Introduction
There is significant morbidity and mortality
resulting from urothelial bladder cancer (UBC) globally; it is the
seventh most common type of cancer in males and the seventeenth in
females (1). Smoking and
occupational exposure to aromatic amines and polycyclic aromatic
hydrocarbons are the most important risk factors for UBC (1,2).
There is significant global variability in the incidence of UBC
with the highest rates occurring in Europe, Egypt and the United
States, while Asia, South America and Sub-Saharan Africa have the
lowest rates (3). Global incidence
appears to coincide with the distribution of risk factors (3). Although the majority of cases of UBC
are non-invasive at the time of diagnosis, up to 25% of cases do
show different degrees of invasiveness at the time of diagnosis
(1). Therefore, understanding the
mechanism by which UBCs acquire invasive properties may aid in the
identification of novel therapeutic targets.
The Rho/Rho-associated protein kinase (ROCK) pathway
has been shown to effect the proliferation and invasion of several
types of cancer cells (1–4). Specifically, ROCK is an important
molecule in metastasis (4). In
addition, the Rho protein is a small GTPase that exhibits a key
biological role in cell division and proliferation through its
downstream molecules. Furthermore, members of the Rho family of
small GTPases regulate microfilament network organization,
intercellular contact and malignant transformation (5). They also regulate cytoskeletal
activity, and are consequently involved in cellular invasion and
migration via epithelial to mesenchymal transition (6). ROCK is the most significant
downstream effector of Rho (7),
and myosin light chain kinase (MLCK) is a downstream effector of
ROCK. The Rho/ROCK/MLCK pathway is important in cell morphology,
motility, invasion, adhesion, polarity formation and mitosis, and
thus participates in the pathogenesis of cancer (8). Therefore, it is hypothesized that the
Rho/ROCK/MLCK pathway may have a role in cancer progression by
regulating the reorganization of the actin cytoskeleton. Moreover,
ROCK inhibitors, such as Y-27632 (a 4-amino pyridine) have been
shown to inhibit the invasion of tumor cells (9,10).
The expression of RhoA and RhoC is significantly
higher in bladder cancer cells, suggesting a role in tumorigenesis
and invasiveness (11). In 5637
and UM-UC-3 bladder cancer cells, Rho/ROCK signaling increased
proliferation and migration, and this was inhibited by treatment
with the ROCK-specific inhibitor, fasudil (HA-1077) (12). In a similar study, inhibition of
the Rho/ROCK pathway by Clostridium difficile toxin B,
HA-1077 and Y-27632, inhibited the migration of T24 and J8 bladder
cancer cells (13). Also, the
inhibition of RhoC by microRNA-493 decreased T24 and J82 bladder
cancer cell migration (14).
Notably, high expression of Rho/ROCK in patients with bladder
cancer is associated with poor tumor differentiation, muscle
invasion, and lymph node metastasis (15).
Elements of the Rho/ROCK/MLCK pathway may therefore
be therapeutic targets. However, relatively few studies have
investigated the effect of the Rho/ROCK pathway on bladder cancer.
In the present study, the ROCK inhibitor, Y-27632, was used to
investigate the effect of the Rho/ROCK pathway on the proliferation
and invasion of T24 and 5634 bladder cancer cells.
Materials and methods
Cell proliferation assay
T24 and 5637 bladder cancer cell lines were
purchased from the Shanghai Institute Cell Bank (Shanghai, China).
Cells (1×104 cells per well) were seeded in 96-well
plates in RPMI-1640 supplemented with 10% fetal bovine serum (FBS)
(both from Gibco-BRL, Carlsbad, CA, USA). Cells were treated with
0, 10, 25, 50, 75, 100, 125 or 150 µmol/l Y-27632 (Tocris,
Bristol, UK) for 24, 48 and 72 h at 37°C with 5% CO2. At
each time point, 10 µl Cell Counting kit-8 solution
(Dojindo, Kumamoto, Japan) was then added to each well, and the
cells were further incubated for 2 h at 37°C with 5%
CO2. Three independent assays were conducted, and the
average was taken.
Cell invasion assay
Transwell chambers (5-µm pore size; Corning,
NY, USA) were pre-coated with BD Matrigel™ (BD Biosciences, San
Jose, CA, USA) according to the manufacturer's instructions. The
cells were harvested and resuspended in media containing FBS, and
200 µl of the suspension containing 5×104 cells
was added to the upper chamber. The cells were then treated with 0,
25, 50 or 75 µmol/l Y-27632, and incubated for 24 h at 37°C.
The transwells were removed and stained with crystal violet
(Sigma-Aldrich, St. Louis, MO, USA) and the cells in the lower
partition were counted under a light microscope (GX41; Olympus,
Tokyo, Japan) at ×200. The percentage of inhibition was calculated
according to the following formula: Percentage inhibition =
(control group penetrated cells-experimental group penetrated
cells) / control group penetrating cells × 100.
Western blot analysis
Cells were treated with various concentrations of
Y-27632 then harvested and lysed in lysis buffer (Thermo Fisher
Scientific, Waltham, MA, USA). After 50 µg of protein was
separated by 7.5% SDS-PAGE (7.5% Mini-Protean TGX™ Precast Protein
gels; Bio-Rad, Hercules, CA, USA) and transferred onto
nitrocellulose membranes (EMD Millipore, Billerica, MA, USA), the
membranes were blocked with 5% bovine serum albumin (Sigma-Aldrich)
in Tris-buffered saline for 60 min and washed three times. The
membranes were then incubated with monoclonal rabbit anti-human
antibodies against P-MLCK (1:1,000; cat. no. ab76092; Abcam,
Cambridge, UK) or polyclonal rabbit anti-human antibodies against
β-actin (1:1,000; cat. no. A2668; Sigma-Aldrich) at 4°C overnight,
and then with polyclonal goat anti-rabbit horseradish
peroxidase-conjugated secondary antibody (1:3,000; Zhongshan Golden
Bridge Biotechnology Co., Beijing, China) at room temperature for
60 min. NIH Image J analysis software V.1 (National Institutes of
Health, Bethesda, MD, USA) was used to detect the optical density
of P-MLCK bands, and was normalized to the values obtained for
β-actin to determine the relative expression of P-MLCK.
Statistical analysis
Continuous variables was presented as the mean ±
standard deviation. Repeated measurement analysis of variance
(ANOVA) with Bonferroni post hoc tests were performed to compare
the differences between different time points at each concentration
of Y-27632 in T24 and 5637 cell proliferation assays. One-way ANOVA
with Bonferroni post hoc tests were performed to compare the
differences among the different concentrations of Y-27632 in T24
and 5637 cell proliferation, cell count, and inhibition rate at
each time point. P<0.05 was considered to indicate a
statistically significant difference. SPSS 17.0 statistics software
(SPSS Inc., Chicago, IL, USA) was used for the statistical
analyses.
Results
Effect of Y-27632 on T24 and 5637 cell
proliferation
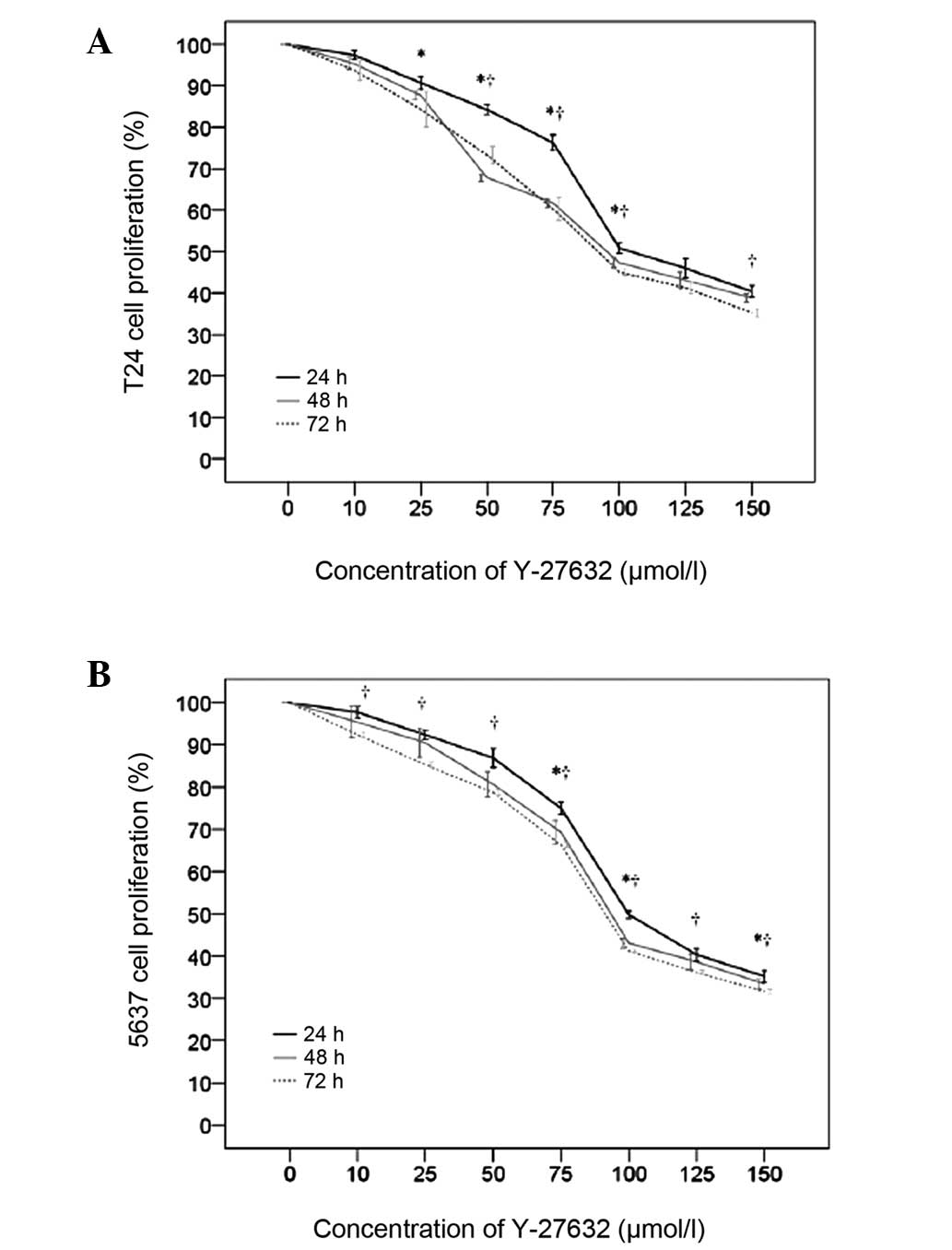
T24 and 5637 bladder cancer cells were subjected to
Y-27632 concentration-response and time course assays. Y-27632
significantly suppressed the cell proliferation of T24 and 5637
cells in a concentration-dependent manner (Fig. 1, Tables I and II). At 24 h, the proliferation of T24
cells significantly decreased from 0 to 150 µmol/l Y-27632
(P<0.001). Similar decreases in T24 cell proliferation were
observed at 48 and 72 h (Table I).
In addition, T24 cell proliferation decreased significantly with
time from 0 to 72 h (Fig. 1A). At
24 h, the proliferation of 5637 cells decreased significantly from
0 to 150 µmol/l (P<0.015). Similar decreases in 5637 cell
proliferation were observed at 48 and 72 h (Table II).
 | Table IT24 cell proliferation in
concentrations of Y-27632 ranging from 0 to 150 mmol/l at 24, 48
and 72 h. |
Table I
T24 cell proliferation in
concentrations of Y-27632 ranging from 0 to 150 mmol/l at 24, 48
and 72 h.
| Y-27632 (mmol/l) | T24 cell
proliferation (%)
|
|---|
| 24 h | 48 h | 72 h |
|---|
| 0 | 100 | 100 | 100 |
| 10 | 97.39±1.1 | 95.3±1.38a | 93.74±2.44a |
| 25 | 90.72±1.55a,b | 87.7±0.98a,b | 84.35±4.27a,b |
| 50 | 84.27±1.11a–c | 67.88±0.9a–c | 73.32±2.11a–c |
| 75 | 76.25±1.89a–d | 61.73±1.02a–d | 60.31±2.81a–d |
| 100 | 50.83±1.23a–e | 47.29±1.21a–e | 45.07±0.9a–e |
| 125 | 45.97±2.33a–f | 43.12±2.03a–f | 41.26±1.43a–e |
| 150 | 40.38±1.38a–g | 38.81±1a–g | 35.22±1.04a–g |
| P-value | <0.001 | <0.001 | <0.001 |
 | Table II5637 cell proliferation in
concentrations of Y-27632 ranging from 0 to 150 mmol/l at 24, 48,
and 72 h. |
Table II
5637 cell proliferation in
concentrations of Y-27632 ranging from 0 to 150 mmol/l at 24, 48,
and 72 h.
| Y-27632 (mmol/l) | 5637 cell
proliferation (%)
|
|---|
| 24 h | 48 h | 72 h |
|---|
| 0 | 100 | 100 | 100 |
| 10 | 97.7±1.33 | 95.39±3.68 | 92.37±0.71a |
| 25 | 92.36±1.16a,b | 90.49±3.51a,b | 85.3±0.63a,b |
| 50 | 86.9±2.27a–c | 80.64±3.01a–c | 78.72±0.57a–c |
| 75 | 75.02±1.47a–d | 69.32±2.8a–d | 66.3±0.53a–d |
| 100 | 49.8±0.86a–e | 43.06±1.23a–e | 41.2±0.27a–e |
| 125 | 40.32±1.49a–f | 38.63±1.83a–e | 36.12±0.48a–f |
| 150 | 35.2±1.36a–g | 33.4±1.18a–g | 31.56±0.56a–g |
| P-value | <0.001 | <0.001 | <0.001 |
Effect of Y-27632 on T24 and 5637 cell
invasion
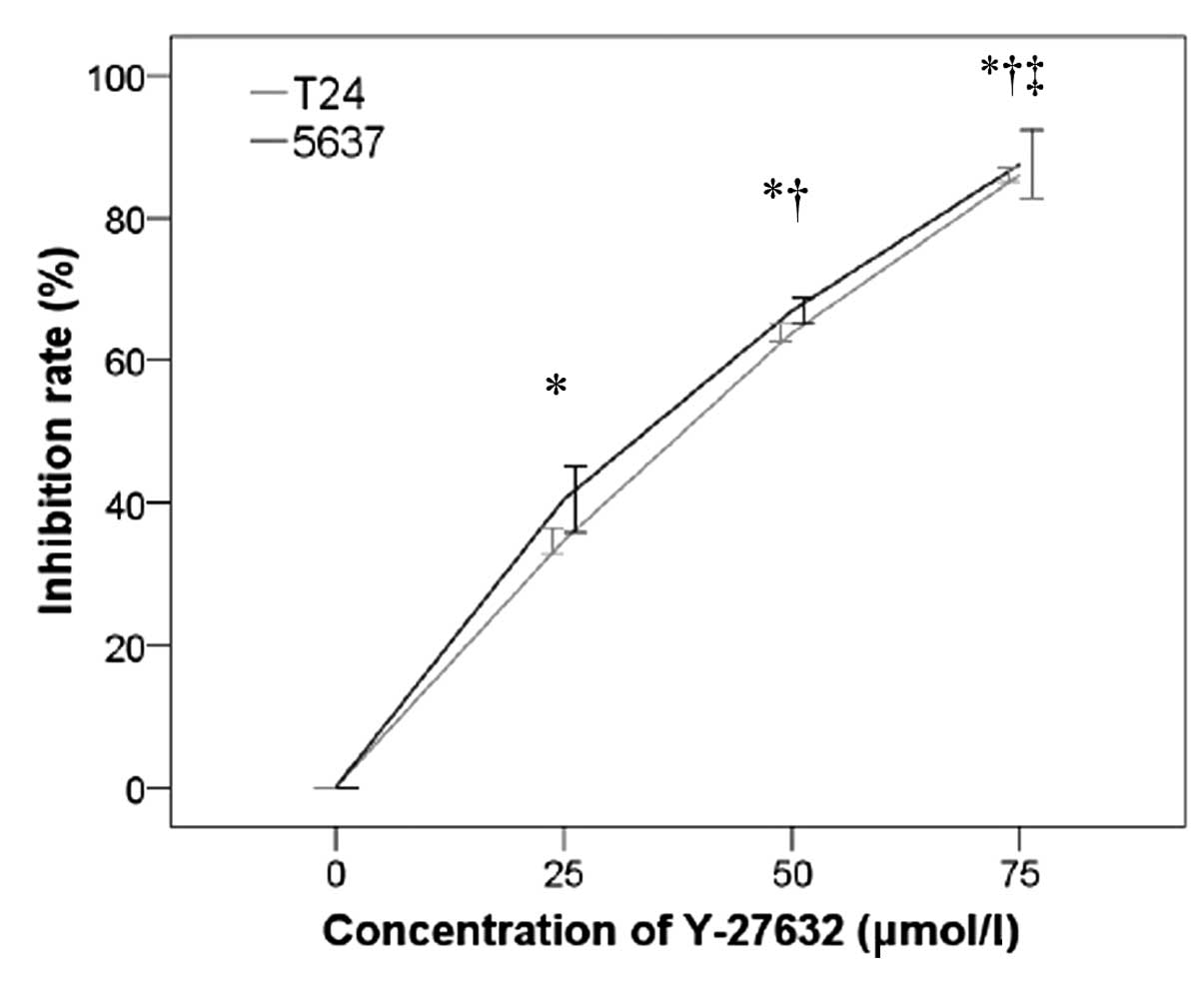
Y-27632 inhibited the invasion of T24 and 5637 cells
in a concentration-dependent manner (P<0.001; Fig. 2). At 24 h, the inhibition of
cellular invasion increased significantly with increasing
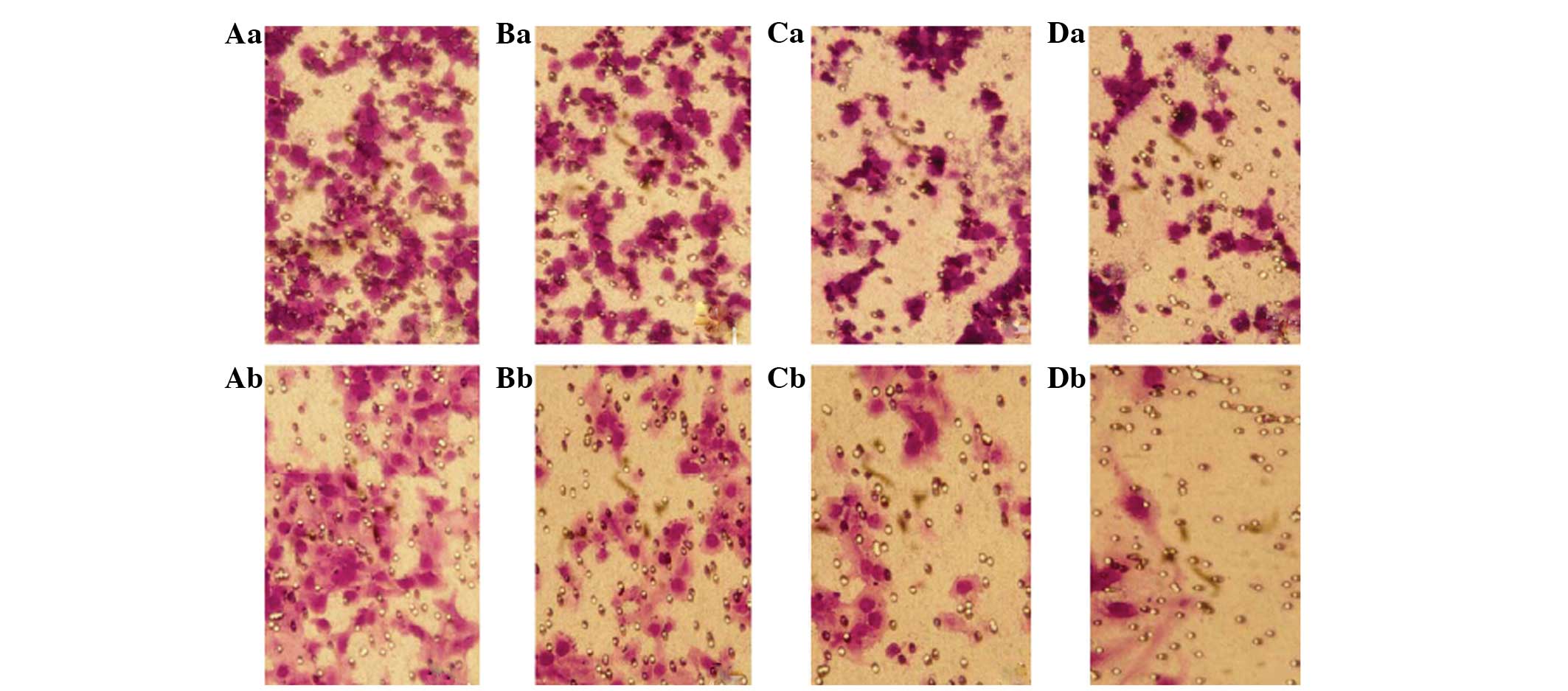
concentrations of Y-27632. Representative images from the invasion
assays are shown in Fig. 3.
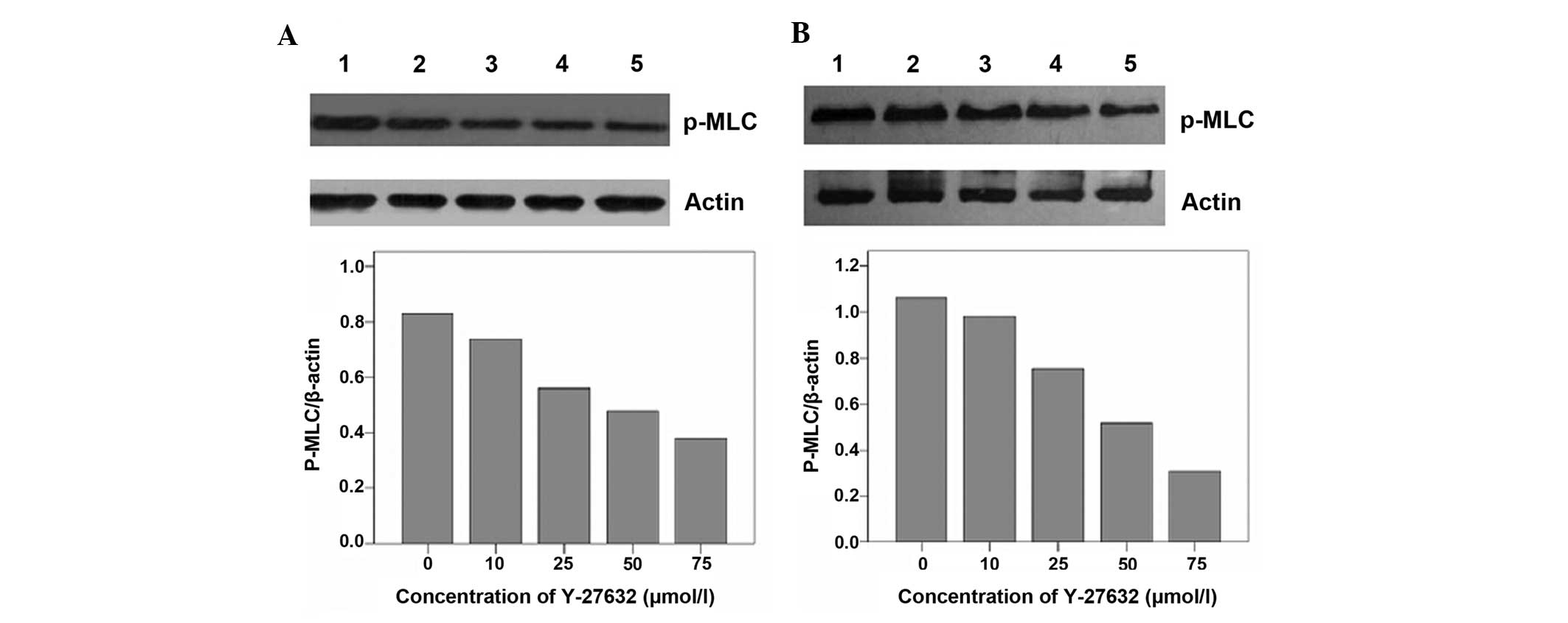
Suppression of P-MLCK expression by
Y-27632 in T24 and 5637 cells
MLCK is a known downstream effector of the Rho/ROCK
pathway in several cells. Therefore, P-MLCK protein levels were
assessed in response to various concentrations of Y-27632 by
western blot analysis. As shown in Fig. 4, Y-27632 suppressed P-MLCK protein
expression in T24 and 5637 cells, confirming that it is also a
downstream effector of the Rho/ROCK pathway in T24 and 5637 bladder
cancer cells.
Discussion
Evidence from several studies indicates that
signaling pathways downstream of Rho GTPases are vital in tumor
development and progression (16,17).
Consequently, there has been considerable interest in the
possibility that specific proteins in the Rho/ROCK signal
transduction pathway could represent potential cancer therapeutic
targets. Relatively little investigation has been conducted,
however, regarding the transduction mechanisms of the Rho/ROCK
pathway in bladder cancer. The Rho/ROCK signal transduction
mechanisms shown in other types of cancers could be common to all
types of cancer or idiosyncratic to the cancer types investigated.
It is, therefore, important to study these mechanisms in bladder
cancer to generate a knowledge base for the potential development
of bladder cancer-specific, Rho pathway-derived, therapeutic
targets. In the present study, it was demonstrated that Y-27632
inhibited T24 and 5637 cell proliferation and invasion in a
concentration-dependent manner. It also suppressed MLCK
phosphorylation in T24 and 5637 cells.
In this study, the specific ROCK inhibitor, Y-27632,
was used to compare proliferation and invasion of bladder cancer
cells when the Rho/ROCK pathway was inhibited by increasing
concentrations of Y-27632. Cell proliferation of the T24 and 5637
cancer cell lines was inhibited by Y-2763 in a
concentration-dependent manner. The inhibition of bladder cancer
cell proliferation by Y-27632 is in contrast to what has been
reported of non-tumor cells. For example, Horani et al
(18) reported an increase in
airway epithelial basal cell proliferation upon Rho inhibition, and
Yu et al (19) also
reported an increase in astrocyte proliferation upon inhibiting
ROCK with Y-27632. The fact that inhibition of the Rho/ROCK pathway
inhibits bladder cell proliferation while sparing or enhancing the
proliferation of normal tissues renders the proteins in this
pathway an attractive therapeutic target in bladder cancer.
However, further studies are required to assess the mechanism by
which Y-27632 alters bladder cancer proliferation as well as assess
its effects in vivo.
ROCK is a serine/threonine protein kinase that is an
important downstream target of Rho. It is involved in several
biological activities, including cell adhesion, mitosis,
cytoskeletal reorganization, muscle cell contraction, and invasion
of tumor cells (20). It was also
demonstrated that inhibition of ROCK by Y-27632 suppressed T24 and
5637 cell invasion. The inhibition of invasion was
concentration-dependent and in agreement with the findings of other
groups. For example, Huang et al (21) reported the inhibition of TSGH
urothelial cancer cell invasion upon inhibiting ROCK with Y-27632,
and Imamura et al (10)
reported a similar effect with ascites hepatoma cells.
ROCK performs these biological functions by
phosphorylating a variety of downstream substrates, including MLCK,
connexin and LIM kinase (22–24).
Therefore, MLCK was investigated as a possible downstream effector.
Y-27632 inhibited the phosphorylation of MLCK in a
concentration-dependent manner, indicating that MLCK is a
downstream effector of the Rho/ROCK pathway for the enhancement of
proliferation and invasion in these bladder cancer cells. This
expands the number of potential bladder cancer therapeutic targets
in the Rho/ROCK pathway. However, further studies are required to
analyze whether the inhibition of bladder cancer cell proliferation
and invasion by Y-27632 was mediated by its effect on MLCK
phosphorylation.
This study was limited in its scope as it
investigated the effects of Y-27632 on in vitro cell
proliferation and invasion only. Thus, further in vivo
studies are warranted to confirm these findings as well as uncover
the underlying mechanism. In addition, the expression of these
proteins may be analyzed in bladder cancer samples isolated from
patients to determine the prognostic value of analyzing this
pathway.
In conclusion, this study showed that T24 and 5637
bladder cancer cell proliferation and invasion were inhibited with
the Rho kinase inhibitor, Y-27632. Furthermore, Y-27632 suppressed
P-MLCK protein expression in T24 and 5637 cells. Thus, the
Rho/ROCK/P-MLCK pathway may be important in tumor cell metastasis
in bladder cancer.
Abbreviations:
|
BSA
|
bovine serum albumin
|
|
FBS
|
fetal bovine serum
|
|
MLCK
|
myosin light chain kinase
|
|
ROCK I and II
|
Rho-associated protein kinase I and
II
|
|
SD
|
standard deviation
|
|
UBC
|
urothelial bladder cancer
|
References
|
1
|
Burger M, Catto JW, Dalbagni G, Grossman
HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C,
Shariat S and Lotan Y: Epidemiology and risk factors of
urothelialbladder cancer. Eur Urol. 63:234–241. 2013. View Article : Google Scholar
|
|
2
|
Ferreccio C, Yuan Y, Calle J, Benítez H,
Parra RL, Acevedo J, Smith AH, Liaw J and Steinmaus C: Arsenic,
tobacco smoke and occupation: Associations of multiple agents with
lung and bladder cancer. Epidemiology. 24:898–905. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chavan S, Bray F, Lortet-Tieulent J,
Goodman M and Jemal A: International variations in bladder cancer
incidence and mortality. Eur Urol. 66:59–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Somlyo AP and Somlyo AV: Ca2+
sensitivity of smooth muscle and nonmuscle myosin II: Modulated by
G proteins, kinases and myosin phosphatase. Physiol Rev.
83:1325–1358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hall A: Rho GTPases and the actin
cytoskeleton. Science. 279:509–514. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gómez del Pulgar T, Benitah SA, Valerón
PF, Espina C and Lacal JC: Rho GTPase expression in tumourigenesis:
Evidence for a significant link. Bioessays. 27:602–613. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsui T, Amano M, Yamamoto T, Chihara K,
Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A and Kaibuchi K:
Rho-associated kinase, a novel serine/threonine kinase, as a
putative target for small GTP binding protein Rho. EMBO J.
15:2208–2216. 1996.PubMed/NCBI
|
|
8
|
Duan WG, Yuan ST, Liao H, Yan M and Zhang
LY: Advances in the study of Rho kinase and its inhibitors. Yao Xue
Xue Bao. 42:1013–1022. 2007.In Chinese.
|
|
9
|
Itoh K, Yoshioka K, Akedo H, Uehata M,
Ishizaki T and Narumiya S: An essential part for Rho-associated
kinase in the transcellular invasion of tumor cells. Nat Med.
5:221–225. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Imamura F, Mukai M, Ayaki M and Akedo H:
Y-27632, an inhibitor of rhoassociated protein kinase, suppresses
tumor cell invasion via regulation of focal adhesion and focal
adhesion kinase. Jpn J Cancer Res. 91:811–816. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Volanis D, Zaravinos A, Kadiyska T,
Delakas D, Zoumpourlis V and Spandidos DA: Expression profile of
Rho kinases in urinary bladder cancer. J BUON. 16:511–521.
2011.PubMed/NCBI
|
|
12
|
Abe H, Kamai T, Hayashi K, Anzai N,
Shirataki H, Mizuno T, Yamaguchi Y, Masuda A, Yuki H, Betsunoh H,
et al: The Rho-kinase inhibitor HA-1077 suppresses
proliferation/migration and induces apoptosis of urothelial cancer
cells. BMC Cancer. 14:4122014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
von Dorp F, Sanders H, Boergermann C,
Lümmen G, Rübben H, Jakobs KH and Schmidt M: Inhibition of
Rho-kinase abrogates migration of human transitional cell carcinoma
cells: Results of an in vitro study. Urol Int. 86:220–227. 2011.
View Article : Google Scholar
|
|
14
|
Ueno K, Hirata H, Majid S, Yamamura S,
Shahryari V, Tabatabai ZL, Hinoda Y and Dahiya R: Tumor suppressor
microRNA-493 decreases cell motility and migration ability in human
bladder cancer cells by downregulating RhoC and FZD4. Mol Cancer
Ther. 11:244–253. 2012. View Article : Google Scholar
|
|
15
|
Kamai T, Tsujii T, Arai K, Takagi K, Asami
H, Ito Y and Oshima H: Significant association of Rho/ROCK pathway
with invasion and metastasis of bladder cancer. Clin Cancer Res.
9:2632–2641. 2003.PubMed/NCBI
|
|
16
|
Rösel D, Brábek J, Tolde O, Mierke CT,
Zitterbart DP, Raupach C, Bicanová K, Kollmannsberger P, Panková D,
Vesely P, et al: Upregulation of Rho/ROCK signaling in sarcoma
cells drives invasion and increased generation of protrusive
forces. Mol Cancer Res. 6:1410–1420. 2008. View Article : Google Scholar
|
|
17
|
Ying H, Biroc SL, Li WW, Alicke B, Xuan
JA, Pagila R, Ohashi Y, Okada T, Kamata Y and Dinter H: The Rho
kinase inhibitor fasudil inhibits tumor progression in human and
rat tumor models. Mol Cancer Ther. 5:2158–2164. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Horani A, Nath A, Wasserman MG, Huang T
and Brody SL: Rho-associated protein kinase inhibition enhances
airway epithelial Basal-cell proliferation and lentivirus
transduction. Am J Respir Cell Mol Biol. 49:341–347. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu Z, Liu M, Fu P, Xie M, Wang W and Luo
X: ROCK inhibition with Y27632 promotes the proliferation and cell
cycle progression of cultured astrocyte from spinal cord. Neurochem
Int. 61:1114–1120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mueller BK, Mack H and Teusch N: Rho
kinase, a promising drug target for neurological disorders. Nat Rev
Drug Discov. 4:387–398. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang HP, Wang CJ, Tsai JP, Wu SW, Hung
TW, Lian JD and Chang HR: Y27632 attenuates the aristolochic
acid-promoted invasion and migration of human urothelial cancer
TSGH cells in vitro and inhibits the growth of xenografts in vivo.
Nephrol Dial Transplant. 27:565–575. 2012. View Article : Google Scholar
|
|
22
|
Heneweer C, Kruse LH, Kindhäuser F,
Schmidt M, Jakobs KH, Denker H and Thie M: Adhesiveness of human
uterine epithelial RL95-2 cells to trophoblast: Rho protein
regulation. Mol Hum Reprod. 8:1014–1022. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fukata M, Nakagawa M and Kaibuchi K: Roles
of Rho-family GTPases in cell polarisation and directional
migration. Curr Opin Cell Biol. 15:590–597. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song Y, Hoang BQ and Chang DD:
ROCK-II-induced membrane blebbing and chromatin condensation
require actin cytoskeleton. Exp Cell Res. 278:452–521. 2002.
View Article : Google Scholar
|