Introduction
Microglia are one of the resident populations of
mononuclear phagocytes in the central nervous system (CNS) and
account for 10% of the total glial cell population in the brain
(1). Increasing evidence has
suggested that microglia have neurotoxic effects when they are
overactivated following severe injury or during neurodegenerative
disease (2,3). The neurotoxic effects of the
activated microglia in the aged neurodegenerative brain may result
in age-associated microglia senescence (4). Microglia senescence, which underlies
the alterations of microglial function and incorrect responses to
stimuli, can promote eventual neurodegeneration (5,6).
Emerging evidence has indicated that microglial senescence can
contribute to age-associated neurodegenerative disease, including
Parkinson's disease (PD), which is characterized by the
degeneration of dopaminergic neurons in the substantia nigra pars
compacta (7–9). The most prominent features of
microglial senescence are morphological alterations, described as
dystrophy, and alterations in the inflammatory profile (4,7). In
addition, a previous study indicated that the expression levels of
proinflammatory cytokines, including tumor necrosis factor (TNF)-α
and interleukin (IL)-1β are increased in the brains of
senescence-accelerated mice (10).
Senescence is a state of irreversible cell
withdrawal from the proliferative pool, and occurs at the
exhaustion of the proliferative lifespan (11). Cellular senescence is characterized
by elevated endogenous β-galactosidase activity, which is commonly
used as a marker for cellular senescence (12). In addition, the induction of p21,
the first identified inhibitor of cyclin/cyclin-dependent kinase
complexes, is essential for the onset of cell cycle arrest in cell
senescence (13,14). Senescence can also be caused by
pathological stimulation, including the activation of oncogenes
(oncogene-induced senescence; OIS) (15). Phorbol myristate acetate (PMA),
commonly known as 12-O-Tetradecanoylphorbol-13-acetate, is a potent
tumor promoter, which is often applied in biomedical investigations
to activate the signal transduction enzyme, protein kinase C (PKC)
(16,17), which is involved in oncogene
activation. In addition, it has also been reported that senescence
can be driven by the activation of PKC (18–20).
Therefore, the present study hypothesized that PMA, as a
carcinogen, can also induce microglial senescence. In the present
study, the expression levels of TNF-α, IL-1β, β-galactosidase and
p21 in the substantia nigra of rats were investigated, as were the
effects on these levels of expression following PMA
administration
Materials and methods
Animals
Male Sprague-Dawley rats (12 weeks old; 220–260 g)
were provided by the Biological Science Animal House (Liaoning,
China). The animals were maintained in a temperature-controlled
environment at 22–24°C on a 12:12 h light-dark cycle, and were
provided with free access to food and water. All animal experiments
were performed in accordance with the National Institutes of Health
Guide for the Care and Use of Laboratory Animals (National
Institutes of Health, Bethesda, MA, USA) and the present study was
approved [no. SCXK (Liao) 2008–0005] by the ethics committee of
China Medical University (Shenyang, China).
Treatment groups
A total of 58 rats were used in the present study.
Firstly, 10 of the 58 rats were randomly selected and divided into
two groups: PMA injection group (n=5) and control group (n=5). In
each group, injection was performed once, and all 10 rats were
sacrificed by an intracardiac injection of sodium pentobarbital (75
mg of 390 mg/ml solution) 1 week following injection to determine
whether a single PMA injection allowed the microglia to enter a
resting state. In the control group, normal saline (NS; 0.9%saline
solution), rather than PMA, was injected providing a single
injection control group. The remaining 48 rats were randomly
divided into three groups: i) repeated injection control group
(n=16); ii) single PMA injection group (n=16) and iii) repeated PMA
injection group (n=16). In the single PMA injection group, the rats
were injected with PMA once in the first week. For the repeated PMA
injection group, the rats were injected with PMA four times, once a
week. In the repeated injection control group, NS, rather than PMA,
was injected four times, once a week. All 48 rats were sacrificed,
as described above, at the fifth week following the first injection
(Fig. 1).
Intra-nigrostriatal injection of PMA
To observe the microglial changes in the substantia
nigra, PMA (Sigma-Aldrich, St. Louis, MO, USA) was injected into
the substantia nigra by performing stereotaxic surgery. For the
stereotaxic surgery, the rats were anesthetized with an
intraperitoneal injection of pentobarbital (50 mg/kg;
Apoteksbolaget, Stockholm, Sweden). Once the animals were deeply
anesthetized, they were placed in a stereotaxic apparatus (David
Kopf Instruments, Tujunga, CA, USA). Subsequently, the rats were
injected with a sub-toxic concentration of PMA (0.5
µg/µl; 2 µl at each site, into the right
nigrostriatal pathway (medial forebrain bundle) at stereotaxic
coordinates (anteroposteriorly 4.4 mm from the bregma;
mediolaterally +1.0 mm from the midline; dorsoventrally −7.2 mm
from the skull), as adapted from Grealish et al (21). The control group was injected with
the same volume of NS (2 µl at each site). At the end of
each injection, the syringe needle remained in position for 5 min
and was then slowly withdrawn to prevent solution reflux.
Tissue sample collection
Following the appropriate time-periods
post-injection, the animals were deeply anesthetized with
pentobarbital. A number of the brains from the PMA injection group
(n=3) and control group (n=3) were immediately removed, and the
whole striatum and substantia nigra were rapidly dissected and
placed on the ice, which was used for enzyme-linked immunosorbent
assay (ELISA) analysis of TNF-α and IL-1β. The remaining animals
were perfused through the aorta with 0.9% saline, followed by
ice-cold fixative consisting of 4% paraformaldehyde
(Sigma-Aldrich,) in 100 mM phosphate-buffered saline (PBS). The
brains were then dissected (3–4 mm thickness) and post-fixed for 24
h with the same fixative. Following fixation, a number of the brain
samples were transferred into 15% sucrose (Sigma-Aldrich) solution
overnight at 4°C, and were subsequently to a 30% sucrose solution
until the brain samples sunk to the tube bottom. Subsequently,
brain samples from all the remaining animals in the PMA injection
group and control group, and samples from the animals in the other
three groups were sectioned using a cryostat (CM3050S; Leica
Microsystems GmbH, Nussloch, Germany) at a thickness of 25
µm. These sections were then mounted onto
poly-l-lysine-coated slides (CEL Associates, Pearland, TX, USA),
which were prepared for immunofluorescence. The remaining brain
samples (n=8/group) from the repeated injection control group,
single PMA injection group and repeated PMA injection group were
embedded in paraffin (Sigma-Aldrich), and 5-µm coronal
sections were obtained using a microtome, which were processed for
β-galactosidase staining and immunohistochemistry.
Assessment of the effect of single PMA
injection on microglia
To determine the effect of a single PMA injection on
the microglia, immunofluorescence staining for Iba-1 was performed,
and the levels of TNF-α and IL-1β were detected using ELISA. The
brain samples from the rats in the PMA injection group and control
group were used for this assessment. For the immunofluorescence
staining, the frozen sections from the PMA injection group and
control group were permeabilized using 0.3% Triton X-100/PBS
(Sigma-Aldrich) for 10 min at room temperature. Following hydration
with ethanol and being fixed with 10% formaldehyde, the sections
were microwave-heated for 6 min in sodium citrate buffer (10 mM; pH
6.0) for antigen retrieval. The sections were then blocked with 10%
bovine serum albumin (Sigma-Aldrich) in PBS for 30 min. The
sections were incubated overnight with polyclonal rabbit anti-rat
Iba-1 (cat. no. 019-19741; 1:100; Wako Pure Chemicals Industries,
Osaka, Japan) at 4°C. Following several washes with PBS, the
sections were incubated for 2 h at room temperature with Alexa-488
(green fluorescence)-conjugated goat anti-rabbit IgG polyclonal
antibody (cat. no. A-11034; 1:800; Invitrogen Life Technologies,
Carlsbad, CA, USA). Fluorescence images were captured using a
digital camera attached to a fluorescent inverted microscope (Nikon
Eclipse E1000; Nikon Corporation, Tokyo, Japan). Green color
indicated positive expression of Iba-1.
The target proteins of TNF-α and IL-1β were
extracted, according to the manufacturer's instruction of the Rat
TNF-α and IL-1β ELISA kits (Invitrogen Life Technologies). Briefly,
the samples were homogenized in lysis buffer containing protease
and phosphatase inhibitors, and 1% Triton X-100, and then
centrifuged at 13,000 x g for 30 min at 4°C. The resultant
supernatants were collected and frozen at −80°C until analysis. For
each reaction in the 96-well plate, 50 µg of proteins were
used, and ELISA were performed according to the manufacturer's
instructions, as described by Koziorowski et al (22). The total proteins were extracted
using RIPA lysis buffer (Beyotime Institute of Biotechnology,
Haimen, China). Following centrifugation, the supernatant was
loaded onto precoated DNA-binding protein wells for ELISA. The
absorbance was measured at 450 nm on an MRX 96-well plate reader
(Dynex Technologies, Inc., Chantilly, VA, USA).
Assessment of the effect of repeated PMA
injection on microglia
The expression of p21 was measured using double
immunofluorescence staining with Iba-1. For double
immunofluorescence, following the permeabilization, hydration,
fixation, antigen retrieval and blocking steps described above,
sections from the repeated injection control group, single PMA
injection group and repeated PMA injection group were incubated
with a mixture of polyclonal rabbit anti-rat Iba-1 (cat. no.
019-19741; 1:1,00; Wako Pure Chemicals Industries) and polyclonal
mouse anti-rat p21 (cat. no. ab80633; 1:80; Abcam, Cambridge, UK)
primary antibodies followed by a mixture of Alexa-488 (green
fluorescence) and Alexa-594 (red fluorescence)-conjugated
polyclonal goat anti-rabbit IgG (cat. no. A-11034; 1:800) and
polyclonal goat anti-mouse IgG (cat. no. A-11029; 1:1,000;
Invitrogen Life Technologies). The red staining of the positive
expression of p21 enabled differentiation from the green positive
expression of Iba-1.
Double staining for β-galactosidase and Iba-1 was
performed, as previously described by Itahana et al
(23). Following dewaxing and
hydration, the rest sections from the repeated injection control
group, single PMA injection group and repeated PMA injection group
were also permeabilized for 10 min and blocked for another 30 min,
as described above. The sections were then incubated overnight at
37°C without CO2 in freshly prepared staining buffer,
containing 1 mg/ml X-gal, 40 mM citric acid/sodium phosphate (pH
6.0), 5 mM potassium ferricyanide, 150 mM NaCl and 2 mM
MgCl2 (all purchased from Sigma-Aldrich), which was
substituted with normal saline prior to observation. Following
β-galactosidase staining, immunohistochemical staining of Iba-1 was
performed according to the avidin-biotin-peroxidase complex method
(Vector Laboratories, Burlingame, CA, USA). Briefly, the stained
sections were incubated overnight at 4°C with polyclonal rabbit
anti-rat Iba-1 antibody (cat. no. 019-19741; 1:100; Wako Pure
Chemicals Industries) and then with biotinylated polyclonal goat
anti-rabbit IgG antibody (cat. no. A-11034; 1:800; Invitrogen Life
Technologies) for 30 min at room temperature, followed by
horseradish peroxidase-conjugated avidin for 30 min at room
temperature. Finally, the colors were developed with
3-amino-9-ethylearbazole substrate (Vector Laboratories, Inc.) for
Iba-1. The positive β-galactosidase cells were stained blue,
enabling differentiation from the red-brown color of the
Iba-1-positive cells.
Unbiased stereological estimation of the number of
cells were performed in all double staining sections throughout the
substantia nigra area using StereoInvestigator analysis software
(version 6.0; MicroBrightField, Williston, VT, USA) combined with a
Nikon Eclipse E600 microscope (Nikon Corporation) and the optical
fractionator method, according to previously published reports
(24,25). The boundaries of the substantia
nigra were defined according to previously published anatomical
analysis in rats (area of counting frame, 64,000 mm3;
guard height, 2 µm; spaced 300 µmm apart in the
x-direction, and 200 µm apart in the y-direction) (26), and the cells were counted in 24
sections (n=8 for each group) along the entire substantia nigra,
including pars reticulate and compacta. The cells were counted by
an observer in a blinded-manner.
For identifying the double stained-positive
senescent microglias, 10 high power microscopic fields throughout
the substantia nigra were randomly selected. The color of the
double-positive cells of Iba-1 and β-galactosidase was confirmed as
violet-black, which formed from the merge of blue (β-galactosidase)
and red-brown (Iba-1). The color of the double-positive cells of
Iba-1 and p21 was defined as yellow, which formed from the merge of
red (p21) and green (Iba-1). Only the microglias with clearly
visible cell bodies were counted. The percentage of senescent
microglia in each high power field (magnification, x400) was
presented as a graph.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Differences between groups were determined using one-way
analysis of variance followed by Bonferroni's t-test for multiple
comparisons. All data were analyzed using SPSS 11.5 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 were considered to indicate a
statistically significant difference.
Results
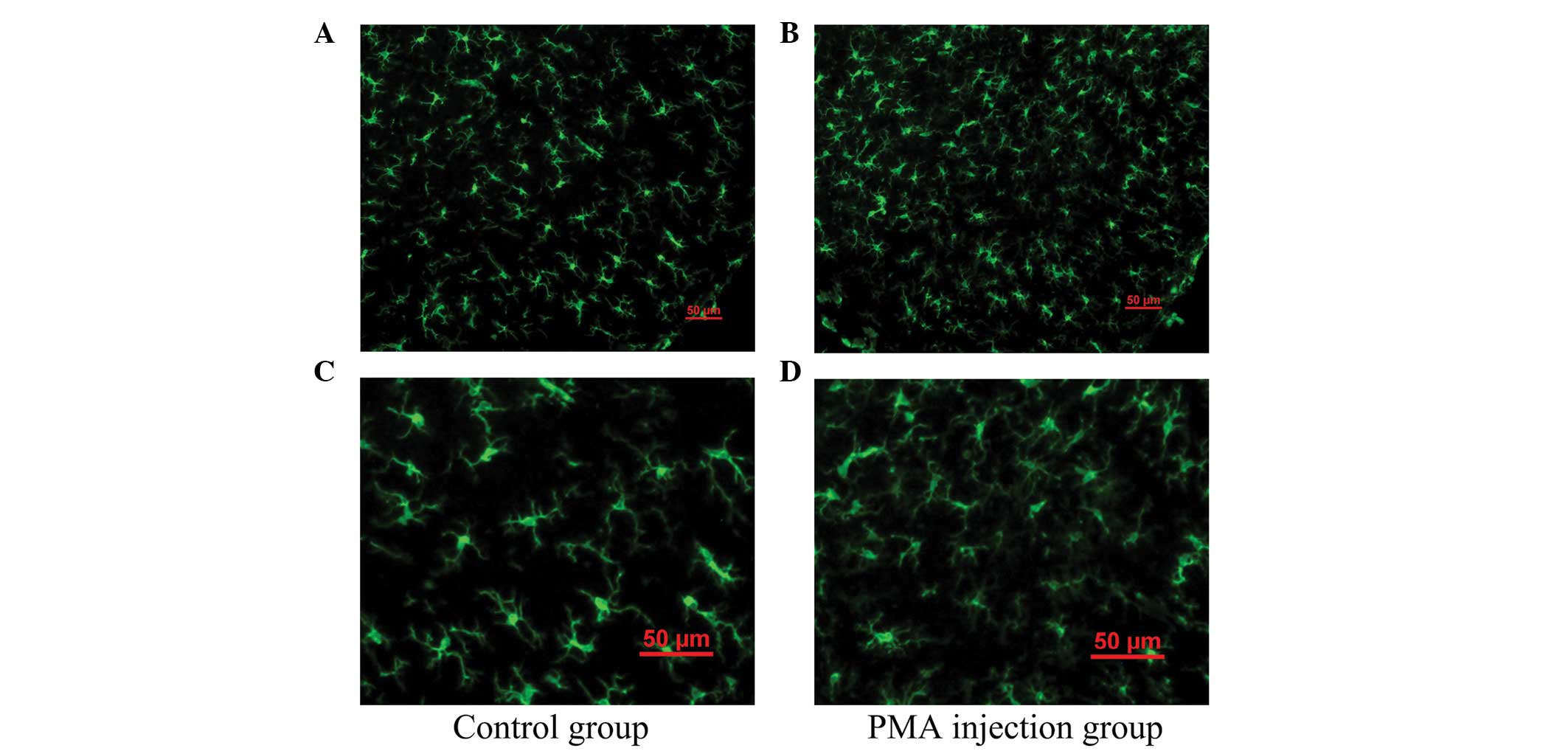
Resting state of microglia 1 week
following single PMA injection
As shown in Fig. 2,
the majority of the microglia were in the resting state with
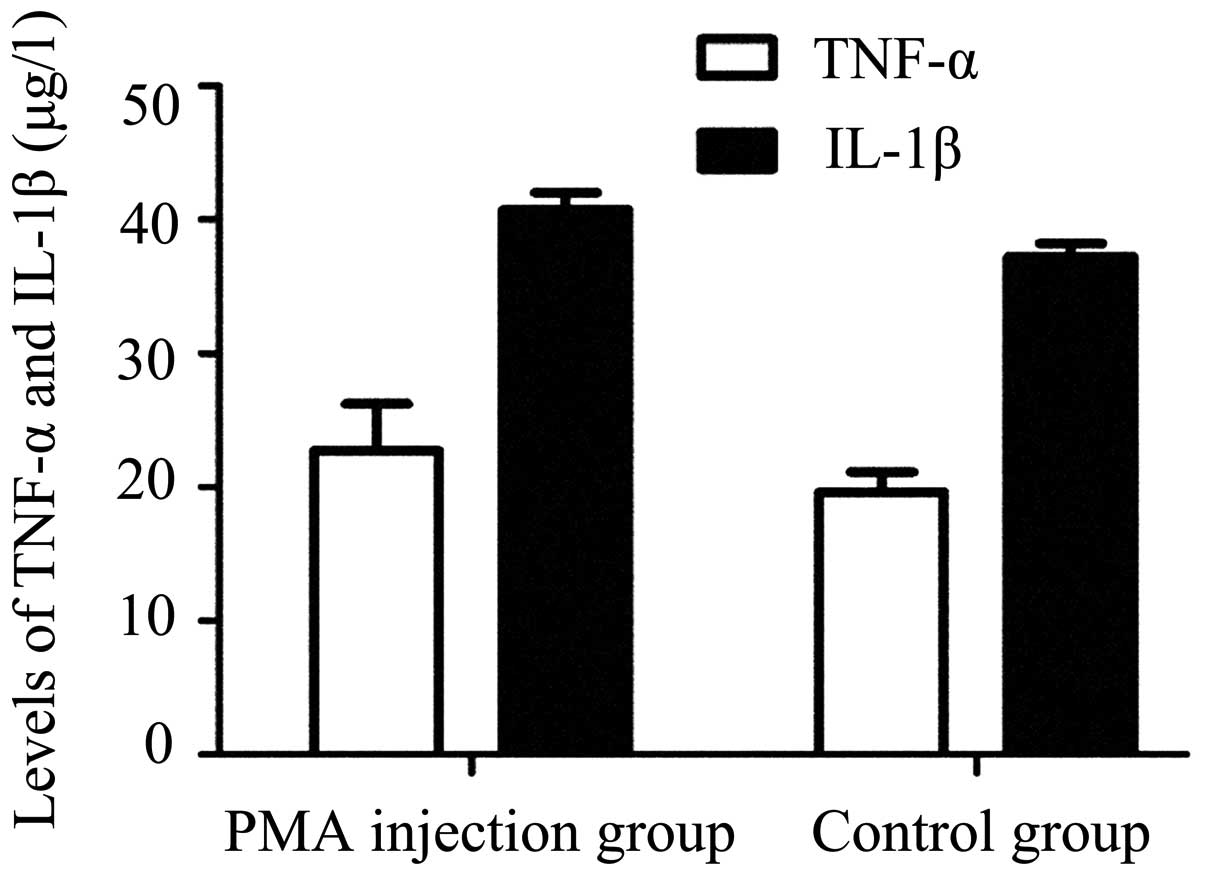
typical ramified branches in the PMA-injected group (Figs. 2B and D). Analysis of ELISA
demonstrated no significant difference in the expression levels of
TNF-α or IL-1β between the control group and PMA injected group
(P>0.05; Fig. 3). These results
indicated that PMA had no significant effect on the microglia
following a single injection at one week.
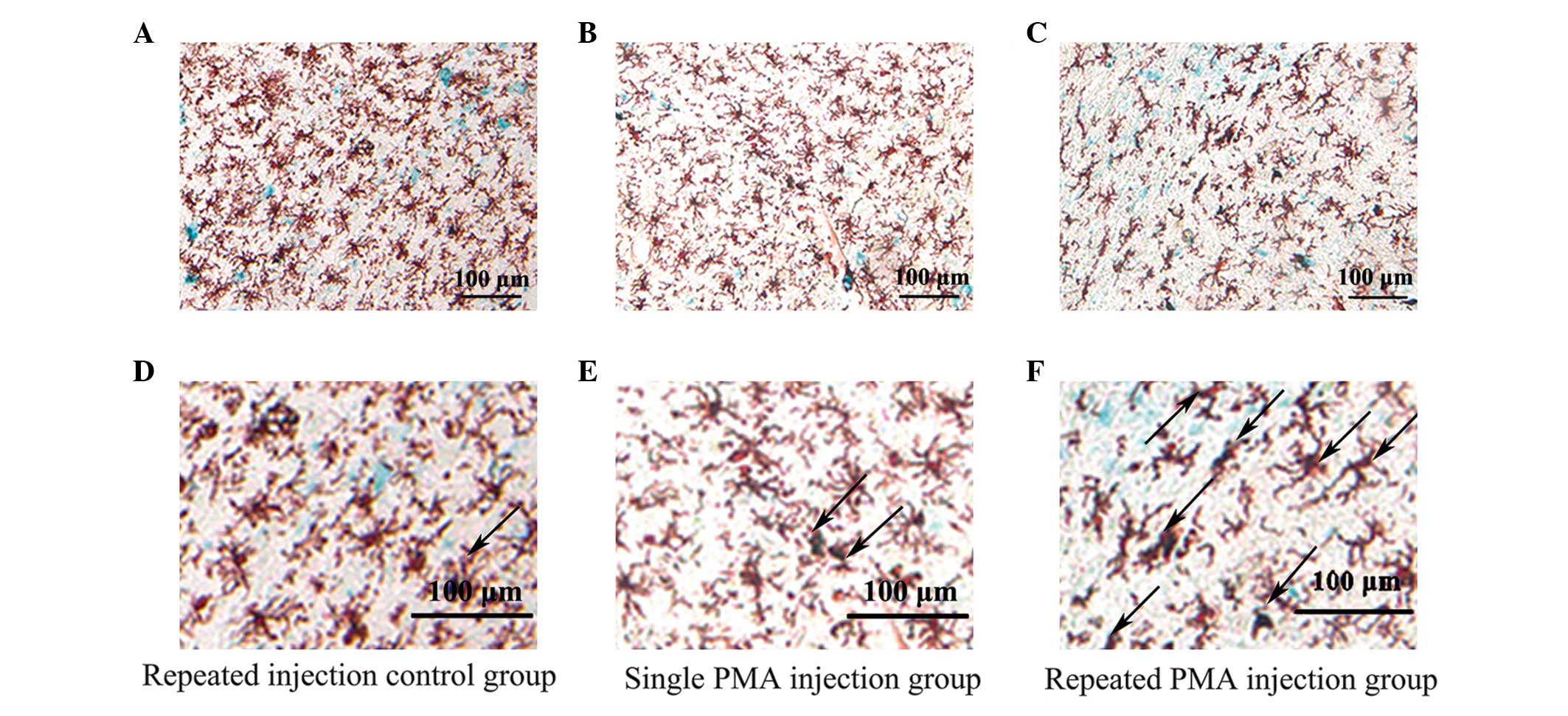
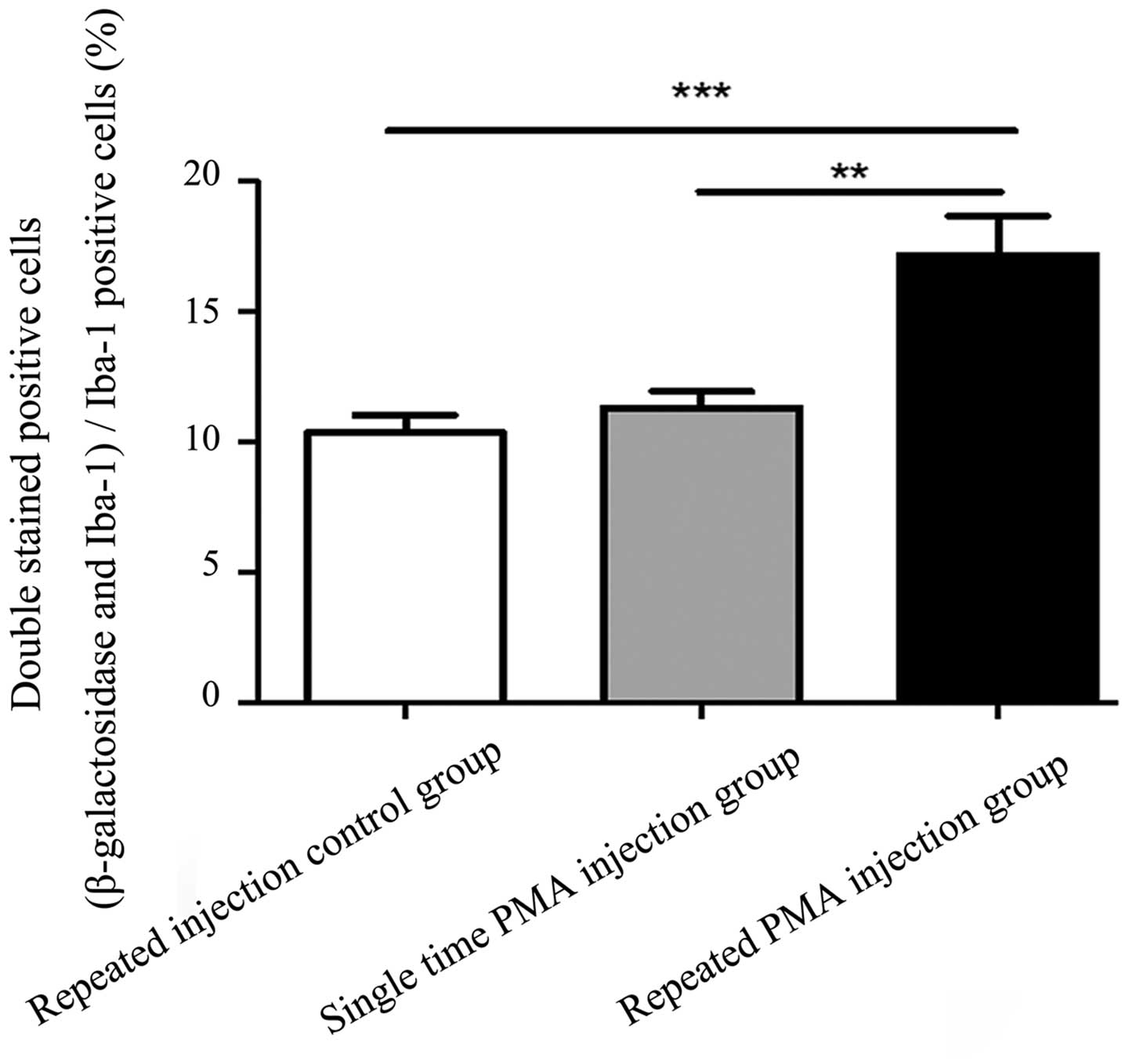
Expression of β-galactosidase is
increased in microglia following repeated injections of PMA
Images of the double immunofluorescence staining are
shown in Fig. 4. There were more
double-stained positive cells in the repeated PMA injection group,
compared with the repeated injection control group (P<0.001) and
single PMA injection group (P=0.002), as shown in Fig. 5. However, the numbers of
double-stained positive cells in the single PMA injection group
were not significantly increased, compared with those in the
repeated injection control group (P=0.777). The percentage of
double-stained positive cells in the repeated PMA injection group
(17.15±4.25%) was significantly increased, compared with the
repeated injection control group (10.34±1.86%; P<0.001) and
single PMA injection group (11.32±1.76%; P=0.002), respectively.
These results suggested that repeated injection of PMA increased
the expression of β-galactosidase in the microglia in the
substantia nigra of the rats.
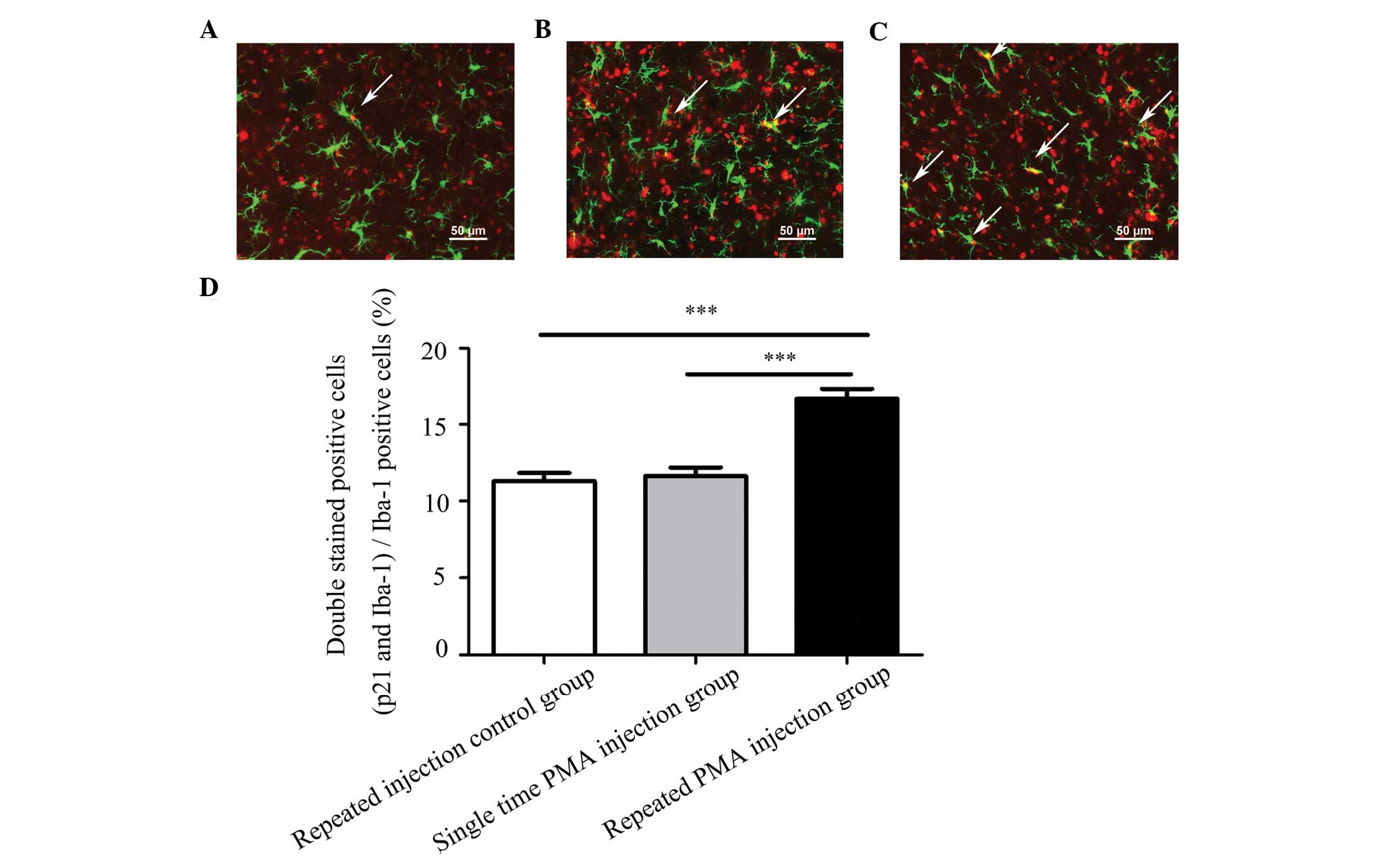
Expression of p21 increases in microglia
following repeated injections of PMA
Representative double-staining images of p21 and
Iba-1 are shown in Fig. 6A–C. The
numbers of microglia exhibiting positive expression of p21 were
significantly higher in the repeated PMA injection group, compared
with the repeated injection control group (P<0.001) and the
single PMA injection group (P<0.001), as shown in Fig. 6D. The numbers of double-stained
positive cells in the single PMA injection group were not
significantly increased, compared to the repeated injection control
group (P=0.912). The percentage of double-stained positive cells in
the repeated PMA injection group (16.67±2.17%) was significantly
increased, compared with the repeated injection control group
(11.31±1.76%; P<0.001) and single PMA injection group
(11.64±1.87%; P<0.001), respectively. These results indicated
that repeated injection of PMA increased the expression of p21 in
the microglia in the substantia nigra of the rats.
Discussion
In the present study, the effect of
intra-nigrostriatal injection of PMA on microglia senescence was
investigated. The results demonstrated that PMA had no significant
effect on the microglia following a single injection at one week.
Following four repeated injections of PMA, microglia in the
substantia nigra exhibited certain senescence characteristics,
including increased expression levels of β-galactosidase and p21.
These results demonstrated that repeated injection of PMA resulted
in microglia senescence, whereas a single injection of PMA did not
induce microglia senescence in the substantia nigra of the
rats.
Microglia senescence is manifested by morphological
changes and alterations in inflammatory profile (4). The observation of morphological
alterations, characterized by cytoplasmic fragmentation, twisting
processes and clusters, has been used in several studies to
identify dystrophic microglia (27,28).
In the present study, the majority of the microglia presented with
no dystrophic microglia features in the PMA injection group,
indicating that a single PMA injection did not lead to
morphological changes, which is one of the characteristics of
microglia senescence. Microglia belong to the macrophage lineage
and are the predominant form of active immune defense in the CNS.
TNF-α and IL-1β are two predominant proinflammatory cytokines
produced by microglia during CNS inflammation (29). In rodents, reports have suggested
that production of the TNF-α and IL-1β proinflammatory cytokines
are increased in the brain of the senescence accelerated mouse
(10,30), while others have suggested that the
proinflammatory cytokines are decreased in macrophages with aging
(31–33). The results of the present study
demonstrated that the levels of TNF-α and IL-1β were not
significantly altered 1 week following a single PMA injection.
These results suggested that microglia senescence did not occur in
the substantia nigra 1 week-post single PMA injection.
Previous studies have reported that the expression
level of β-galactosidase is increased during senescence (12,34).
In addition, the expression of p21 is increased during cell
senescence (13,14). A previous study found that, in
response to repeated lipopolysaccharide administration to mimic
chronic inflammation, cultured BV2 microglial cells exhibited signs
of senescence, including growth arrest and enhanced β-galactosidase
activity (35). In the present
study, repeated administration of PMA increased the expression
levels of β-galactosidase and p21 in the substantia nigra of the
rats, which indicated that repeated administration of PMA induced
microglia senescence in the substantia nigra of the rats. PMA, a
potent tumor promoter, can activate PKC in vivo and in
vitro by binding to PKC, resulting in several types of cellular
effects (16,36). Studies have indicated that PMA can
activate oncogenes, including Ras, in vivo and in
vitro (37,38). In addition, Serrano et al
first reported that the expression of oncogenic Ras results in a
permanent G1 arrest in primary human or rodent cells in OIS
(39). Studies have also indicated
that the accumulation of p21 can mediate G1 arrest (40). Several studies also have
demonstrated that the activation of oncogenes can induce OIS, and
melanocytic nevi provide a clear example of OIS (15,41–43).
Thus, PMA induced microglia senescence may be associated with
oncogenic Ras-induced G1 arrest, which also mediated by p21 in
OIS.
PMA can also lead to oxidative stress in
vitro (44). Studies have
demonstrated that various forms of oxidative stress can induce
senescence, including exposure to reactive oxygen species (45–47).
Cultured astrocytes can undergo cellular senescence with the
development of characteristics of senescence, including growth
arrest, expression of β-galactosidase and increased expression of
the cell-cycle inhibitor p21 in response to a variety of stressors,
including oxidative stress (48–50).
Thus, oxidative stress-induced senescence may be another potential
mechanism of PMA-induced microglia senescence.
PMA, as a carcinogen, can induce activation of
endogenic oncogenes, and resulted in microglia senescence in the
substantia nigra in the present study. Oncogenes can be activated
by various stimulants, which can cause tumorigenesis (51). Microglia senescence is a major
factor contributing to the development of age-associated
neurodegenerative diseases (52).
There is a close connection between tumorigenesis and
neurodegeneration (53), PD, as a
neurodegenerative disease, has been reported to be associated with
a decreased risk of developing cancer (54,55).
Therefore, the result of the present study that PMA induced
microglia senescence, provided further evidence supporting the
interaction between tumorigenesis and neurodegeneration.
In conclusion, the present study demonstrated that
repeated intra-nigrostriatal treatment with PMA for carcinogen
stimulation induced microglia senescence, which may be associated
with OIS- and oxidative stress-induced senescence. In addition,
these results provide novel evidence for the link between
tumorigenesis and neurodegeneration.
Acknowledgments
This study was funded by the China National Nature
Science Fund (grant. nos. 30973153, 81371421 and 81000623), the
Foundation of the Liaoning Educational Committee (grant. nos.
L202013136 and L2010560), the Liaoning Doctoral Starting Fund
(grant. no. 20071042) and the Liaoning S&T Project Fund (grant.
no. 2011225020).
References
|
1
|
Kielian T: Microglia and chemokines in
infectious diseases of the nervous system: Views and reviews. Front
Biosci. 9:732–750. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kanaan NM, Kordower JH and Collier TJ: Age
and region-specific responses of microglia, but not astrocytes,
suggest a role in selective vulnerability of dopamine neurons after
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure in monkeys.
Glia. 56:1199–1214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Overmyer M, Helisalmi S, Soininen H,
Laakso M, Riekkinen P Sr and Alafuzoff I: Reactive microglia in
aging and dementia: An immunohistochemical study of postmortem
human brain tissue. Acta Neuropathol. 97:383–392. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luo XG, Ding JQ and Chen SD: Microglia in
the aging brain: Relevance to neurodegeneration. Mol Neurodegener.
5:122010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sawada M, Sawada H and Nagatsu T: Effects
of aging on neuroprotective and neurotoxic properties of microglia
in neuro-degenerative diseases. Neurodegener Dis. 5:254–256. 2008.
View Article : Google Scholar
|
|
6
|
Conde JR and Streit WJ: Effect of aging on
the microglial response to peripheral nerve injury. Neurobiol
Aging. 27:1451–1461. 2006. View Article : Google Scholar
|
|
7
|
Streit WJ, Sammons NW, Kuhns AJ and Sparks
DL: Dystrophic microglia in the aging human brain. Glia.
45:208–212. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Streit WJ, Miller KR, Lopes KO and Njie E:
Microglial degeneration in the aging brain-bad news for neurons?
Front Biosci. 13:3423–3438. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Olanow CW and Tatton WG: Etiology and
pathogenesis of Parkinson's disease. Annu Rev Neurosci. 22:123–144.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tha KK, Okuma Y, Miyazaki H, Murayama T,
Uehara T, Hatakeyama R, Hayashi Y and Nomura Y: Changes in
expressions of proinflammatory cytokines IL-1beta, TNF-alpha and
IL-6 in the brain of senescence accelerated mouse (SAM) P8. Brain
Res. 885:25–31. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Campisi J: The biology of replicative
senescence. Eur J Cancer. 33:703–709. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee BY, Han JA, Im JS, Morrone A, Johung
K, Goodwin EC, Kleijer WJ, DiMaio D and Hwang ES:
Senescence-associated beta-galactosidase is lysosomal
beta-galactosidase. Aging Cell. 5:187–195. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roninson IB: Oncogenic functions of tumour
suppressor p21 (Waf1/Cip1/Sdi1): Association with cell senescence
and tumour-promoting activities of stromal fibroblasts. Cancer
Lett. 179:1–14. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Macip S, Igarashi M, Fang L, Chen A, Pan
ZQ, Lee SW and Aaronson SA: Inhibition of p21-mediated ROS
accumulation can rescue p21-induced senescence. EMBO J.
21:2180–2188. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Campisi J and d'Adda di Fagagna F:
Cellular senescence: When bad things happen to good cells. Nat Rev
Mol Cell Biol. 8:729–740. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Blumberg PM: Protein kinase C as the
receptor for the phorbol ester tumor promoters: Sixth Rhoads
memorial award lecture. Cancer Res. 48:1–8. 1988.PubMed/NCBI
|
|
17
|
Li YH, Bi HC, Huang L, Jin J, Zhong GP,
Zhou XN and Huang M: Phorbol 12-myristate 13-acetate inhibits
P-glycoprotein-mediated efflux of digoxin in MDCKII-MDR1 and Caco-2
cell monolayer models. Acta Pharmacologica Sin. 35:283–291. 2014.
View Article : Google Scholar
|
|
18
|
O'neill AK, Gallegos LL, Justilien V,
Garcia EL, Leitges M, Fields AP, Hall RA and Newton AC: Protein
kinase Cα promotes cell migration through a PDZ-dependent
interaction with its novel substrate discs large homolog 1 (DLG1).
J Biol Chem. 286:43559–43568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mason SA, Cozzi SJ, Pierce CJ, Pavey SJ,
Parsons PG and Boyle GM: The induction of senescence-like growth
arrest by protein kinase C-activating diterpene esters in solid
tumor cells. Invest New Drugs. 28:575–586. 2010. View Article : Google Scholar
|
|
20
|
Akakura S, Nochajski P, Gao L, Sotomayor
P, Matsui S and Gelman IH: Rb-dependent cellular senescence,
multinucleation and susceptibility to oncogenic transformation
through PKC scaffolding by SSeCKS/AKAP12. Cell Cycle. 9:4656–4665.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grealish S, Xie L, Kelly M and Dowd E:
Unilateral axonal or terminal injection of 6-hydroxydopamine causes
rapid-onset nigrostriatal degeneration and contralateral motor
impairments in the rat. Brain Res Bull. 77:312–319. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koziorowski D, Friedman A, Arosio P,
Santambrogio P and Dziewulska D: ELISA reveals a difference in the
structure of substantia nigra ferritin in Parkinson's disease and
incidental lewy body compared to control. Parkinsonism Relat
Disord. 13:214–218. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Itahana K, Zou Y, Itahana Y, Martinez JL,
Beausejour C, Jacobs JJ, Van Lohuizen M, Band V, Campisi J and
Dimri GP: Control of the replicative life span of human fibroblasts
by p16 and the polycomb protein Bmi-1. Mol Cell Biol. 23:389–401.
2003. View Article : Google Scholar :
|
|
24
|
West MJ, Slomianka L and Gundersen HJ:
Unbiased stereological estimation of the total number of neurons in
thesubdivisions of the rat hippocampus using the optical
fractionator. Anat Rec. 231:482–497. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan J, Wang G, Yang HQ, Hong Z, Xiao Q,
Ren RJ, Zhou HY, Bai L and Chen SD: K252a prevents nigral
dopaminergic cell death induced by 6-hydroxydopamine through
inhibition of both mixed-lineage kinase 3/c-Jun NH2-terminal kinase
3 (JNK3) and apoptosis-inducing kinase 1/JNK3 signaling pathways.
Mol Pharmacol. 72:1607–1618. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
German DC and Manaye KF: Midbrain
dopaminergic neurons (nuclei A8, A9 and A10): Three-dimensional
reconstruction in the rat. J Comp Neurol. 331:297–309. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Streit WJ, Braak H, Xue QS and Bechmann I:
Dystrophic (senescent) rather than activated microglial cells are
associated with tau pathology and likely precede neurodegeneration
in Alzheimer's disease. Acta Neuropathol. 118:475–485. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo XG and Chen SD: The changing phenotype
of microglia from homeostasis to disease. Transl Neurodegener.
1:92012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim YS and Joh TH: Microglia, major player
in the brain inflammation: Their roles in the pathogenesis of
Parkinson's disease. Exp Mol Med. 38:333–347. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Franceschi C, Bonafé M, Valensin S,
Olivieri F, De Luca M, Ottaviani E and De Benedictis G:
Inflamm-aging. An evolutionary perspective on immunosenescence. Ann
N Y Acad Sci. 908:244–254. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Inamizu T, Chang MP and Makinodan T:
Influence of age on the production and regulation of interleukin-1
in mice. Immunology. 55:447–455. 1985.PubMed/NCBI
|
|
32
|
Corsini E, Battaini F, Lucchi L,
Marinovich M, Racchi M, Govoni S and Galli CL: A defective protein
kinase C anchoring system underlying age–zassociated impairment in
TNF-alpha production in rat macrophages. J Immunol. 163:3468–3473.
1999.PubMed/NCBI
|
|
33
|
Plackett TP, Boehmer ED, Faunce DE and
Kovacs EJ: Aging and innate immune cells. J Leukoc Biol.
76:291–299. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dimri GP, Lee X, Basile G, Acosta M, Scott
G, Roskelley C, Medrano EE, Linskens M, Rubelj I and Pereira-Smith
O: A biomarker that identifies senescent human cells in culture and
in aging skin in vivo. Proc Natl Acad Sci USA. 92:9363–9367. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu HM, Zhao YM, Luo XG, Feng Y, Ren Y,
Shang H, He ZY, Luo XM, Chen SD and Wang XY: Repeated
lipopolysac-charide stimulation induces cellular senescence in BV2
cells. Neuroimmunomodulation. 19:131–136. 2012. View Article : Google Scholar
|
|
36
|
Petiti JP, De Paul AL, Gutiérrez S,
Palmeri CM, Mukdsi JH and Torres AI: Activation of PKC epsilon
induces lactotroph proliferation through ERK1/2 in response to
phorbol ester. Mol Cell Endocrinol. 289:77–84. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Inoue S, Hao Z, Elia AJ, Cescon D, Zhou L,
Silvester J, Snow B, Harris IS, Sasaki M, Li WY, et al:
Mule/Huwe1/Arf-BP1 suppresses Ras-driven tumorigenesis by
preventing c-Myc/Miz1-mediated down-regulation of p21 and p15.
Genes Dev. 27:1101–1114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mowla S, Pinnock R, Leaner VD, Goding CR
and Prince S: PMA-induced up-regulation of TBX3 is mediated by AP-1
and contributes to breast cancer cell migration. Biochem J.
433:145–153. 2011. View Article : Google Scholar
|
|
39
|
Serrano M, Lin AW, Mccurrach ME, Beach D
and Lowe SW: Oncogenic ras provokes premature cell senescence
associated with accumulation of p53 and p16INK4a. Cell. 88:593–602.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
John R, Chand V, Chakraborty S, Jaiswal N
and Nag A: DNA damage induced activation of Cygb stabilizes p53 and
mediates G1 arrest. DNA Repair (Amst). 2014:107–112. 2014.
View Article : Google Scholar
|
|
41
|
Sarkisian CJ, Keister BA, Stairs DB, Boxer
RB, Moody SE and Chodosh LA: Dose-dependent oncogene-induced
senescence in vivo and its evasion during mammary tumorigenesis.
Nat Cell Biol. 9:493–505. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Courtois-Cox S, Genther Williams SM,
Reczek EE, Johnson BW, McGillicuddy LT, Johannessen CM, Hollstein
PE, MacCollin M and Cichowski K: A negative feedback signaling
network underlies oncogene-induced senescence. Cancer Cell.
10:459–472. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chinta SJ, Lieu CA, Demaria M, Laberge RM,
Campisi J and Andersen JK: Environmental stress, ageing and glial
cell senescence: A novel mechanistic link to Parkinson's disease? J
Intern Med. 273:429–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kumar A, Chen SH, Kadiiska MB, Hong JS,
Zielonka J, Kalyanaraman B and Mason RP: Inducible nitric oxide
synthase is key to peroxynitrite-mediated, LPS-induced protein
radical formation in murine microglial BV2 cells. Free Radical Bio
Med. 73:51–59. 2014. View Article : Google Scholar
|
|
45
|
Collado M and Serrano M: Senescence in
tumours: Evidence from mice and humans. Nat Rev Cancer. 10:51–57.
2010. View Article : Google Scholar
|
|
46
|
Parrinello S, Samper E, Krtolica A,
Goldstein J, Melov S and Campisi J: Oxygen sensitivity severely
limits the replicative lifespan of murine fibroblasts. Nat Cell
Biol. 5:741–747. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen JH, Ozanne SE and Hales CN: Methods
of cellular senescence induction using oxidative stress. Methods
Mol Biol. 371:179–189. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bitto A, Sell C, Crowe E, Lorenzini A,
Malaguti M, Hrelia S and Torres C: Stress-induced senescence in
human and rodent astrocytes. Exp Cell Res. 316:2961–2968. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Inoue Y, Matsuda T, Sugiyama KI, Izawa S
and Kimura A: Genetic analysis of glutathione peroxidase in
oxidative stress response of Saccharomyces cerevisiae. J Biol Chem.
274:27002–27009. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kaneto H, Kajimoto Y, Fujitani Y, Matsuoka
T, Sakamoto K, Matsuhisa M, Yamasaki Y and Hori M: Oxidative stress
induces p21 expression in pancreatic islet cells: Possible
implication in beta-cell dysfunction. Diabetologia. 42:1093–1097.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Croce CM: Oncogenes and cancer. N Engl J
Med. 358:502–511. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Streit WJ and Xue QS: Human CNS immune
senescence and neurodegeneration. Curr Opin Immunol. 29:93–96.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lehmann AR and Carr AM: The
ataxia-telangiectasia gene: A link between checkpoint controls,
neurodegeneration and cancer. Trends Genet. 11:375–377. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Vanacore N, Spila-Alegiani S, Raschetti R
and Meco G: Mortality cancer risk in parkinsonian patients: A
population-based study. Neurology. 52:395–398. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ong EL, Goldacre R and Goldacre M:
Differential risks of cancer types in people with Parkinson's
disease: A national record-linkage study. Eur J Cancer.
50:2456–2462. 2014. View Article : Google Scholar : PubMed/NCBI
|