Introduction
Breast cancer is the most common malignancy in women
worldwide presenting as a key factor affecting women's health, and
ranks second (to lung cancer) in the rate of mortality of women in
2013 (1). With the accelerating
urbanization of China, breast cancer incidence and mortality are
increasing (2,3). The development of comprehensive
treatments has led to improved therapeutic efficacy in breast
cancer, however there remains a considerable number of patients
experiencing recurrence or resistance to conventional treatment
(4).
Matrix metalloproteinases (MMPs) are zinc dependent
neutral proteases, which exhibit combined actions to degrade all
extracellular and basement membrane components (5). The activity regulation of MMP serves
an important role in the process of tissue reconstruction and
inflammation, and in the growth, invasion and metastasis of tumors
(1). Previous studies demonstrated
that MMP-2 and -9 are overexpressed in a variety of solid malignant
tumors, potentially promoting tumor invasion, metastasis and
angiogenesis through the disruption of the basement membrane and
the extracellular matrix (6). In
addition, studies have demonstrated that levels of MMP-2 and 9 are
correlated with the proliferation of tumor cells and apoptosis of
endothelial cells (7,8). MMP-15, -16 and -17 are membrane-type
MMPs and are able to degrade the ECM and activate MMP-2 and -13
(4,5). Additionally, MMP-16 has been
demonstrated to be an enhancer of breast cancer cell invasiveness
(9,10).
MicroRNAs (miRNAs) are a class of non-coding
single-stranded RNA molecules with a length of approximately 22
nucleotides, which exist widely in eukaryotic cells. miRNAs bind to
specific mRNA molecules to inhibit protein translation or regulate
protein expression, with certain varieties able to degrade mRNA
(11). At present, approximately
500 miRNAs have been identified in the human genome, and 200 miRNA
sequences have been identified to be associated with cancer
(12). Specific alterations in
miRNA expression are able to initiate and promote the occurrence of
cancer, and certain miRNAs may affect oncogenes and tumor
suppressor genes (13,14). Expression of microRNA-146 has been
demonstrated to reduce metastatic potential in breast cancer cells
through the downregulation of nuclear factor κB (15). Furthermore, the expression levels
of miRNA-146a (miR-146a) are significantly higher in patients with
breast cancer, when compared with healthy controls (16).
Catalpol is one of the key active ingredients of
Rehmannia, which has effects on the nervous and
cardiovascular systems, in addition to exhibiting
antihyperglycemic, antitumor and antiproliferative activities
(17–19). However, previous studies have only
demonstrated that catalpol is able to reproduce the diuretic,
laxative and hypoglycemic effects of Rehmannia (20). The present study aimed to
investigate whether catalpol inhibited cancer growth by
upregulating microRNA-146a and downregulating MMP-16
expression.
Materials and methods
Chemicals and reagents
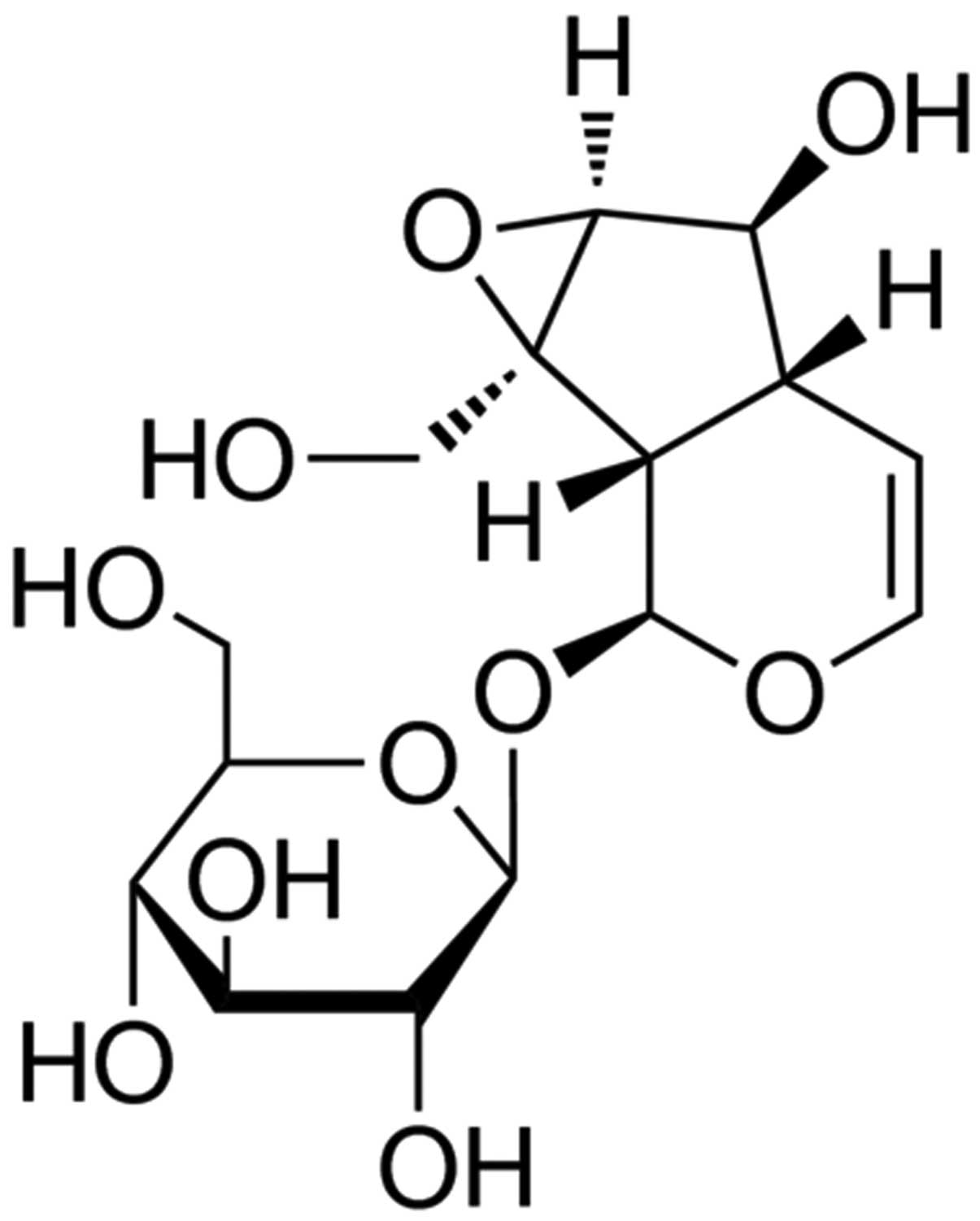
Catalpol (purity ≥ 98%) was obtained from
Sigma-Aldrich (St. Louis, MO, USA) and the chemical structure is
presented in Fig. 1. Dulbecco's
modified Eagle's medium (DMEM) and fetal calf serum (FCS) were
obtained from Gibco Life Technologies (Carlsbad, CA, USA).
3-3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) and the Caspase-3 Colorimetric Assay kit were purchased from
Sangon Biotech Co., Ltd. (Shanghai, China). The Annexin V-Propidium
Iodide (PI) Apoptosis Detection kit was obtained from BestBio
(Shanghai, China). TRIzol was obtained from Tiandz, Inc. (Beijing,
China). TaqMan®, the ABI 7900 Real-Time Polymerase Chain
Reaction (PCR) machine (Applied Biosystems Life Technologies,
Foster City, CA, USA) and SYBR® Green kit were obtained
from Qiagen, Inc. (Valencia, CA, USA).
Cell culture
The human breast cancer MCF-7 cell line was
purchased from the Animal Experiment Center of Binzhou Medical
University (Yantai, China) and cultured with DMEM containing 10%
FCS with penicillin (100 U/ml) and streptomycin (100 U/ml), which
were both purchased from Sigma-Aldrich. The cells were maintained
in a humidified chamber at 37°C in 5% CO2 and culture
media was renewed every 2–3 days.
MTT assay
Following culture of MCF-7 cells with catalpol (0,
25, 50 and 100 µg/ml) (21)
for 0, 24, 48 and 72 h, the viability of MCF-7 cells was determined
using the MTT assay. MTT (~10 µl, 5 mg/ml; Sangon Biotech
Co., Ltd.) was added to each well and cells were incubated for 4 h
in a humidified chamber at 37 °C in 5% CO2.
Subsequently, the culture medium was removed and 150 µl
dimethyl sulfoxide (Invitrogen Life Technologies, Carlsbad, CA,
USA) was added to each well, which were then agitated for 20 min at
room temperature. Cell viability was determined using an ELISA
reader (Thermo Fisher Scientific, Waltham, MA, USA) at 570 nm.
Caspase-3 activity assays
Following culture of MCF-7 cells with catalpol (0,
25, 50 and 100 µg/ml) for 48 h, the activity of caspase-3
was measured using the Caspase-3 Colorimetric Assay kit (Sangon
Biotech Co., Ltd.). The level of caspase-3 activity was measured at
405 nm with the ELISA reader.
Flow cytometry
Following culture of MCF-7 cells with catalpol (0,
25, 50 and 100 µg/ml) for 48 h, apoptosis was measured using
the Annexin V-PI Apoptosis Detection kit according to the
manufacturer's instructions (BestBio) and the fluorescence
intensity was detected using flow cytometry (Beckman Coulter, Inc.,
Brea, CA, USA).
Gelatin zymography assays of MMP-16
The MMP-16 level in MCF-7 cells was measured by
gelatin zymography. Samples (~20 µl) were collected and
placed into new centrifuge tubes. Equal volumes (50 µl) of
vitreous samples were mixed with sodium dodecyl sulfate (SDS)
sample buffer (Invitrogen Life Technologies). The miscible liquids
were run through 10% SDS-PAGE gels (Invitrogen Life Technologies)
polymerized with 1 mg/ml gelatin. Subsequent to electrophoresis,
the gel was washed three times for 20 min at room temperature in
2.5% Triton X-100 (Invitrogen Life Technologies) to remove SDS, and
incubated in a reaction buffer (Invitrogen Life Technologies) at
37°C for 12 h. Following incubation, the gel was stained with 0.05%
Coomassie® Brilliant Blue R-250 (Amresco, LLC, Solon,
OH, USA) followed by destaining with washing buffer (45% methanol,
10% acetic acid) until the bands were clear. MMP-16 was quantified
through densitometer measurement using an image-analysis system
(Image Lab™ software; Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Reverse transcription-quantitative PCR
analysis of miR-146a expression
Total RNA was extracted from MCF-7 cell samples
using TRIzol according to the manufacturer's protocol. cDNAs were
synthesized using TaqMan® and the ABI 7900 Real-Time PCR
machine, according to the manufacturer's instructions. The
expression of miR-146b was detected with the SYBR® Green
kit according to the manufacturer's instructions. The primer
sequences were as follows: miR-146a, forward 5′-CTA GCT AGC GGC CGC
TAG TAA CCC ATG GAA TTC AGT TCT CAG-3′ and reverse 5′-TCG ACT GAG
AAC TGA ATT CCA TGG GTT ACT AGC GGC CGC TAG-3′; U6, forward 5′-TGA
CTT CAA CAG CGA CAC CCA-3′ and reverse 5′-CAC CCT GTT GCT GTA GCC
AAA-3′. The cycling conditions were as follows: 95°C for 60 sec, 40
cycles of 95°C for 30 sec and 60°C for 45 sec, then 72°C for 30
sec. The U6 gene was used as an internal control to normalize
variances.
Transfection of miR-146a and
anti-miR-146a
miR-146a and anti-miR-146a plasmids were designed by
and purchased from BioSune Biotechnology Co, Ltd. (Shanghai,
China). The plasmids were transfected into MCF-7 cells using
Lipofectamine 2000 (Invitrogen Life Technologies) according to the
manufacturer's instructions. Following transfection for 24 h, MCF-7
cells were treated with catalpol (50 µg/ml) for 48 h.
Statistical analysis
The data were analyzed with SPSS software, version
17.0 (SPSS, Inc., Chicago, IL, USA) in the current study. To
compare the two groups, Student's t-test was used. P<0.05 was
considered to indicate a statistically significant difference.
Results
MTT analysis and the activity of
caspase-3
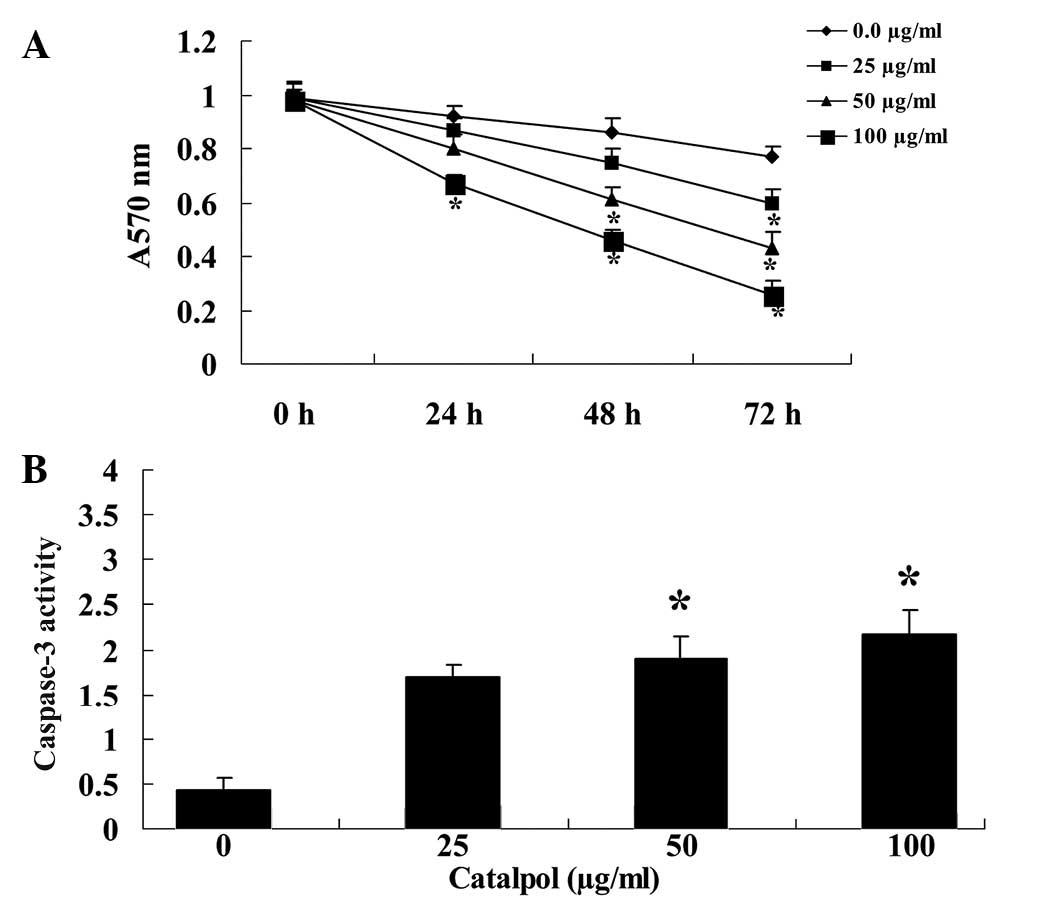
An MTT assay was used to determine whether the
antiproliferative effect of catalpol (0, 25, 50 and 100
µg/ml) reduced the proliferation of MCF-7 cells. The effect
of catalpol was dose-dependent, as increasing concentrations of
catalpol further reduced the proliferation of MCF-7 cells (Fig. 2A). In addition, the effect of
catalpol was time-dependent (Fig.
2A). Following treatment with catalpol (50 and 100
µg/ml) for 48 h, the activity of caspase-3 in MCF-7 cells
was significantly increased (P<0.01; Fig. 2B).
Flow-cytometric analysis for the
detection of apoptosis
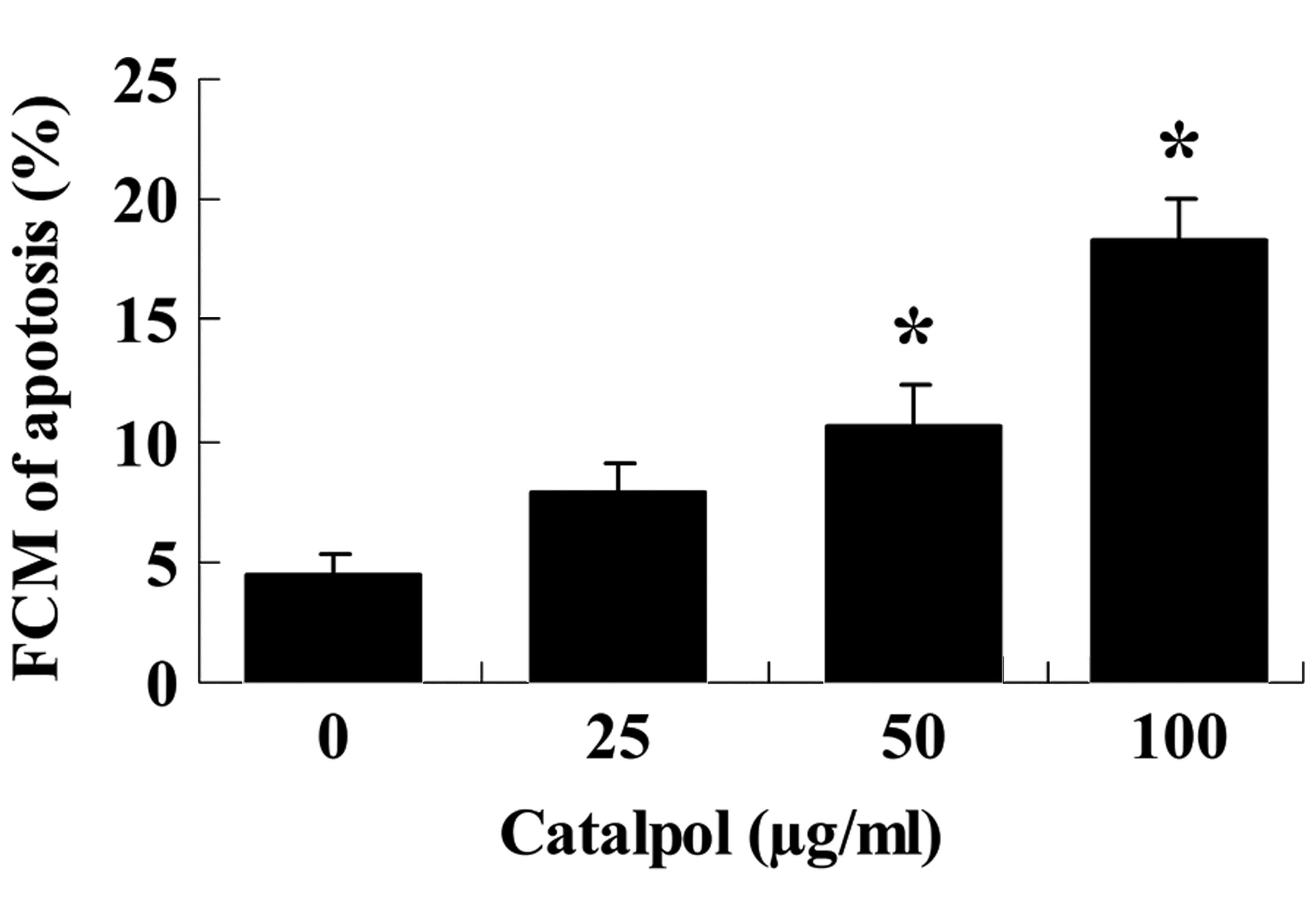
To determine whether MCF-7 cells undergo apoptosis
upon treatment with catalpol (0, 25, 50 and 100 µg/ml) for
48 h, annexin V-IP was used to measure the apoptotic cells. The
addition of catalpol (0, 25, 50 and 100 µg/ml) for 48 h
induced concentration-dependent apoptosis of MCF-7 cells (Fig. 3). The percentage of apoptotic MCF-7
cells in the 50 and 100 µg/ml catalpol groups was
significantly increased (P<0.01; Fig. 3).
Catalpol inhibits expression of
MMP-16
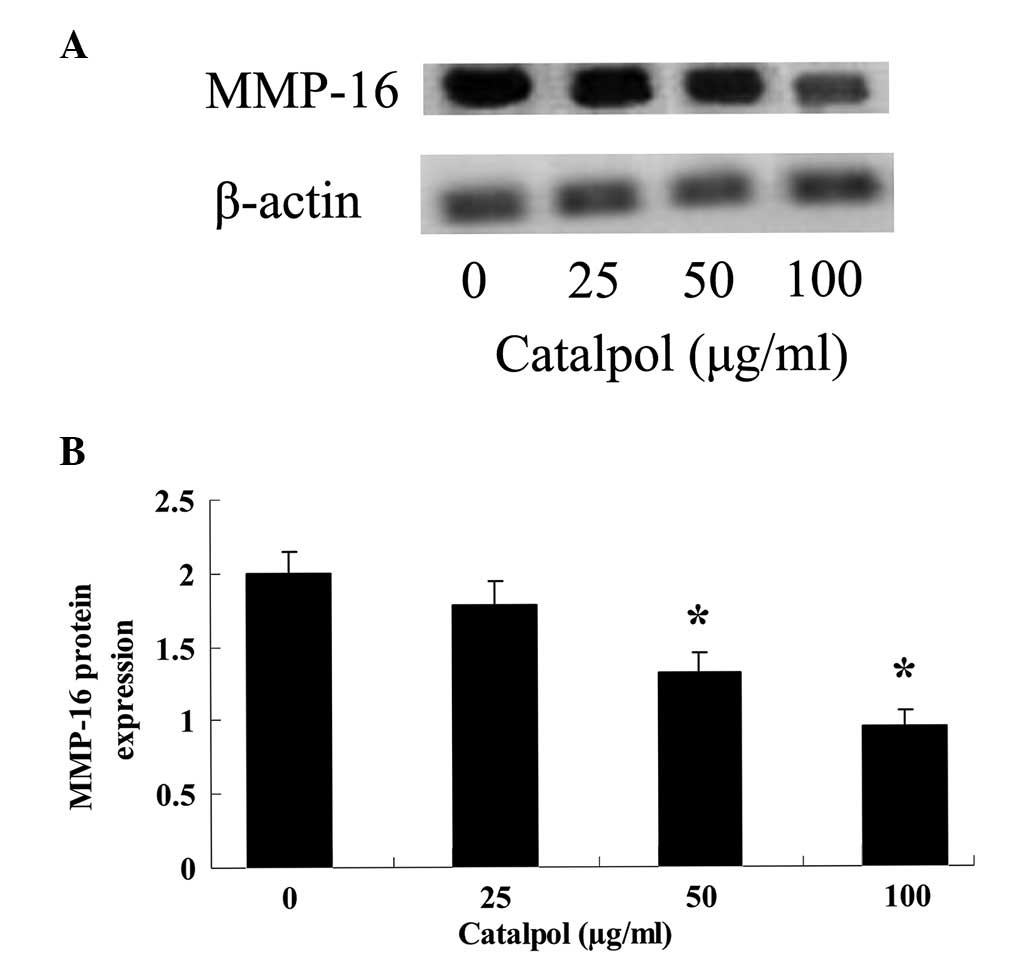
To investigate the effect of catalpol upon MMP-16, a
gelatin zymography assay was used to analyze the MMP-16 protein
levels in MCF-7 cells (Fig. 4).
When treated with catalpol (50 and 100 µg/ml) for 48 h, the
MMP-16 protein levels in MCF-7 cells were significantly reduced
(P<0.01; Fig. 4).
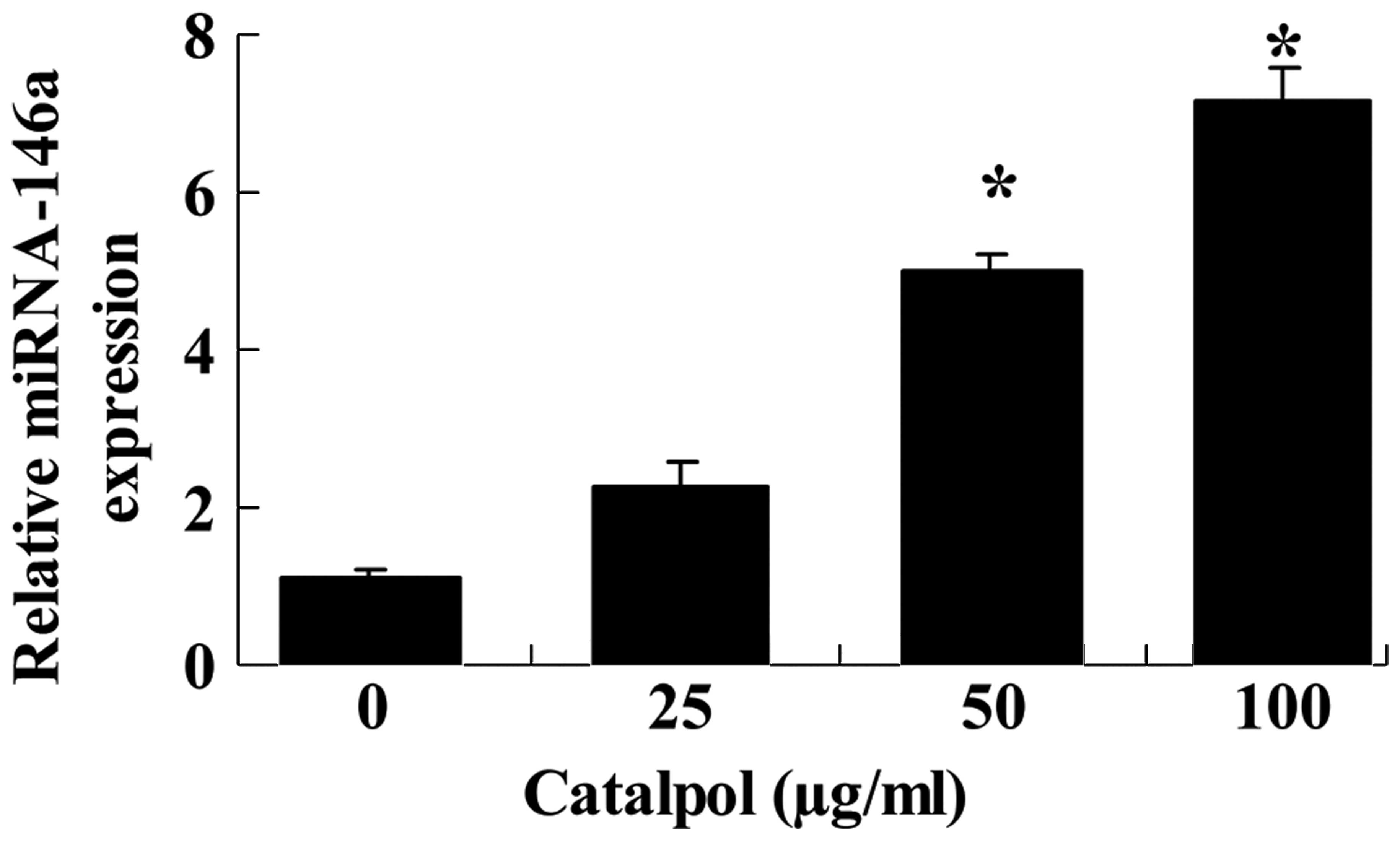
Catalpol increases miR-146a
expression
As expression of miR-146a had been previously
observed in MCF-7 cells (22), its
expression was investigated following treatment with catalpol. The
expression of miR-146a in MCF-7 cells was demonstrated to increase
following treatment with catalpol (0, 25, 50 and 100 µg/ml)
for 48 h (Fig. 5). Treatment with
50 and 100 µg/ml catalpol resulted in a significant increase
in the level of miR-146a expression in MCF-7 cells (P<0.01;
Fig. 5).
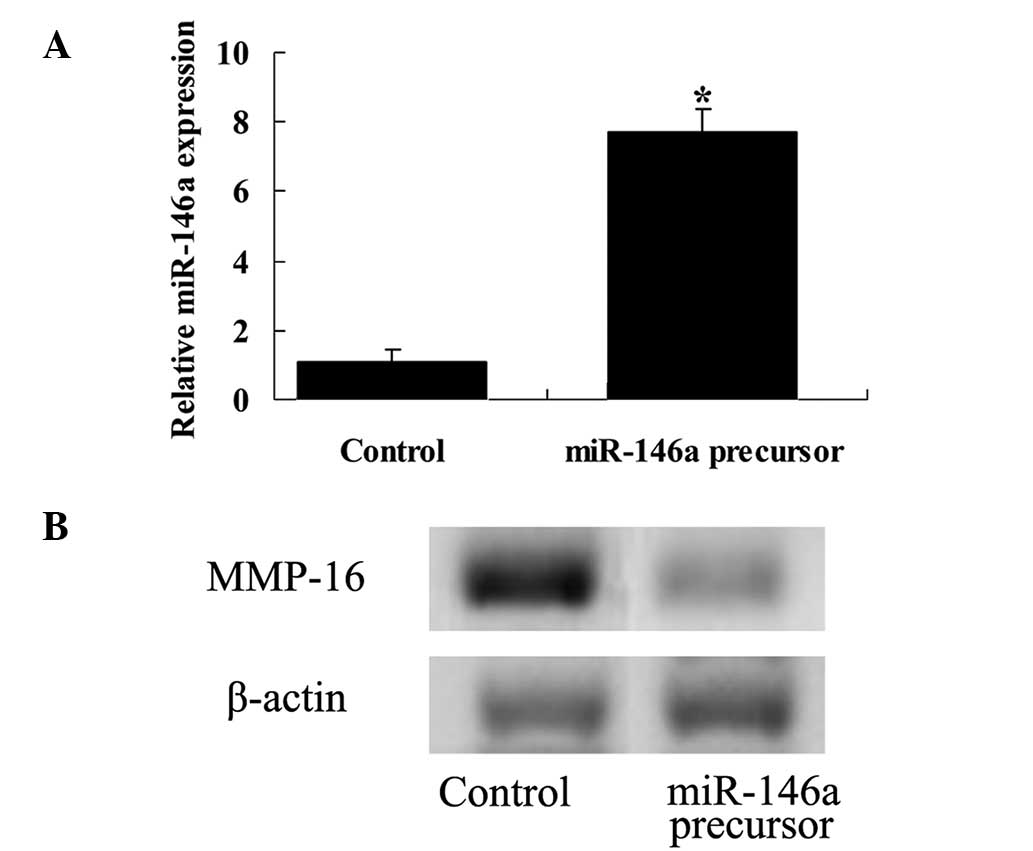
Overexpression of miR-146a and MMP-16
expression
To further investigate the role of miR-146a
expression in MCF-7 cells, cells were transfected with miR-146a.
The results demonstrated that overexpression of miR-146a
significantly increased the expression of miR-146a in MCF-7 cells
(P<0.01; Fig. 6A). In addition,
overexpression of miR-146a also reduced the expression of MMP-16 in
MCF-7 cells (Fig. 6B).
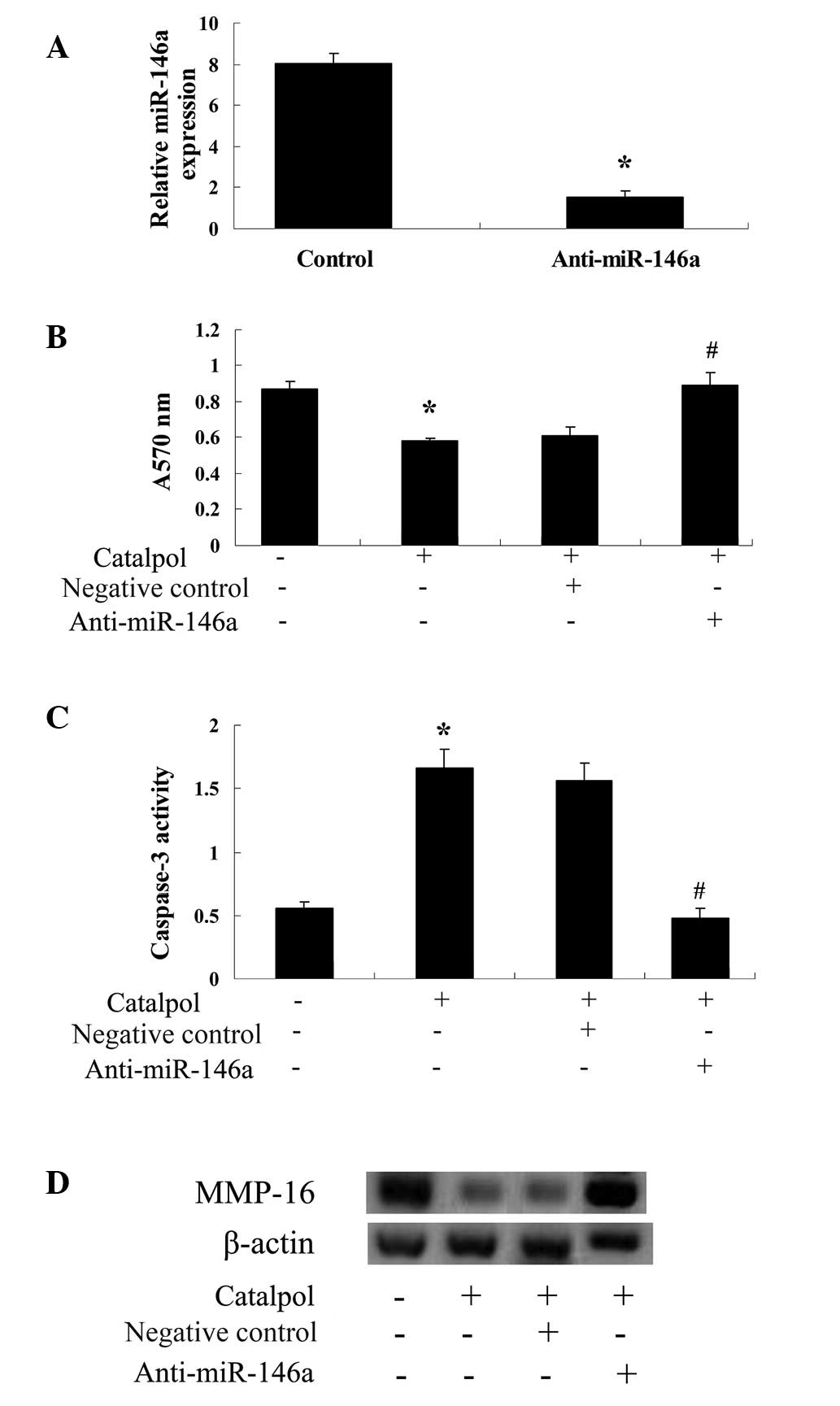
Anti-miR-146a is able to reverse the
effect of catalpol
To further investigate the association between
catalpol and miR-146a expression, anti-miR-146a was transfected
into MCF-7 cells that were subsequently treated with catalpol.
Transfection of MCF-7 cells with anti-miR-146a resulted in a
significant reduction in miR-146a expression (P<0.01; Fig. 7A). Following treatment with 50
µg/ml catalpol, expression of anti-miR-146a significantly
reduced the effect of catalpol on cellular proliferation
(P<0.01; Fig. 7B) and apoptosis
in MCF-7 cells (P<0.01; Fig.
7C). Furthermore, anti-miR-146a prevented the reduction in
MMP-16 levels following catalpol treatment of MCF-7 cells
(P<0.01; Fig. 7D).
Discussion
Breast cancer is the most common malignancy in women
in numerous countries and regions, presenting a serious threat to
women's health (9). In China, a
trend towards rapid growth in incidence rates has been observed. In
the present study, the effect of catalpol was demonstrated to
reduce the proliferation of MCF-7 cells in a time- and
dose-dependent manner. Previously, it was observed that catalpol is
able to inhibit the A2780 sensitive ovarian cancer cell line
(23). The present study
demonstrated that catalpol is additionally able to promote
apoptosis and increase the activity of caspase-3 in MCF-7 cells.
Wang et al (24) reported
that catalpol attenuated ischemia-induced apoptotic death via
suppressing the activation of caspase-3. Liang et al
(25) observed that treatment with
catalpol attenuated neuronal apoptosis also through regulating the
activity of caspase-3 and -9.
The growth, invasion and metastasis of tumor cells
is a complex process with multiple steps and stages. MMPs are
involved in the process by which tumor cells degrade the basement
membrane, infiltrate into the surrounding matrix to induce
angiogenesis, approach adjacent lymphatic vessels, penetrate
endothelial cells of the basement membrane and form secondary
tumors (26). Different tumor
types express different MMPs, and the degree to which breast cancer
is malignant is positively correlated with the overexpression of
MMP-2 and -9 (27). In the present
study, it was demonstrated that treatment with catalpol was able to
reduce the level of MMP-16 protein expression in MCF-7 cells.
Previous studies indicate that catalpol possesses activity against
human epidermoid carcinoma, human rhabdomyosarcoma, transgenic
murine L-cells cancer cell lines and African green monkey kidney
cells (28,29).
Previous studies have demonstrated that miRNA is
involved in the occurrence and development of tumors by targeting
important tumor-associated genes (30–32).
miR-101, -129, -130, -133a and -144 have been reported to
potentially inhibit the migration of MDA-MB-231 to various degrees
(33). miR-106b-25 gene clusters
have been demonstrated to promote drug resistance in breast cancer
cells, and the effect is not achieved by altering the expression of
P-gp (34). Sandhu et al
(35) reported that miR-146a
resulted in reduced proliferation and increased apoptosis of breast
cancer cells and Wang et al (36) indicated that miR-146a expression
suppresses CXCR4-mediated human breast cancer migration. The
present study suggested that treatment with catalpol may
effectively increase the miR-146a expression in MCF-7 cells.
Notably, the upregulation of expression of miR-146a may control the
expression of MMP-16 in MCF-7 cells. In addition, downregulation of
miR-146a expression was observed to induce MMP-16 expression in
MCF-7 cells. Therefore, miR-146a may regulate and control the
expression of MMP-16 levels in MCF-7 cells. In support of this, the
present study demonstrated that downregulation of expression of
miR-146a reduced the effect of catalpol on cellular proliferation
and apoptosis of MCF-7 cells.
In conclusion, the current study demonstrated the
effects of catalpol on cellular proliferation and cell death in
breast cancer cells. Catalpol treatment resulted in the
upregulation of miR-146a expression and downregulation of MMP-16
expression in MCF-7 cells. Furthermore, downregulation of miR-146a
expression resulted in an upregulation of the expression levels of
MMP-16 in catalpol-treated MCF-7 cells. Taken together, these
results suggest that catalpol may be therapeutically beneficial in
breast cancer through the upregulation of miR-146a expression and
the downregulation of MMP-16 expression.
References
|
1
|
Hu XC, Zhang J, Xu BH, et al: Cisplatin
plus gemcitabine versus paclitaxel plus gemcitabine as first-line
therapy for metastatic triple-negative breast cancer (CBCSG006): A
randomised, open-label, multicentre, phase 3 trial. Lancet Oncol.
16:436–446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fu JF, Chen HL, Yang J, Yi CH and Zheng S:
Feasibility and accuracy of sentinel lymph node biopsy in
clinically node-positive breast cancer after neoadjuvant
chemotherapy: A meta-analysis. PLoS One. 9:e1053162014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nie XC, Dong DS, Bai Y and Xia P:
Meta-analysis of black tea consumption and breast cancer risk:
Update 2013. Nutr Cancer. 66:1009–1014. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Noh EM, Chung EY, Youn HJ, Jung SH, Hur H,
Lee YR and Kim JS: Cis-guggulsterone inhibits the IKK/NF-κB
pathway, whereas trans-guggulsterone inhibits MAPK/AP-1 in MCF-7
breast cancer cells: Guggulsterone regulates MMP-9 expression in an
isomer-specific manner. Int J Mol Med. 31:393–399. 2013.
|
|
5
|
Cai KQ, Yang WL, Capo-Chichi CD,
Vanderveer L, Wu H, Godwin AK and Xu XX: Prominent expression of
metalloproteinases in early stages of ovarian tumorigenesis. Mol
Carcinog. 46:130–143. 2007. View
Article : Google Scholar
|
|
6
|
Fan SH, Wang YY, Lu J, et al: CERS2
Suppresses Tumor Cell Invasion and Is Associated with Decreased
V-ATPase and MMP-2/MMP-9 activities in Breast Cancer. J Cell
Biochem. 116:502–513. 2015. View Article : Google Scholar
|
|
7
|
Xie M, Hu A, Luo Y, Sun W, Hu X and Tang
S: Interleukin-4 and melatonin ameliorate high glucose and
interleukin-1beta stimulated inflammatory reaction in human retinal
endothelial cells and retinal pigment epithelial cells. Mol Vis.
20:921–928. 2014.
|
|
8
|
Tang C, Chen L, Gu W, et al: Cyclosporin A
enhances the ability of trophoblasts to displace the activated
human umbilical vein endothelial cell monolayers. Int J Clin Exp
Pathol. 6:2441–2450. 2013.PubMed/NCBI
|
|
9
|
Delassus GS, Cho H, Park J and Eliceiri
GL: New pathway links from cancer-progression determinants to gene
expression of matrix metalloproteinases in breast cancer cells. J
Cell Physiol. 217:739–744. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hegedüs L, Cho H, Xie X and Eliceiri GL:
Additional MDA-MB-231 breast cancer cell matrix metalloproteinases
promote invasiveness. J Cell Physiol. 216:480–485. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hede K: Studies define role of microRNA in
cancer. J Natl Cancer Inst. 97:1114–1115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Croce CM and Calin GA: miRNAs, cancer, and
stem cell division. Cell. 122:6–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gregory RI and Shiekhattar R: MicroRNA
biogenesis and cancer. Cancer Res. 65:3509–3512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bhaumik D, Scott GK, Schokrpur S, Patil
CK, Campisi J and Benz CC: Expression of microRNA-146 suppresses
NF-kappaB activity with reduction of metastatic potential in breast
cancer cells. Oncogene. 27:5643–5647. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kumar S, Keerthana R, Pazhanimuthu A and
Perumal P: Overexpression of circulating miRNA-21 and miRNA-146a in
plasma samples of breast cancer patients. Indian J Biochem Biophys.
50:210–214. 2013.PubMed/NCBI
|
|
17
|
Xiao WQ, Yin GJ, Fan YT, Qiu L, Cang XF,
Yu G, Hu YL, Xing M, Wu Q, Wang XP, et al: Catalpol ameliorates
sodium taurocholate-induced acute pancreatitis in rats via
inhibiting activation of nuclear factor kappa B. Int J Mol Sci.
15:11957–11972. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang WJ, Niu HS, Lin MH, Cheng JT and Hsu
FL: Antihyperglycemic effect of catalpol in streptozotocin-induced
diabetic rats. J Nat Prod. 73:1170–1172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lü J, Wang Y, Zhao W, Li N, Li H, Lu J,
Zeng W, Bao S and Bai Y: Effects of catalpol, L-shikonin and
paeonol extracted from radix rehmanniae, radix arnebiae and cortex
moutan on KGF-induced HaCaT cell proliferation. Zhonghua Yi Xue Za
Zhi. 94:1265–1269. 2014.In Chinese.
|
|
20
|
García C, León LG, Pungitore CR, Ríos-Luci
C, Daranas AH, Montero JC, Pandiella A, Tonn CE, Martín VS and
Padrón JM: Enhancement of antiproliferative activity by molecular
simplification of catalpol. Bioorg Med Chem. 18:2515–2523. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen C, Chen Z, Xu F, Zhu C, Fang F, Shu
S, Li M and Ling C: Radio-protective effect of catalpol in cultured
cells and mice. J Radiat Res (Tokyo). 54:76–82. 2013. View Article : Google Scholar
|
|
22
|
Pogribny IP, Filkowski JN, Tryndyak VP,
Golubov A, Shpyleva SI and Kovalchuk O: Alterations of microRNAs
and their targets are associated with acquired resistance of MCF-7
breast cancer cells to cisplatin. Int J Cancer. 127:1785–1794.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pungitore CR, León LG, García C, Martín
VS, Tonn CE and Padrón JM: Novel antiproliferative analogs of the
Taq DNA polymerase inhibitor catalpol. Bioorg Med Chem Lett.
17:1332–1335. 2007. View Article : Google Scholar
|
|
24
|
Wang Z, An LJ, Duan YL, Li YC and Jiang B:
Catalpol protects rat pheochromocytoma cells against oxygen and
glucose deprivation-induced injury. Neurol Res. 30:106–112. 2008.
View Article : Google Scholar
|
|
25
|
Liang JH, Du J, Xu LD, Jiang T, Hao S, Bi
J and Jiang B: Catalpol protects primary cultured cortical neurons
induced by Abeta(1–42) through a mitochondrial-dependent caspase
pathway. Neurochem Int. 55:741–746. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Loo WT, Chen JP, Chow LW and Chou JW:
Effects of Shugansanjie Tang on matrix metalloproteinases 1, 3 and
9 and telomerase reverse transcriptase expression in human breast
cells in vitro. Biomed Pharmacother. 61:601–605. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fagan-Solis KD, Schneider SS, Pentecost
BT, Bentley BA, Otis CN, Gierthy JF and Arcaro KF: The RhoA pathway
mediates MMP-2 and MMP-9-independent invasive behavior in a
triple-negative breast cancer cell line. J Cell Biochem.
114:1385–1394. 2013. View Article : Google Scholar
|
|
28
|
Liu YF, Zhao Y, Wen XS and Dong QT:
Advances in research on pharmacodynamics and chemical conversion of
catalpol. Zhongguo Zhong Yao Za Zhi. 32:1128–1130. 2007.In Chinese.
PubMed/NCBI
|
|
29
|
Saracoglu I and Harput US: In vitro
cytotoxic activity and structure activity relationships of iridoid
glucosides derived from Veronica species. Phytother Res.
26:148–152. 2012. View
Article : Google Scholar
|
|
30
|
Adams BD, Kasinski AL and Slack FJ:
Aberrant regulation and function of microRNAs in cancer. Curr Biol.
24:R762–R776. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maxwell GL, Shoji Y, Darcy K, et al:
MicroRNAs in endometrial cancers from black and white patients. Am
J Obstet Gynecol. 212:e191–e110. 2015.
|
|
32
|
Seven M, Karatas OF, Duz MB and Ozen M:
The role of miRNAs in cancer: from pathogenesis to therapeutic
implications. Future Oncol. 10:1027–1048. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
D'Ippolito E and Iorio MV: MicroRNAs and
triple negative breast cancer. Int J Mol Sci. 14:22202–22220. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou Y, Hu Y, Yang M, Jat P, Li K,
Lombardo Y, Xiong D, Coombes RC, Raguz S and Yagüe E: The
miR-106b~25 cluster promotes bypass of doxorubicin-induced
senescence and increase in motility and invasion by targeting the
E-cadherin transcriptional activator EP300. Cell Death Differ.
21:462–474. 2014. View Article : Google Scholar :
|
|
35
|
Sandhu R, Rein J, D'Arcy M, Herschkowitz
JI, Hoadley KA and Troester MA: Overexpression of miR-146a in
basal-like breast cancer cells confers enhanced tumorigenic
potential in association with altered p53 status. Carcinogenesis.
35:2567–2575. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang D, Liu D, Gao J, Liu M, Liu S, Jiang
M, Liu Y and Zheng D: TRAIL-induced miR-146a expression suppresses
CXCR4-mediated human breast cancer migration. FEBS J.
280:3340–3353. 2013. View Article : Google Scholar : PubMed/NCBI
|