013.PubMed/NCBI
|
|
13
|
Motadi LR, Bhoola KD and Dlamini Z:
Expression and function of retinoblastoma binding protein 6 (RBBP6)
in human lung cancer. Immunobiology. 216:1065–1073. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cattoretti G, Becker MH, Key G, et al:
Monoclonal antibodies against recombinant parts of the Ki-67
antigen (MIB 1 and MIB 3) detect proliferating cells in
microwave-processed formalin-fixed paraffin sections. J Pathol.
168:357–363. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gerdes J, Li L, Schlueter C, et al:
Immunobiochemical and molecular biologic characterization of the
cell proliferation-associated nuclear antigen that is defined by
monoclonal antibody Ki-67. Am J Pathol. 138:867–873.
1991.PubMed/NCBI
|
|
16
|
Hui AM, Shi YZ, Li X, et al: Proliferative
marker Ki-67 in gallbladder carcinomas: high expression level
predicts early recurrence after surgical resection. Cancer Lett.
176:191–198. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xuan YH, Choi YL, Shin YK, et al: An
immunohistochemical study of the expression of cell-cycle-regulated
proteins p53, cyclin D1, RB, p27, Ki67 and MSH2 in gallbladder
carcinoma and its precursor lesions. Histol Histopathol. 20:59–66.
2005.
|
|
18
|
Haraguchi M, Yamamoto M, Saito A, et al:
Prognostic value of depth and pattern of stomach wall invasion in
patients with an advanced gastric carcinoma. Semin Surg Oncol.
10:125–129. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Japanese Classification of Gastric
Carcinoma - 2nd English Edition. Gastric Cancer. 1:10–24. 1998.
View Article : Google Scholar
|
|
20
|
Maehara Y, Oshiro T, Adachi Y, Ohno S,
Akazawa K and Sugimachi K: Growth pattern and prognosis of gastric
cancer invading the subserosa. J Surg Oncol. 55:203–208. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okada K, Kijima H, Imaizumi T, et al:
Wall-invasion pattern correlates with survival of patients with
gallbladder adenocarcinoma. Anticancer Res. 29:685–691.
2009.PubMed/NCBI
|
|
22
|
Song KY, Hur H, Jung CK, et al: Impact of
tumor infiltration pattern into the surrounding tissue on prognosis
of the subserosal gastric cancer (pT2b). Eur J Surg Oncol.
36:563–567. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kong KY, Park JY, Kim DY, et al:
Prognostic significance of stromal microinvasion in the intestinal
type of ovarian mucinous adenocarcinoma. Ann Surg Oncol.
18:3462–3468. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Masuda R, Kijima H, Imamura N, et al:
Tumor budding is a significant indicator of a poor prognosis in
lung squamous cell carcinoma patients. Mol Med Rep. 6:937–943.
2012.PubMed/NCBI
|
|
25
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumors. 7th edition. Wiley;
Hoboken, New Jersey, NJ, USA: 2010
|
|
26
|
Travis WD, Brambilla E, Muller-Hermedin HK
and Harris CC: Pathology and Genetics Tumours of the Lung, Pleura,
Thymus and Heart. IARC Press; Lyon, France: 2004
|
|
27
|
Kawano K and Yanagisawa S: Predictive
value of laminin-5 and membrane type 1-matrix metalloproteinase
expression for cervical lymph node metastasis in T1 and T2 squamous
cell carcinomas of the tongue and floor of the mouth. Head Neck.
28:525–533. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Soomro IN and Whimster WF: Growth fraction
in lung tumours determined by Ki67 immunostaining and comparison
with AgNOR scores. J Pathol. 162:217–222. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scagliotti GV, Micela M, Gubetta L, et al:
Prognostic significance of Ki67 labelling in resected non small
cell lung cancer. Eur J Cancer. 29A:363–365. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hommura F, Dosaka-Akita H, Mishina T, et
al: Prognostic significance of p27KIP1 protein and ki-67 growth
fraction in non-small cell lung cancers. Clin Cancer Res.
6:4073–4081. 2000.PubMed/NCBI
|
|
31
|
Nguyen VN, Mirejovský P, Mirejovský T,
Melinova L and Mandys V: Expression of cyclin D1, Ki-67 and PCNA in
non-small cell lung cancer: prognostic significance and comparison
with p53 and bcl-2. Acta Histochem. 102:323–338. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maddau C, Confortini M, Bisanzi S, et al:
Prognostic significance of p53 and Ki-67 antigen expression in
surgically treated non-small cell lung cancer: immunocytochemical
detection with imprint cytology. Am J Clin Pathol. 125:425–431.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ishida H, Irie K, Itoh T, Furukawa T and
Tokunaga O: The prognostic significance of p53 and bcl-2 expression
in lung adenocarcinoma and its correlation with Ki-67 growth
fraction. Cancer. 80:1034–1045. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Martin B, Paesmans M, Mascaux C, et al:
Ki-67 expression and patients survival in lung cancer: systematic
review of the literature with meta-analysis. Br J Cancer.
91:2018–2025. 2004. View Article : Google Scholar : PubMed/NCBI
|