Introduction
Following spinal cord injury (SCI),
ischemia-hypoxia, reperfusion injury, lipid peroxidation and
inhibition of the expression of various molecules in local tissues
lead to neuronal cell necrosis and axonal demyelination, and glial
scar formation, which seriously impede axonal regeneration and
myelination, and impact neurological recovery (1,2).
A substantial number of inflammatory cells in SCI
exhibit characteristic pathological changes, which is a multi-step
process with complex multifactorial involvement. Chemokines have
been reported to be key in this process. Macrophage inflammatory
protein-2 (MIP-2) is one member of the CXC chemokine family, which
leads to specific chemotaxis of neutrophils and lymphocytes to
sites of inflammation, and is important in the development of
chronic bronchitis, hepatitis and other inflammatory disorders
(3). Pineau et al indicated
that astrocytes initiate inflammation through the modulation of
monocyte chemotactic protein 1 (MCP-1; CCL2), keratinocyte
chemoattractant (KC; CXCL1) and MIP-2 (CXCL2) in the injured mouse
spinal cord (4). Berghmans et
al demonstrated that chlorite-oxidized oxyamylose protects
significantly against hyperacute spinal cord homogenate-induced
experimental autoimmune encephalomyelitis via suppression of the
MIP-2/CXCL2 signal pathway (5).
Matrix metalloproteinases (MMPs) are a family of
metalloprotein endonucleases dependent on Zn++, which
are involved in fibrosis, arthritis, tumor growth, migration,
invasion and metastasis in pathological conditions (6–8).
MMP, particularly MMP-9, is an important factor involved in acute
spinal cord injury (9). Feng et
al reported that ulinastatin protects against experimental
autoimmune encephalomyelitis through downregulation of the
expression of MMP-9 (10), and
Zhang et al indicated that nutrient mixture attenuated
SCI-induced impairment by negatively affecting the promoter
activity of MMP-2 and MMP-9 in mice (11).
In previous years, the role of TNF-α in spinal cord
injury has gained increasing attention, TNF-α is regarded to be an
initiation factor among several cytokines, upregulating the
generation of other cytokines, which is significant in the
amplification of local inflammation. Protein kinase B (Akt) as a
serine/threonine protein kinase, is a key central effector protein
for multiple signal transduction pathways and, as downstream target
protein for phosphoinositide 3-kinase (PI3K), it is the core of
PI3K/Akt signaling pathway (12).
Felix et al demonstrated that the inhibition of medulla Akt
phosphorylation (p-Akt) signaling prevented the spontaneous
respiratory recovery observed following partial cervical SCI in
adult rats (13).
Rutin is a common food flavonoid belonging to the
flavonols. Studies have demonstrated that flavonoid compounds have
anti-inflammatory, antioxidant, antitumor, antiviral,
anticardiovascular disease and immunomodulatory effects (14–16).
Each phenyl ring of flavonoid compounds can be connected to a
hydroxy group, sugar group or substituent group, including
polysaccharides and, as the types and connection positions of the
sugar are different, various flavonoid glycosides may be formed
(17). However, the mechanism
through which rutin regulates neurological function of SCI remains
to be fully elucidated. In the present study, the predominant focus
was to investigate whether rutin has a positive effect on
neurological function in SCI rats, and to determine the mechanisms
involved in the regulation of expression of MIP-2, activation of
MMP-9 and phosphorylation of Akt in SCI rats.
Materials and methods
Drugs and chemicals
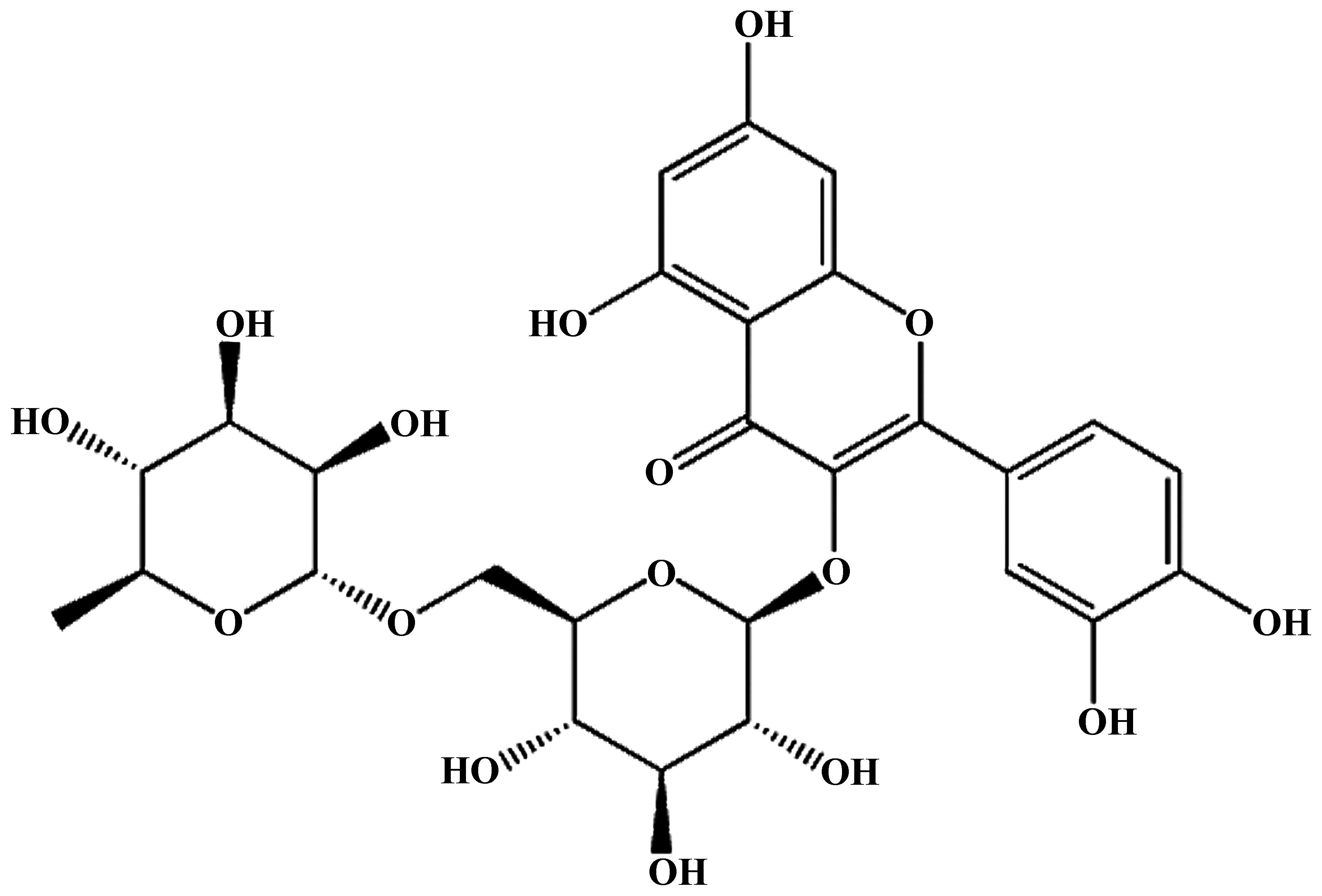
Rutin (purity >95%; Fig. 1) was purchased from Nanjing
Traditional Chinese Medicine Institute of Chinese Material Medica
(Nanjing, China). Methylprednisolone (MPSS) was supplied by the
Affiliated Shanxi Da Yi Hospital of Shanxi Medical University
(Taiyuan, China). The MIP-2 ELISA assay kit was supplied by Cayman
Chemicals, (Ann Arbor, MI, USA). The Bicinchoninic Acid (BCA)
protein assay was supplied by Beyotime Institute of Biotechnology
(Nanjing, China).
Materials
A total of 40 healthy male adult Sprague-Dawley (SD)
rats, aged 2 months and weighing 250±20 g, were purchased from the
Chinese Academy of Medical Sciences Animal Laboratory (Beijing,
China). All SD rats were allowed free access to food and water and
were housed in individual cages at 23±2°C with a humidity of ~56%
under a 12-h light/dark cycle. The present study was approved by
the ethics committee of The Affiliated Shanxi Da Yi Hospital of
Shanxi Medical University. The protocols were performed in
accordance with the Chinese National Natural Science Foundation
animal research regulations (18)
and the animal care guidelines of the National Institutes of Health
(Bethesda, MA, USA).
Preparation of the rat model of SCI
The experiment rats were fixed on an operating table
in a supine position and administered intraperitoneally (i.p) with
pentobarbital (50 mg/kg) chloral hydrate for anesthesia. Following
sterilization, the skin of the rats above the vertebral column was
shaved carefully, an abdominal midline incision was made, and a
20-mm midline incision was made in the thoracic region for the
purpose of exposing the vertebral column. Laminectomy was performed
at vertebral level T-10, and the dura remaining intact was used to
expose the dorsal cord surface.
Drug treatment and grouping
According to a previous report (19), the dosage and dosing frequency of
rutin were selected. The rats were randomly divided into five
groups, each containing eight rats, as follows: (i) control group
(Con), in which normal rats received physiological saline (0.1
ml/100 g, i.p.); (ii) SCI group, in which SCI model rats received
physiological saline (0.1 ml/100 g, i.p.); (iii) MPSS group, in
which SCI model rats received 100 mg/kg MPSS (i.p.); (iv) 1
µmol/kg rutin group (RT group), in which SCI model rats
received 1 µmol/kg rutin; (v) 10 µmol/kg rutin group
(RT group), in which SCI model rats received 10 µmol/kg
rutin. After 6 h, the rats were sacrificed by cervical dislocation,
and samples were collected for further analysis.
Evaluation of Basso, Beattie and
Bresnahan (BBB) scores for evaluating neurological function
After 6 h, the locomotor recovery of the rats were
evaluated using the BBB scoreing system, in which locomotion was
scored on a rating scale between 0 (complete paralysis) and 21
(normal locomotion) (20).
Evaluation of the water content of spinal
cord tissue
The rats were administered for edema at 3 days
post-SCI. The spinal cord of the rats were cut into 10 mm segments
and dried for 48 h at −80°C prior to the wet weight being measured
(21). The percentage of tissue
water content was then calculated using the following equation:
Water Content (%) = (wet weight − dry weight) / wet weight) ×
100.
Evaluation of programmed cell death using
hematoxylin and eosin (H&E) staining
Following treatment with rutin, the spinal cord
tissues were collected and the sections of the spinal cord were
washed with distilled water and stained with hematoxylin solution
for 10 min (Shanghai Research Company Biological Technology
Co.,Ltd., Shanghai, China). These sections were differentiated in
1% acid-alcohol for 30 sec and were then sections placed into eosin
for 30 sec and dehydrated with alcohol (70, 80, 90 and 100%) for 2
min each. Subsequently, the sections were covered with xylene-based
mounting medium (Invitrogen Life Technologies, Carlsbad, CA, USA)
following two changes of xylene. The cells were visualized using
microscopy (IX71; Olympus, Tokyo, Japan).
Evaluation of the expression of MIP-2
using ELISA assay kits
Following treatment with rutin, the spinal cord
samples were collected and were homogenized in a glass homogenizer
into tissue homogenates for the estimation of MIP-2. According to
the manufacturer's instructions (Cayman Chemicals), the evaluation
of MIP-2 was performed using commercially available ELISA assay
kits (Cayman Chemicals). The protein content was determined using a
BCA protein assay (Beyotime Institute of Biotechnology).
Evaluation of the activation of MMP-9
using zymographic analysis
As previously described (22), the activation of MMP-9 was measured
using a gelatin zymography protease assay. Following treatment with
rutin, the spinal cord tissues were collected and homogenized for
the estimation of MMP-9. The samples were prepared using SDS sample
buffer and then subjected to 8% SDS-PAGE (containing 0.1% gelatin;
Invitrogen Life Technologies). Following electrophoresis, the gels
were washed twice with 2.5% Triton X-100 (Beyotime Institute of
Biotechnology) for 1 h at room temperature. The gels were then
incubated at 37°C for 16–18 h in reaction buffer (Beyotime
Institute of Biotechnology). Finally, the gels were stained with
Coomassie Brilliant R-250 (Amresco, Inc., Framingham, MA, USA) to
stain the gels.
Evaluation of the phosphorylation of Akt
using western blot analysis
Following treatment with rutin, the spinal cord
tissue samples were collected homogenized for the estimation of the
protein expression levels of phosphorylated (p)-Akt. Briefly, 10 mg
of the exposed spinal cord tissue samples were removed and
incubated with 100 µl tissue lysis buffer (pH 7.5; Beyotime
Institute of Biotechnology) for 20–30 min on ice. Subsequently, the
homogenates were centrifuged at 12,000 g for 20 min at 4°C. The
tissue extracts were determined using a BCA protein assay (Beyotime
Institute of Biotechnology). Equal quantities of protein (100
µg) were fractioned by 10% SDS-PAGE (Invitrogen Life
Technologies), followed by transferring onto polyvinylidene
fluoride membranes (EMD Millipore, Bedford, MA, USA). The membranes
were blocked with phosphate-buffered saline containing 0.1%
Tween-20 (PBST) and 5% non-fat milk to inhibit nonspecific binding
sites. Following washing with PBST, the membranes were incubated
with monoclonal anti-p-Akt (cat. no. sc-293125; 1:1,500; Santa Cruz
Biotechnology, Inc, Santa Cruz, CA, USA) and monoclonal
anti-β-actin (AC106; 1:500; Beyotime Institute of Biotechnology)
overnight at 4°C. The membranes were then washed twice with PBST
for 2 h at room temperature, and antibody binding was detected by
incubating with a dilution of horseradish peroxidase-conjugated IgG
(cat. no. sc-52336; 1:1,000; Santa Cruz Biotechnology, Inc.) for 2
h at room temperature. The western blots were developed using
enhanced chemiluminescence western blotting reagents (E-CS-0050c;
Wuhan Elabscience, Wuhan, China).
Statistical analysis
Statistical analyses were performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). Results are expressed as
the mean ± standard deviation. Statistical analysis was evaluated
using two-way analysis of variance. P<0.05 was considered to
indicate a statistically significant difference.
Results
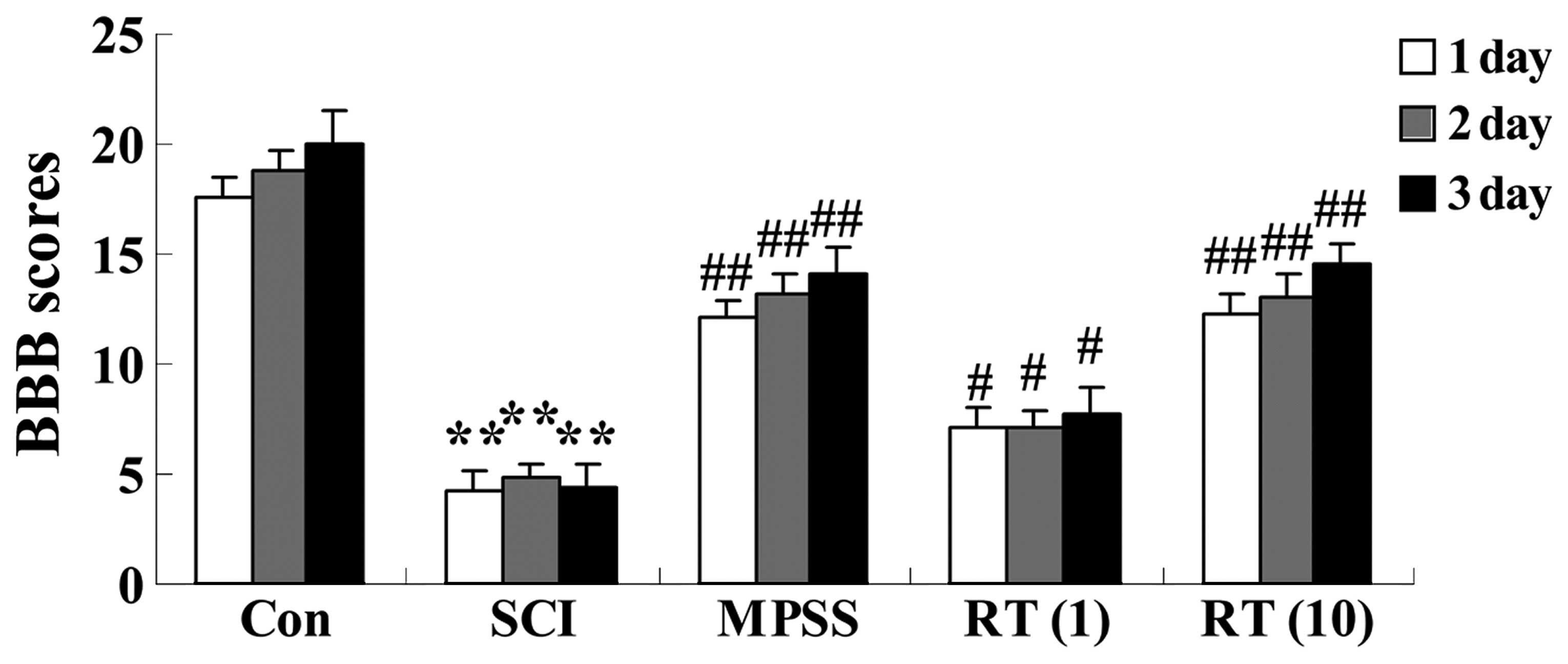
Effects of rutin on SCI-induced BBB
scores for evaluating neurological function
To measure the effect of rutin on the neurological
function of the SCI rats, BBB scores were assigned to the
functional abilities of the rats. Following injury, significant
(P<0.01) decreases in BBB scores were observed in the SCI group
at 24, 48 and 72 h post-surgery, respectively, compared with those
ofthe control group (Fig. 2). The
SCI rats in the rutin-treated groups (1 and 10 µmol/kg)
exhibited increased BBB scores (P<0.05 and P<0.01,
respectively), compared with the SCI group (Fig. 2). No significant differences were
observed between The MPSS group and 10 µmol/kg rutin-treated
group (P>0.05; Fig. 2).
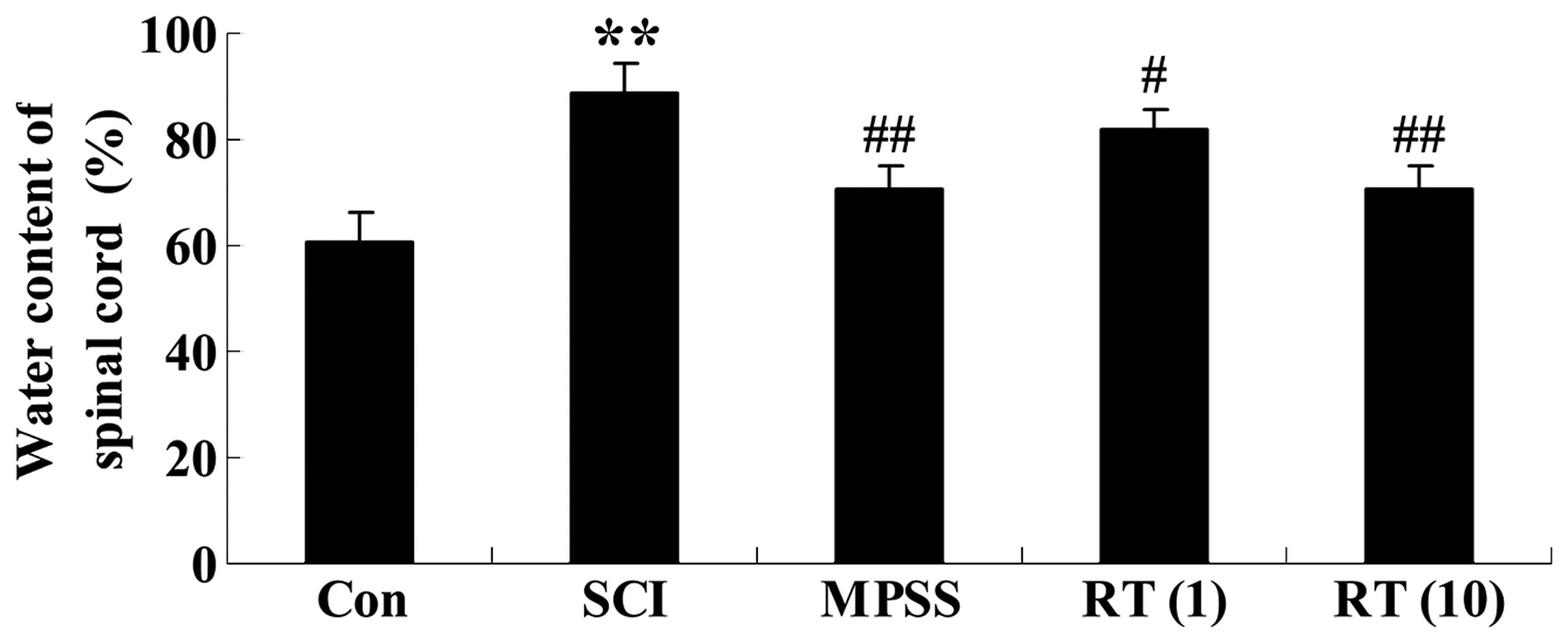
Effects of rutin on the water content of
the spinal cord tissue
At 6 h in the vehicle SCI animals, the spinal cord
water content had increased significantly, compared with the
control group (Fig. 3). The SCI
rats in the rutin-treated (1 and 10 µmol/kg) groups had
reduced spinal cord water content (P<0.05 and P<0.01,
respectively), compared with the SCI group (Fig. 3). Additionally, as shown in
Fig. 3, the water content of the
spinal cord in the 10 µmol/kg rutin-treated group was
similar to that in the MPSS group (P>0.05).
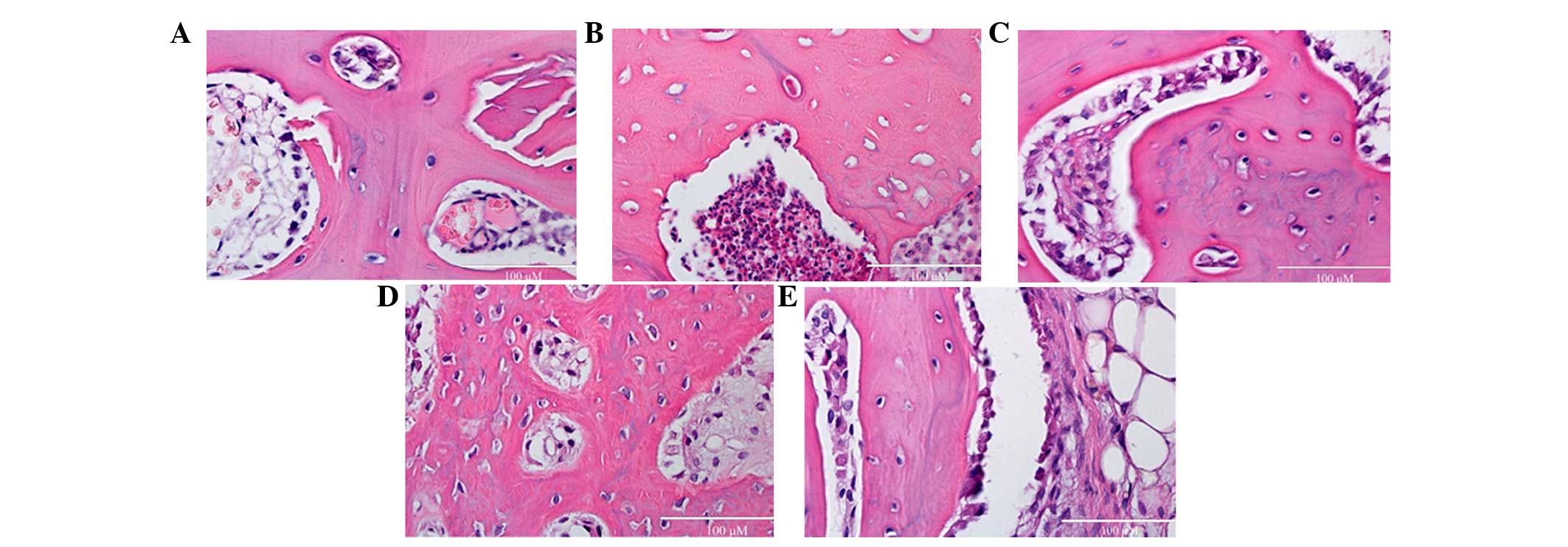
Effects of rutin on SCI-induced
programmed cell death
To assess effects of rutin on SCI-induced programmed
cell death, cell death was detected using H&E staining. The
levels of programmed cell death in the SCI group was markedly
augmented, compared with the control group (Fig. 4A and B). No significant differences
were observed between the MPSS group and 10 µmol/kg
rutin-treated group (Fig. 4C;
P>0.05). However, the rutin-treated (1 and 10 µmol/kg)
animals exhibited reduced programmed cell, compared with the SCI
group (Fig. 4D and E).
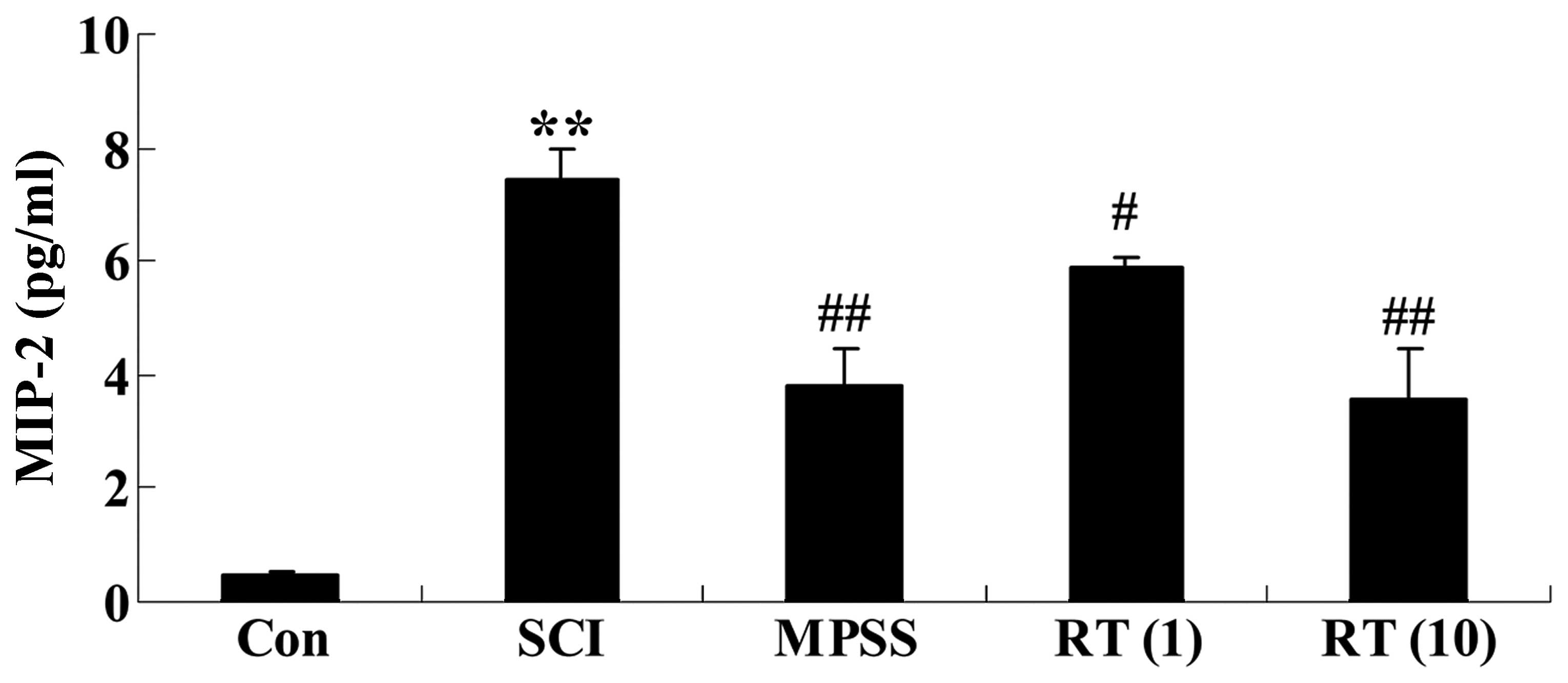
Effects of rutin on SCI-induced MIP-2
generation
To investigate the effects of rutin on SCI-induced
MIP-2 generation, the expression levels of MIP-2 in the spinal cord
of the SCI rats were detected using ELISA assay kits. As shown in
Fig. 5, the levels of MIP-2 in the
SCI rats were augmented, compared with those of control rats. In
the rutin-treated (1 and 10 µmol/kg) animals, lower levels
of MIP-2 were observed, compared with the SCI group (P<0.05 and
P<0.01, respectively; Fig. 5).
No significant inter-group differences were observed between the
MPSS group and rutin-treated (10 µmol/kg) group in the
levels of MIP-2 in the SCI rats (P>0.05).
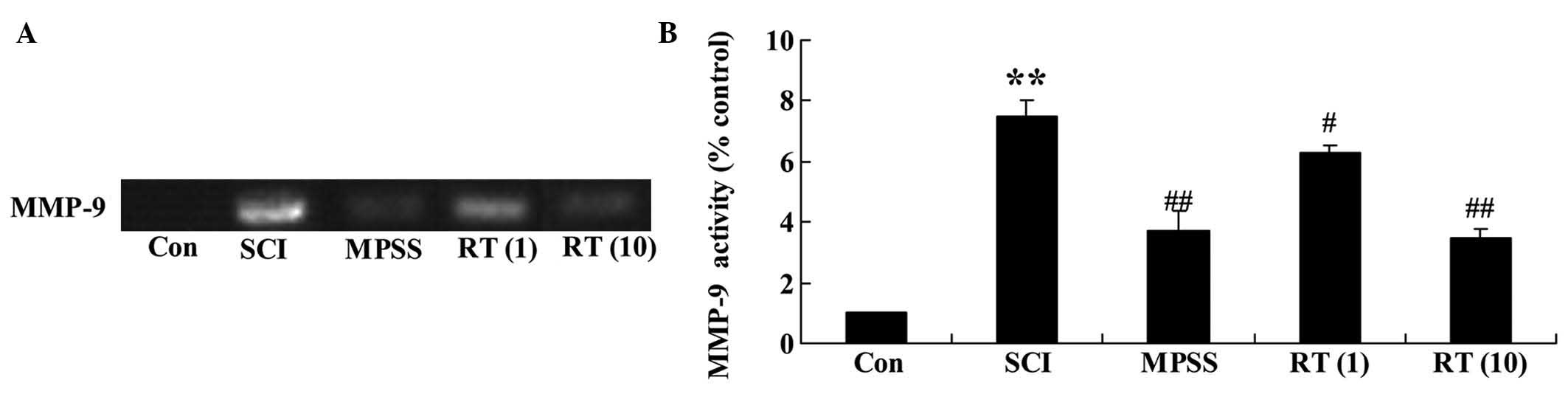
Effects of rutin on the SCI activation of
MMP-9
To confirm the effects of rutin on the SCI
activation of MMP-9, the present study detected the activation of
MMP-9 using zymographic analysis. Zymography revealed that the
activation of MMP-9 in the SCI rat group was significantly higher,
compared with that in the control group (Fig. 6A and B). Rutin treatment (1 and 10
µmol/kg) led to a reduction in the activation of MMP-9 in
the SCI rats (P<0.05 and P<0.01, respectively), compared with
the SCI group (Fig. 6A and B). By
contrast, no significant changes in the activation of MMP-9 were
observed between the MPSS group and 10 µmol/kg rutin-treated
group (P>0.05).
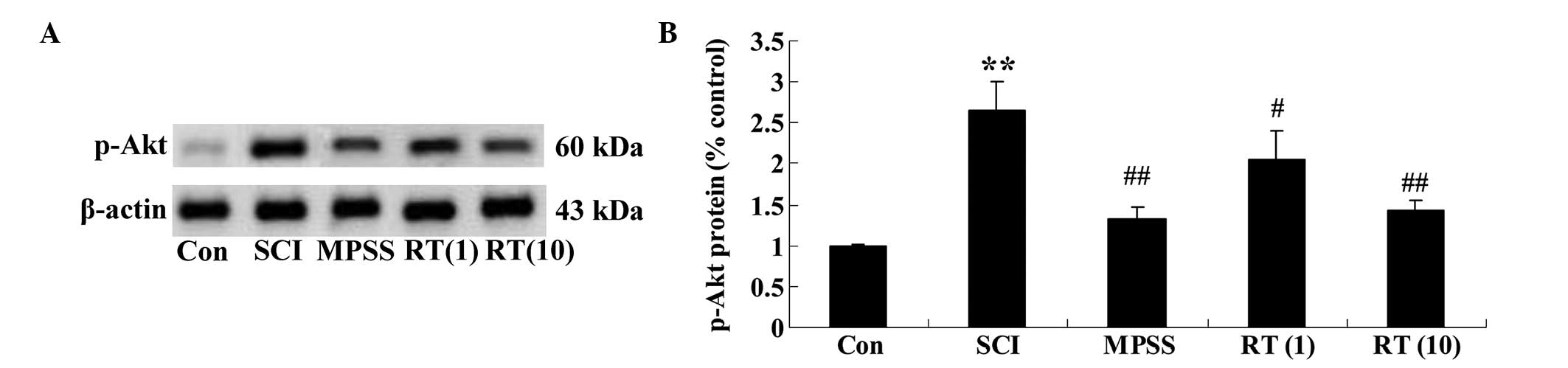
Effects of rutin on the SCI-induced
protein expression of p-Akt
To determine the protein expression levels of p-Akt
in the SCI rats, the protein expression of p-Akt was determined
using western blot analysis. The results demonstrated that the
protein expression of p-Akt in the SCI group was significant higher
than in the control group (Fig. 7A and
B). These data clearly indicated that rutin (1 and 10
µmol/kg) modulated the protein expression of p-Akt in the
SCI rats (P<0.05 and P<0.01, respectively), compared with the
SCI group (Fig. 7A and B).
However, no significant difference was observed between the MPSS
group and the 10 µmol/kg rutin-treated group
(P>0.05).
Discussion
The clinical morbidity rates of SCI are high, and
paraplegia caused by SCI is one of the medical problems, which has
remained unresolved, with no effective treatment (23). The pathological process underlying
the development of SCI remains to be fully elucidated (24). Previous studies have suggested that
cell necrosis is the predominant manifestation of SCI, however,
with further investigation, it has been reported that the
inflammatory reaction and necrosis in spinal cord nerve cells
following primary mechanical tissue damage are accompanied by
apoptosis, or programmed cell death (25–27).
In the present study, it was demonstrated that rutin markedly
augmented the BBB scores and decreased spinal cord water content in
the SCI rats. In addition, rutin was observed to prevent
SCI-induced programmed cell death. Xu et al indicated that
rutin improves spatial memory and improves neurological function in
Alzheimer's disease-transgenic mice (28). Rodrigues et al reported that
rutin caused a reduction of neurodegeneration in the periphery of
cortical injury (29). Aruna et
al revealed that rutin reduces programmed cell death by
affecting the downregulation of apoptosis-associated speck-like
protein containing-NOD-like receptor pyrin domain containing 3
(30). These curative effects of
rutin (10 µmol/kg) treatment were similar to those observed
in the MPSS group.
MIP-2 is derived from a variety of cells, including
macrophages, neutrophils and endothelial cells (31). The predominant biological function
of MIP-2 is the chemotaxis and activation of neutrophils and
lymphocytes for involvement in inflammatory reactions (32). The heparin-binding sites of MIP-2
can interact with endothelial cells, upregulate the expression of
leukocyte adhesion molecules CD11b/CD18, and ultimately guide
leukocytes through the vessel wall to reach the site of
inflammation, thus being important in the occurrence and
development of many inflammatory diseases (33). The present study observed that
rutin reduced the expression levels of MIP-2 in SCI rats.
Similarly, Chen et al reported that rutin is a potential
protective agent for acute lung injury via downregulation in the
expression of MIP-2 and activation MMP-9 (19).
MMPs are a class of highly conserved endogenous
zinc-dependent proteases in natural evolution, and are widely
distributed in plants, vertebrates and invertebrates (34). The predominant physiological role f
MMPs is to degrade extracellular matrix components, including
collagen, gelatin, elastin, fibronectin and proteoglycans, in which
MMP-9 is the most important in degrading the endothelial basement
membrane to open the blood-brain barrier (35). Studies have reported that MMP-9 is
associated with apoptosis (36,37).
In the present study, a decrease in the activation of MMP-9 was
observed in response to rutin, which occurred in a dose-dependent
manner. Jang et al suggested that rutin improved functional
outcome via reducing the level of MMP-9 in a photothrombotic focal
ischemic model of rats (38), and
Chen et al reported that rutin is a potential protective
agent for acute lung injury via downregulation of activation of
MMP-9 and inhibition of the expression of p-Akt (19).
As an important downstream signaling molecule for
PI3K, Akt is a serine/threonine protein kinase, which is important
in the proliferation, differentiation and apoptosis of cells
(39). Previous studies have
demonstrated that Akt is also important in the nociceptive
information transfer process, and is involved in peripheral and
central pain modulation at different levels, with inhibition of the
PI3K-Akt signaling pathway resulting in a significant
antinociceptive effect (40–42).
Following peripheral nerve injury, p-Akt is predominantly
distributed in the superficial dorsal horn of the spinal cord
(43). The Akt signaling pathway
is involved in the genesis and development of neuropathic pain,
which is associated with its effect on the activation of
nociceptive sensory neurons (44).
In addition, studies have demonstrated that the Akt signaling
pathway is involved in several regulatory processes of neural
plasticity, and is important in the process of change in spinal
cord dorsal horn neuronal plasticity caused by nerve damage
(45,46). The Akt signaling pathway is
important role in the genesis and development of neuropathic pain.
In the present study, rutin was observed to modulate the protein
expression of p-Akt in the SCI rats. Hu et al suggested that
rutin ameliorates activation of the renal NOD-like receptor 3
inflammasome by mediating Akt signaling (47). In addition, Jeong et al
reported that rutin inhibits myocardial
ischemia/reperfusion-induced apoptosis via extracellular
signal-regulated kinase 1/2 and PI3K/Akt signals in vitro
(48).
In conclusion, the predominant finding of the
present study was that the mediated delivery of rutin successfully
decreased neuropathic function behavior and associated protein
expression levels. Rutin appeared to inhibit the expression of
MIP-2 and activation of MMP-9, and reduce the protein expression of
Akt in the SCI rats. Future investigations on the signaling
pathways to rutin administration aim to provide further insights
into its therapeutic action in terms of SCI-induced neuropathic
function, and provide a starting point for developing novel
strategies for pain control.
References
|
1
|
Casha S, Yu WR and Fehlings MG:
Oligodendroglial apoptosis occurs along degenerating axons and is
associated with FAS and p75 expression following spinal cord injury
in the rat. Neuroscience. 103:203–218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen H, Gong C, Ma C, Zhang X, Xu L and
Lin C: Cardioprotective effects of phosphocreatine on myocardial
cell ultrastructure and calcium-sensing receptor expression in the
acute period following high level spinal cord injury. Mol Med Rep.
10:560–566. 2014.PubMed/NCBI
|
|
3
|
Kollmar O, Menger MD and Schilling MK:
Macrophage inflammatory protein-2 contributes to liver
resection-induced acceleration of hepatic metastatic tumor growth.
World J Gastroenterol. 12:858–867. 2006.PubMed/NCBI
|
|
4
|
Pineau I, Sun L, Bastien D and Lacroix S:
Astrocytes initiate inflammation in the injured mouse spinal cord
by promoting the entry of neutrophils and inflammatory monocytes in
an IL-1 receptor/MyD88-dependent fashion. Brain Behav Immun.
24:540–553. 2010. View Article : Google Scholar
|
|
5
|
Berghmans N, Heremans H, Li S, Martens E,
Matthys P, Sorokin L, Van Damme J and Opdenakker G: Rescue from
acute neuroinflammation by pharmacological chemokine-mediated
deviation of leukocytes. J Neuroinflammation. 9:2432012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chandramohan Reddy T, Bharat Reddy D,
Aparna A, Arunasree KM, Gupta G, Achari C, Reddy GV, Lakshmipathi
V, Subramanyam A and Reddanna P: Anti-leukemic effects of gallic
acid on human leukemia K562 cells: downregulation of COX-2,
inhibition of BCR/ABL kinase and NF-kappaB inactivation. Toxicol In
Vitro. 26:396–405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee S, Lee CH, Moon SS, Kim E, Kim CT, Kim
BH, Bok SH and Jeong TS: Naringenin derivatives as anti-atherogenic
agents. Bioorg Med Chem Lett. 13:3901–3903. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang X, Liu H, Zhang Y, Li J, Teng X, Liu
A, Yu X, Shan Z and Teng W: Effects of isolated positive maternal
thyro-globulin antibodies on brain development of offspring in an
experimental autoimmune thyroiditis model. Thyroid. 25:551–558.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee JY, Choi HY, Na WH, Ju BG and Yune TY:
Ghrelin inhibits BSCB disruption/hemorrhage by attenuating MMP-9
and SUR1/TrpM4 expression and activation after spinal cord injury.
Biochim Biophys Acta. 1842:2403–2412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng M, Shu Y, Yang Y, Zheng X, Li R, Wang
Y, Dai Y, Qiu W, Lu Z and Hu X: Ulinastatin attenuates experimental
autoimmune encephalomyelitis by enhancing anti-inflammatory
responses. Neurochem Int. 64:64–72. 2014. View Article : Google Scholar
|
|
11
|
Zhang H, Chu G, Pan C, Hu J, Guo C, Liu J,
Wang Y and Wu J: A nutrient mixture reduces the expression of
matrix metalloproteinases in an animal model of spinal cord injury
by modulating matrix metalloproteinase-2 and matrix
metal-loproteinase-9 promoter activities. Exp Ther Med.
8:1835–1840. 2014.PubMed/NCBI
|
|
12
|
Bachmeier BE, Nerlich AG, Weiler C,
Paesold G, Jochum M and Boos N: Analysis of tissue distribution of
TNF-alpha, TNF-alpha-receptors and the activating
TNF-alpha-converting enzyme suggests activation of the TNF-alpha
system in the aging intervertebral disc. Ann N Y Acad Sci.
1096:44–54. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Felix MS, Bauer S, Darlot F, Muscatelli F,
Kastner A, Gauthier P and Matarazzo V: Activation of Akt/FKHR in
the medulla oblongata contributes to spontaneous respiratory
recovery after incomplete spinal cord injury in adult rats.
Neurobiol Dis. 69:93–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsunaga K, Yoshimi N, Shimoi K, Yamada
Y, Katayama M, Sakata K, Kumo T, Yoshida K, Qiao Z, Kinae N and
Mori H: Inhibitory effects of dietary monoglucosyl-rutin on
azoxy-methane-induced colon carcinogenesis in rats. Asian Pac J
Cancer Prev. 1:211–216. 2000.
|
|
15
|
Aruna R, Geetha A, Suguna P and Suganya V:
Rutin rich Emblica officinalis Geart. fruit extract ameliorates
inflammation in the pancreas of rats subjected to alcohol and
cerulein administration. J Complement Integr Med. 11:9–18.
2014.PubMed/NCBI
|
|
16
|
Song K, Na JY, Kim S and Kwon J: Rutin
upregulates neuro-trophic factors resulting in attenuation of
ethanol-induced oxidative stress in HT22 hippocampal neuronal
cells. J Sci Food Agric. 95:2117–2123. 2015. View Article : Google Scholar
|
|
17
|
Mascaraque C, Aranda C, Ocón B, Monte MJ,
Suárez MD, Zarzuelo A, Marín JJ, Martínez-Augustin O and de Medina
FS: Rutin has intestinal antiinflammatory effects in the CD4+
CD62L+ T cell transfer model of colitis. Pharmacol Res. 90:48–57.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stemper BD, Shah AS, Pintar FA, McCrea M,
Kurpad SN, Glavaski-Joksimovic A, Olsen C and Budde MD: Head
rotational acceleration characteristics influence behavioral and
diffusion tensor imaging outcomes following concussion. Ann Biomed
Eng. 43:1071–1088. 2015. View Article : Google Scholar
|
|
19
|
Chen WY, Huang YC, Yang ML, Lee CY, Chen
CJ, Yeh CH, Pan PH, Horng CT, Kuo WH and Kuan YH: Protective effect
of rutin on LPS-induced acute lung injury via down-regulation of
MIP-2 expression and MMP-9 activation through inhibition of Akt
phosphorylation. Int Immunopharmacol. 22:409–413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Basso DM, Beattie MS, Bresnahan JC,
Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ,
Nockels R, et al: MASCIS evaluation of open field locomotor scores:
Effects of experience and teamwork on reliability. Multicenter
animal spinal cord injury study. J Neurotrauma. 13:343–359. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vink R, Young A, Bennett CJ, Hu X, Connor
CO, Cernak I and Nimmo AJ: Neuropeptide release influences brain
edema formation after diffuse traumatic brain injury. Acta
Neurochir Suppl. 86:257–260. 2003.
|
|
22
|
Huang CH, Yang ML, Tsai CH, Li YC, Lin YJ
and Kuan YH: Ginkgo biloba leaves extract (EGb 761) attenuates
lipopolysac-charide-induced acute lung injury via inhibition of
oxidative stress and NF-κB-dependent matrix metalloproteinase-9
pathway. Phytomedicine. 20:303–309. 2013. View Article : Google Scholar
|
|
23
|
Tan F, Chen J, Liang Y, Gu M, Li Y, Wang X
and Meng D: Electroacupuncture attenuates cervical spinal cord
injury following cerebral ischemia/reperfusion in stroke-prone
renovascular hypertensive rats. Exp Ther Med. 7:1529–1534.
2014.PubMed/NCBI
|
|
24
|
Beattie MS, Farooqui AA and Bresnahan JC:
Review of current evidence for apoptosis after spinal cord injury.
J Neurotrauma. 17:915–925. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lichte P, Grigoleit JS, Steiner EM,
Kullmann JS, Schedlowski M, Oberbeck R and Kobbe P: Low dose LPS
does not increase TLR4 expression on monocytes in a human in vivo
model. Cytokine. 63:74–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pejcic T, Stankovic I, Petkovic TR,
Borovac DN, Djordjevic I and Jeftovic-Stoimenov T: Peroxisome
proliferator-activated receptor gamma as modulator of inflammation
in pulmonary sarcoidosis. Srp Arh Celok Lek. 141:705–709. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krenz NR and Weaver LC: Nerve growth
factor in glia and inflammatory cells of the injured rat spinal
cord. J Neurochem. 74:730–739. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu PX, Wang SW, Yu XL, Su YJ, Wang T, Zhou
WW, Zhang H, Wang YJ and Liu RT: Rutin improves spatial memory in
Alzheimer's disease transgenic mice by reducing Aβ oligomer level
and attenuating oxidative stress and neuroinflammation. Behav Brain
Res. 264:173–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rodrigues AM, Marcilio Fdos S, Frazão
Muzitano M and Giraldi-Guimarães A: Therapeutic potential of
treatment with the flavonoid rutin after cortical focal ischemia in
rats. Brain Res. 1503:53–61. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aruna R, Geetha A and Suguna P: Rutin
modulates ASC expression in NLRP3 inflammasome: A study in alcohol
and cerulein-induced rat model of pancreatitis. Mol Cell Biochem.
396:269–280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grygiel-Górniak B: Peroxisome
proliferator-activated receptors and their ligands: nutritional and
clinical implications - a review. Nutr J. 13:172014. View Article : Google Scholar
|
|
32
|
Jiang Y, Gu XP, Qiu YD, Sun XM, Chen LL,
Zhang LH and Ding YT: Ischemic preconditioning decreases C-X-C
chemokine expression and neutrophil accumulation early after liver
transplantation in rats. World J Gastroenterol. 9:2025–2029.
2003.PubMed/NCBI
|
|
33
|
Ribeiro A, Almeida VI, Costola-de-Souza C,
Ferraz-de-Paula V, Pinheiro ML, Vitoretti LB, Gimenes-Junior JA,
Akamine AT, Crippa JA, Tavares-de-Lima W and Palermo-Neto J:
Cannabidiol improves lung function and inflammation in mice
submitted to LPS-induced acute lung injury. Immunopharmacol
Immunotoxicol. 37:35–41. 2015. View Article : Google Scholar
|
|
34
|
Zhang J, Huang X and Wang L: Pioglitazone
inhibits the expression of matrix metalloproteinase-9, a protein
involved in diabetes-associated wound healing. Mol Med Rep.
10:1084–1088. 2014.PubMed/NCBI
|
|
35
|
Zhang YM, Zhou Y, Qiu LB, Ding GR and Pang
XF: Altered expression of matrix metalloproteinases and tight
junction proteins in rats following PEMF-induced BBB permeability
change. Biomed Environ Sci. 25:197–202. 2012.PubMed/NCBI
|
|
36
|
Choi JH, Comess KA, Xu C, Park J and Kim
Y: Development of an interactive Coronary Doppler Vibrometry system
for detection of coronary artery disease. Conf Proc IEEE Eng Med
Biol Soc. 2011:7195–7198. 2011.
|
|
37
|
Wang Y, Wang W, Gong F, Fu S, Zhang Q, Hu
J, Qi Y and Xie C: Evaluation of intravenous immunoglobulin
resistance and coronary artery lesions in relation to Th1/Th2
cytokine profiles in patients with Kawasaki disease. Arthritis
Rheum. 65:805–814. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jang JW, Lee JK, Hur H, Kim TW, Joo SP and
Piao MS: Rutin improves functional outcome via reducing the
elevated matrix metalloproteinase-9 level in a photothrombotic
focal ischemic model of rats. J Neurol Sci. 339:75–80. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yi H, Long B, Ye X, Zhang L, Liu X and
Zhang C: Autophagy: A potential target for thyroid cancer therapy
(Review). Mol Clin Oncol. 2:661–665. 2014.PubMed/NCBI
|
|
40
|
Liu Q, Chen L, Hu L, Guo Y and Shen X:
Small molecules from natural sources, targeting signaling pathways
in diabetes. Biochim Biophys Acta. 1799:854–865. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mahmoud AM, Ashour MB, Abdel-Moneim A and
Ahmed OM: Hesperidin and naringin attenuate hyperglycemia-mediated
oxidative stress and proinflammatory cytokine production in high
fat fed/streptozotocin-induced type 2 diabetic rats. J Diabetes
Complications. 26:483–490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu Z, Zhang YY, Zhang QW, Zhao SR, Wu CZ,
Cheng X, Jiang CC, Jiang ZW and Liu H: 3-Bromopyruvate induces
apoptosis in breast cancer cells by downregulating Mcl-1 through
the PI3K/Akt signaling pathway. Anticancer Drugs. 25:447–455. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen H, Wang X, Tong M, Wu D, Wu S, Chen
J, Wang X, Wang X, Kang Y, Tang H, Tang C and Jiang W: Intermedin
suppresses pressure overload cardiac hypertrophy through activation
of autophagy. PLoS One. 8:e647572013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xiong W, Qiu SY, Xu LY, Zhang CP, Yi Y, Wu
Q, Huang LP, Liu SM, Wu B, Peng LC, Song MM, Gao Y and Liang SD:
Effects of intermedin on dorsal root ganglia in the transmission of
neuropathic pain in chronic constriction injury rats. Clin Exp
Pharmacol Physiol. 42:780–787. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Du X, Cao Y, Xue P, Lin Z, Zeng Z and Xia
Q: Protective effect of intermedin on myocardial cell in a rat
model of severe acute pancreatitis. Cell Mol Biol Lett. 16:462–476.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li P, Sun HJ, Han Y, Wang JJ, Zhang F,
Tang CS and Zhou YB: Intermedin enhances sympathetic outflow via
receptor-mediated cAMP/PKA signaling pathway in nucleus tractus
solitarii of rats. Peptides. 47:1–6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hu QH, Zhang X, Pan Y, Li YC and Kong LD:
Allopurinol, quercetin and rutin ameliorate renal NLRP3
inflammasome activation and lipid accumulation in fructose-fed
rats. Biochem Pharmacol. 84:113–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jeong JJ, Ha YM, Jin YC, Lee EJ, Kim JS,
Kim HJ, Seo HG, Lee JH, Kang SS, Kim YS and Chang KC: Rutin from
Lonicera japonica inhibits myocardial ischemia/reperfusion-induced
apoptosis in vivo and protects H9c2 cells against hydrogen
peroxide-mediated injury via ERK1/2 and PI3K/Akt signals in vitro.
Food Chem Toxicol. 47:1569–1576. 2009. View Article : Google Scholar : PubMed/NCBI
|