Introduction
Hepatocellular carcinoma (HCC) has one of the
highest mortality rates worldwide (1). HCC characteristics, such as strong
resistance to anticancer drugs, local metastasis and heterogeneity,
lead to complications in the effective treatment of HCC. Although
multiple clinical trials to treat HCC through systemic chemotherapy
have been conducted, all treatments from Phase 3 clinical trials
were unsuccessful, with the exception of sorafenib (2); however, it is not curative or
approved by the US Food and Drug Administration (3). Sorafenib is a multiple kinase
inhibitor, which blocks various signaling pathways involved in the
proliferation of cancer cells (4)
via the inhibition of multiple kinases, including mitogen-activated
protein kinase (5), extracellular
signal-regulated kinase (6),
vascular endothelial growth factor receptor (7) and platelet-derived growth factor
receptor (8).
Regarding the anticancer effects of combination
therapy with sorafenib, it was reported that treatment with a
combination of sorafenib, cisplatin and fluorouracil (5-FU)
increased survival rates for metastatic nasopharyngeal carcinoma
(9). In HCC, Petrini et al
(10) reported that treatment with
a combination of sorafenib and 5-FU improved the efficacy of
systemic treatment in advanced HCC without side effects.
Cancer stem cells are known as tumor initiating
cells (11) or anticancer
drugresistant cells (12). In HCC
treatment, cancer stem cells are not easily eradicated due to their
drug resistance and proliferative activity (13–15).
HCC cancer stem cells that are resistant to chemotherapy positively
express cluster of differentiation (CD)90, CD133 and ATP-binding
cassette sub-family G member 2 (ABCG2) (16). Notably, ABCG2 expression was
upregulated in HCC cells to remove the anticancer therapeutic
agents from the cancer stem cells following treatment and was
highly expressed in HCC tissues when compared with normal tissues
(17). In addition, cancer stem
cells induced c-Jun N-terminal kinase (JNK) and Wnt signaling
interactions promoting their survival and proliferation in a model
of colorectal carcinogenesis (18). In addition, Mucha et al
(19) reported that JNK signaling
was associated with TNF-related apoptosis-inducing ligand-induced
cell death and resistance to apoptosis in HCC cells.
To date, to the best of our knowledge, there is a
lack of studies regarding the anticancer effects of sorafenib-based
combination therapies on cancer stem cells in HCC, particularly
focusing on ABCG2 and JNK signaling in side population (SP) cells.
In the present study, the anticancer effect of sorafenib was
observed, with a focus on suppression of tumor initiating ability
and drug-resistance in cancer stem cells. Specifically, growth
rates in a fraction of SP cells, the expression of cancer stem cell
markers, the efficacy of sphere-forming cells, and the expression
of relevant signaling molecules following sorafenib and 5-FU
treatment were assessed.
Materials and methods
Cell culture
Huh7 and Huh-BAT cells (Korean Cell Line Bank,
Seoul, Korea) were cultured in Dulbecco's modified Eagle's medium
(DMEM; Gibco Life Technologies, Carlsbad, CA, USA) containing 10%
fetal bovine serum (FBS; Gibco Life Technologies). For adherent
cultures, 5×105 cells were seeded on tissue culture
dishes (Falcon, San Jose, CA, USA). All cultures were maintained at
37°C in a humidified 5% CO2 atmosphere.
Growth rate following 5-FU and sorafenib
treatment
Cells (5×105 cells) were seeded in DMEM
containing 10% FBS. After 24 h, the cells were washed twice with
phosphate-buffered saline (PBS) and fresh media was added. Cells
were treated for 72 h with distilled water (control), 100 nM, 1 or
10 µM 5-FU (Sigma-Aldrich, St. Louis, MO, USA) or 100 nM, 1
or 5 µM of sorafenib (LC Laboratories, Woburn, MA, USA).
Growth rates were estimated by the number of viable cells counted
by positive staining with 0.4% trypan blue dye (Gibco Life
Technologies) in a Neubauer chamber (Marienfeld-Superior,
Lauda-Königshofen, Germany) with an inverted microscope (IX51;
Olympus Corporation, Tokyo, Japan) equipped with a DP50 camera
system (Olympus Corporation) at 72 h time points. Selected cells
treated with 1 µM 5-FU or 5 µM sorafenib were used to
observe cell death, the cell cycle, SP cells, stem cell markers
(CD44, CD24 and CD133), and JNK signaling molecules subsequent to
treatment with these therapeutic agents.
Sphere-formation assay
Cells (1,000 per well) were seeded on
Poly-HEMA-coated 96-well plates (Sigma-Aldrich) in DMEM containing
10% FBS. Cells were incubated for seven days with dimethyl
sulfoxide (DMSO; Sigma-Aldrich; control), 1 µM 5-FU, 3
µM sorafenib or 1 µM 5-FU plus 3 µM sorafenib.
The number of spheres in each well was counted after seven
days.
SP cell analyses
Cells (5×105 cells) were seeded in DMEM
containing 10% FBS. After 24 h, the cells were washed twice with
PBS and fresh media was added. Cells were treated for 72 h with
distilled water (control), 1 µM 5-FU or 5 µM
sorafenib. SP cell analyses were performed as reported previously
(20). Cells were detached and
collected in the form of cell pellets. To analyze the SP fraction,
1×106 cells/ml were incubated for 90 min at 37°C before
vortexing at maximum speed with Hoechst 33342 dye (5 µg/ml;
Sigma-Aldrich) in DMEM containing 10% FBS. The cells were also
incubated with Hoechst dye and 50–100 µM verapamil
(Sigma-Aldrich), an efflux blocker, to confirm the SP cell
population. At the end of the incubation, these cells were
centrifuged at 80 x g and 4°C for 3 min and collected for analysis
of the SP fraction. Propidium iodide (1 µg/ml;
Sigma-Aldrich) was added before fluorescence-activated cell sorting
(FACS) analysis to identify and exclude the dead cells. Samples
were analyzed using a FACSAria™ II (BD Biosciences, Franklin Lakes,
NJ, USA).
Immunoblotting
Cells (5×105 cells) were seeded in DMEM
containing 10% FBS. After 24 h, the cells were washed twice with
PBS and fresh media was added. Cells were treated for 72 h with
distilled water (control), 1 µM 5-FU or 5 µM
sorafenib. Total cell lysates were prepared in 100 µl lysis
buffer (Cell Signaling Technology, Inc., Danvers, MA, USA). Protein
concentrations were measured using a Bio-Rad Protein Assay kit
(Bio-Rad Laboratories Inc., Hercules, CA, USA). Equal quantities of
cell lysates were separated using 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (Bio-Rad Laboratories,
Inc.) and proteins were electrotransferred to Hybond-ECL
nitrocellulose membranes (GE Healthcare Life Sciences, Chalfont,
UK). Blots were blocked for 1 h with blocking buffer (5% skim milk)
and incubated overnight at 4°C with anti-ABCG2 mouse monoclonal
antibodies (1:1,000; cat. no. MABN1108; EMD Millipore, Billerica,
MA, USA), anti-SAPK/JNK mouse monoclonal antibodies (1:1,000; cat.
no. 9252; Cell Signaling Technology, Inc.), anti-phosphorylated
(P)-SAPK/JNK (Thr183/Tyr185) mouse monoclonal antibodies (1:1,000;
cat. no. 9255; Cell Signaling Technology, Inc.), c-Jun mouse
monoclonal antibodies (1:1,000; cat. no. 2315; Cell Signaling
Technology, Inc.), P-c-Jun (ser63) rabbit polyclonal antibodies
(1:1,000; cat. no. 2361; Cell Signaling Technology, Inc.) and
anti-β-actin mouse monoclonal antibodies (1:1,000; cat. no.
sc-47778; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Blots
were washed with Tris-buffered saline containing 0.2% Tween-20
(Sigma-Aldrich) and incubated for 1 h at room temperature with
peroxidase-conjugated AffiniPure rabbit anti-mouse immunoglobulin
(Ig)G (1:2,500; cat. no. 315-005-045; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) or peroxidase-conjugated
AffiniPure mouse anti-rabbit IgG (1:2,500; cat. no. 211-005-109;
Jackson ImmunoResearch Laboratories, Inc.). Labeled proteins were
detected using an enhanced chemiluminescence detection system (GE
Healthcare Life Sciences).
Statistical analyses
At least three replicate experiments were performed
for all analyses. Data were expressed as the mean ± standard error.
Student's t-tests were applied to compare the results of the
treated and control cells. P<0.05 was considered to indicate a
statistically significant difference.
Results
5-FU and sorafenib treatment decreases
Huh7 and Huh-BAT cell growth rate
To observe the effect of 5-FU and sorafenib
treatment on growth rates in Huh7 and Huh-BAT cells after 72 h,
cells were treated with various concentrations of 5-FU and
sorafenib, as indicated in Fig. 1A and
B. Growth rates of Huh7 and Huh-BAT cells were reduced by 5-FU
or sorafenib treatment in a dose-dependent manner (Fig. 1A and B; left and middle panel).
Based on these results, 1 µM 5-FU and 5 µM sorafenib
were selected to observe the synergistic anticancer growth rate
effects in the Huh7 and Huh-BAT cells. The cells were treated with
1 µM 5-FU, 5 µM sorafenib or 1 µM 5-FU plus 5
µM sorafenib for 72 h. However, a synergistic anticancer
effect on growth rates resulting from treatment with 1 µM
5-FU plus 5 µM sorafenib treatment was not observed in the
cells (Fig. 1A and B; right
panel).
 | Figure 1Growth rates in (A) Huh7 and (B)
Huh-BAT cells following 5-FU and sorafenib treatment. Cells
(5×105) were seeded in Dulbecco's modified Eagle's
medium containing 10% fetal bovine serum. Cells were incubated for
72 h with 5-FU, sorafenib or 5-FU plus sorafenib. Growth rates were
assessed by cell counting using trypan blue dye exclusion. Left
panels: Cells were treated with distilled water (CTL), 1, 10 or 100
µM 5-FU; middle panels: Cells were treated with DMSO (CTL),
100 nM, 1 or 5 µM sorafenib; right panels: Cells were
treated with DMSO (control), 1 µM 5-FU, 5 µM
sorafenib or 1 µM 5-FU plus 5 µM sorafenib. The
relative cell survival rate is presented as relative survival
versus the CTL cells. Values are presented as the mean ± standard
error from at least three independent experiments.
*P≤0.05, **P≤0.01, vs. the control group.
5-FU, fluorouracil; DMSO, dimethyl sulfoxide; CTL, control. |
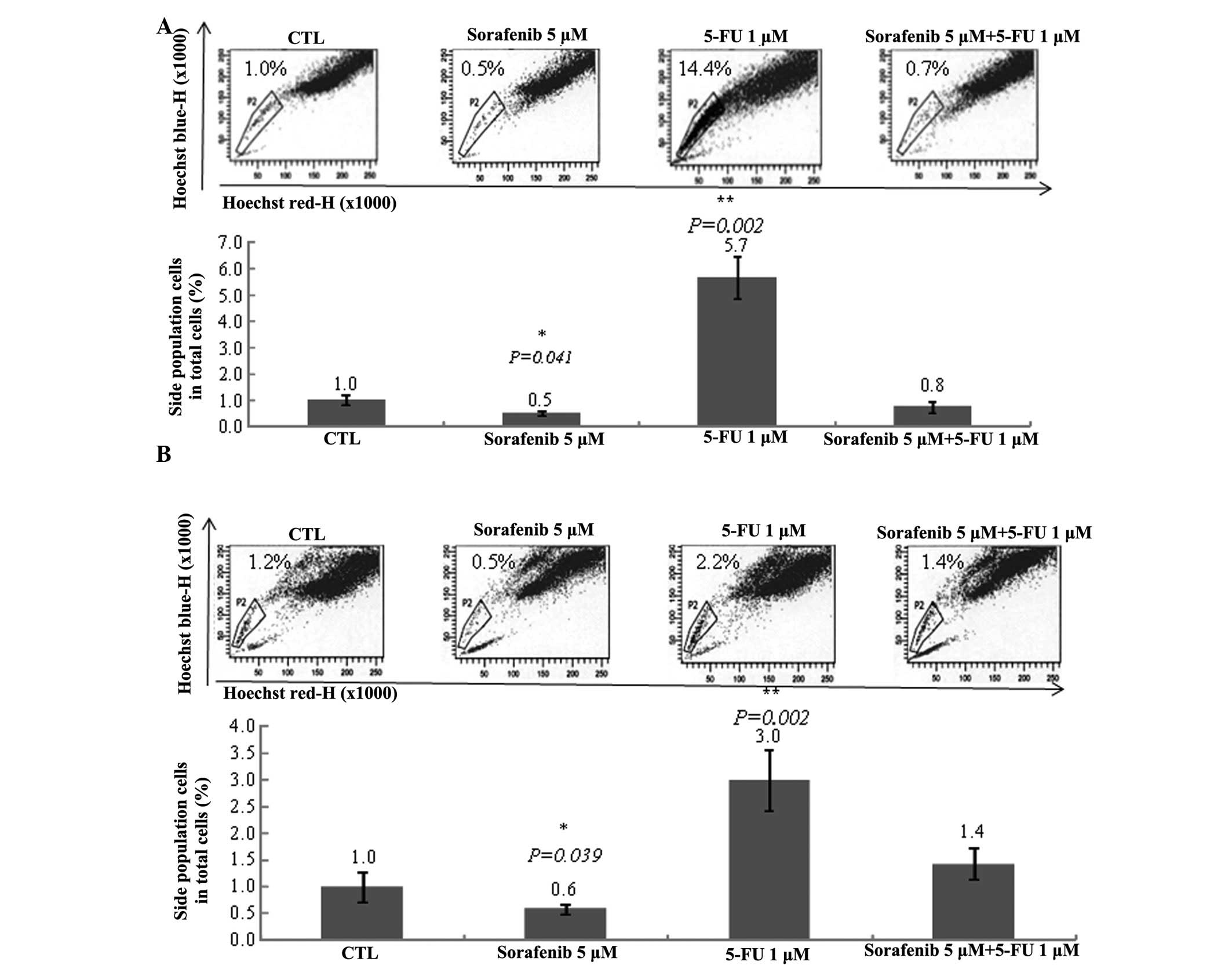
Sorafenib decreases and 5-FU increases
the number of SP cells
To determine the effect of 5-FU and sorafenib
treatment against cancer stem cells induced from Huh7 and Huh-BAT
cells, SP cells were analyzed using Hoechst dye staining followed
by flow cytometry. Sorafenib reduced the number of SP cells,
whereas 5-FU significantly increased SP cell number (Fig. 2). Furthermore, sorafenib plus 5-FU
treatment blocked the 5-FU-mediated increase in SP cell number
(Fig. 2). These results
demonstrate that sorafenib may reduce growth rates by targeting
cancer stem cells.
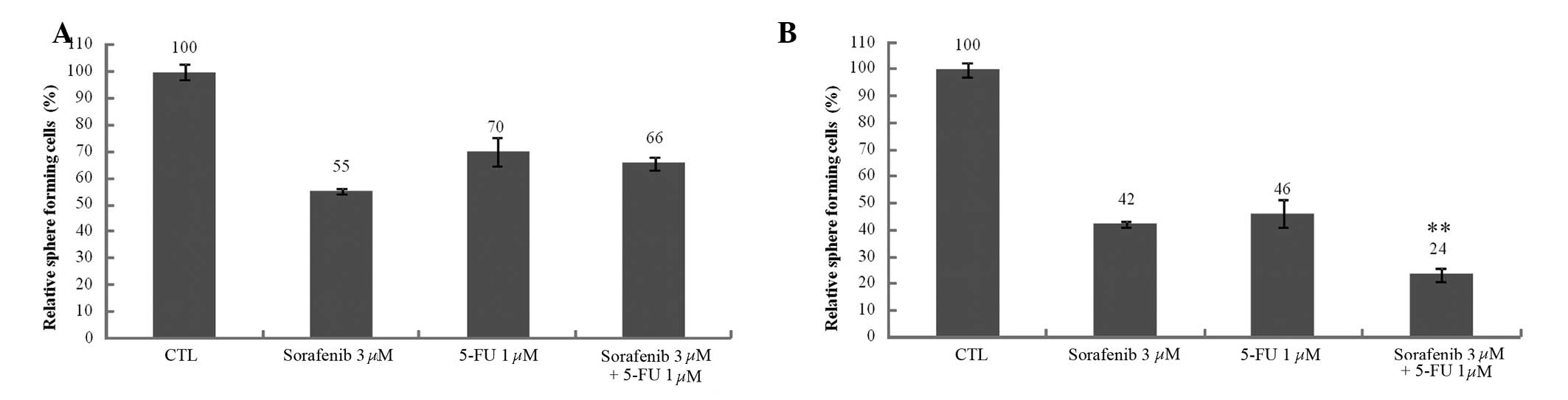
5-FU and sorafenib decrease the number of
Huh7 and Huh-BAT sphere-forming cells
Sphere-forming cells have been proposed as a model
for investigating cancer stem cells (21). It was observed that SP cell number
was decreased following sorafenib treatment and increased following
5-FU treatment. To confirm the effect of these therapeutic agents
against SP cells, sphere-forming cells were observed following 5-FU
and sorafenib treatment in Huh7 and Huh-BAT cells. The number of
sphere-forming cells decreased subsequent to 5-FU and sorafenib
treatment compared with the controls (Fig. 3A and B). No significant differences
were identified in the 5-FU, sorafenib or 5-FU plus sorafenib
treatment groups in Huh7 cells (Fig.
3A). However, a difference was observed between Huh7 and
Huh-BAT cells in the sphere-forming assay. The number of
sphere-forming cells decreased following 5-FU plus sorafenib
treatment compared with 5-FU and sorafenib treatments alone in the
Huh-BAT cells (Fig. 3B). These
results demonstrate that sphere-forming ability is dependent on
varied conditions, such as cell type and therapeutic agent
treatment.
Expression of ABCG2 and JNK signaling
molecules are downregulated in Huh7 and Huh-BAT cells following
sorafenib treatment
The expression of ABCG2, which is associated with
drug-resistance, and JNK signaling molecules, including JNK,
P-SAPK/JNK, c-Jun, and P-c-Jun, in Huh7 and Huh-BAT cells
subsequent to 5-FU and sorafenib treatments was examined. The
expression of ABCG2 and JNK signaling molecules (JNK, P-SAPK/JNK,
c-Jun and P-c-Jun) was downregulated by sorafenib treatment and
sorafenib plus 5-FU treatment in the Huh-7 (Fig. 4A) and Huh-BAT cells (Fig. 4B).
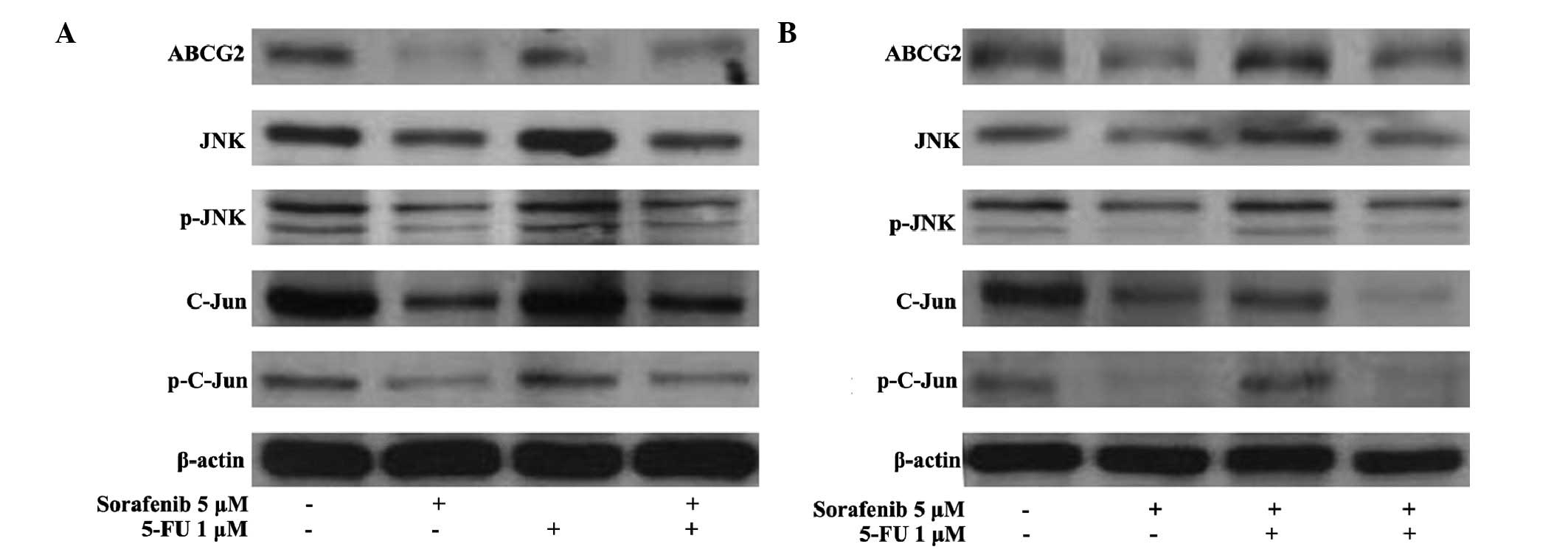 | Figure 4Expression of JNK signaling molecules
(JNK, P-SAPK/JNK, c-Jun, and P-c-Jun) in Huh7 and Huh-BAT cells
following 5-FU and sorafenib treatment. (A) Huh7 or (B) Huh-BAT
cells (5×105) were seeded in Dulbecco's modified Eagle's
medium containing 10% fetal bovine serum. Cells were incubated for
72 h with DMSO (CTL), 1 µM 5-FU, 5 µM sorafenib, or 1
µM 5-FU plus 5 µM sorafenib. Expression levels of
ABCG2, JNK, P-JNK, c-Jun, and P-c-Jun were detected by
immunoblotting. Cells were treated with distilled water and DMSO
(lane 1), 5 µM sorafenib (lane 2), 1 µM 5-FU (lane
3), or 5 µM sorafenib plus 1 µM 5-FU (lane 4). JNK,
c-Jun N-terminal kinase; SAPK, stress-activated protein kinases; P,
phosphorylated; 5-FU, fluorouracil; DMSO, dimethyl sulfoxide;
ABCG2, ATP-binding cassette sub-family G member 2; CTL,
control. |
Discussion
The present study hypothesized that sorafenib
induced anticancer effects on cancer stem cells by blocking ABCG2
transporters and JNK signaling. It was further assessed whether the
anticancer effects of sorafenib were fortified when combined with
conventionally administered anticancer therapeutic agents, such as
5-FU. In the current study, sorafenib decreased the SP fraction,
while 5-FU increased the SP fraction in two HCC cell lines. A
combination treatment of 5-FU plus sorafenib further decreased the
sphere-forming efficacy of Huh-BAT cells when compared with
sorafenib or 5-FU treatment alone. In addition, the expression of
ABCG2 and JNK signaling molecules was identified to be
downregulated by sorafenib or 5-FU plus sorafenib treatment. These
results indicate that sorafenib induces anticancer effects in SP
cells via the inhibition of JNK signaling and ABCG2 transporters.
Although synergistic anticancer effects on tumor growth rates
following treatment with sorafenib plus 5-FU in HCC cells were not
observed, the results indicate that sorafenib treatment may provide
a novel therapeutic strategy for inducing anticancer effects in
cancer stem cells.
According to the cancer stem cell hypothesis,
initiation, aggressive progression, recurrence, metastasis and drug
resistance are unique properties implicit in cancer stem cells.
Thus, targeting cancer stem cells may present important clinical
implications for the effective treatment of HCC. Current strategies
are focused on targeting rapidly proliferating cancer cells, rather
than cancer stem cells. Treatments may initially appear to be
successful, however often fail to provide a long-lasting cure for
the disease. This may be due to the heterogeneity of certain types
of cancer, which contain different cell lines with varying
sensitivities, or due to failing to eradicate the cancer stem cell
population, leading to disease recurrence and tumor progression
(22).
The current study indicates that, although sorafenib
monotherapy is effective, the patterns of response vary between the
SP and non-SP cell lines. This result may demonstrate the superior
efficacy of sorafenib for treating advanced HCC in the clinical
setting, as it effectively kills cancer stem cells. Lee et
al (23) reported that
treatment using sorafenib plus radiation inhibits growth by
targeting cancer stem cells in breast cancer. Furthermore, Carra
et al (24) reported that
sorafenib induces cell death by targeting glioblastoma stem cells
in human glioblastoma.
In conclusion, sorafenib and conventionally-used
anticancer therapeutic agents inhibit cancer cell growth. However,
treatment with sorafenib alone decreased cancer stem cell numbers,
their sphere-forming efficacy, expression of ABCG2, and JNK
signaling, all of which are involved in drug resistance. These
results indicate that sorafenib is effective as an anticancer
therapeutic agent against HCC cancer stem cells and affects the
signaling pathways involved in drug resistance.
Acknowledgments
The present study was supported by grants from
Ildong Pharmaceutical Co., Ltd. (Seoul, South Korea) (grant no.
S72-06001) and the Han Wha Pharma Co., Ltd. (Seoul, South Korea)
[grant no. 0620141500 (2014-1516)].
References
|
1
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JD, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. New Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lang L: FDA approves sorafenib for
patients with inoperable liver cancer. Gastroenterology.
134:3792008.PubMed/NCBI
|
|
4
|
Kane RC, Farrell AT, Saber H, Tang S,
Williams G, Jee JM, Liang C, Booth B, Chidambaram N, Morse D, et
al: Sorafenib for the treatment of advanced renal cell carcinoma.
Clin Cancer Res. 12:7271–7278. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Min L, He B and Hui L: Mitogen-activated
protein kinases in hepatocellular carcinoma development. Semin
Cancer Biol. 21:10–20. 2011. View Article : Google Scholar
|
|
6
|
Wilhelm SM, Carter C, Tang L, Wilkie D,
McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al:
BAY 43-9006 exhibits broad spectrum oral antitumor activity and
targets the RAF/MEK/ERK pathway and receptor tyrosine kinases
involved in tumor progression and angiogenesis. Cancer Res.
64:7099–7109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scartozzi M, Faloppi L, Svegliati Baroni
G, Loretelli C, Piscaglia F, Iavarone M, Toniutto P, Fava G, De
Minicis S, Mandolesi A, et al: VEGF and VEGFR genotyping in the
prediction of clinical outcome for HCC patients receiving
sorafenib: The ALICE-1 study. Int J Cancer. 135:1247–1256. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu L, Cao Y, Chen C, Zhang X, McNabola A,
Wilkie D, Wilhelm S, Lynch M and Carter C: Sorafenib blocks the
RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor
cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer
Res. 66:11851–11858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xue C, Huang Y, Huang PY, Yu QT, Pan JJ,
Liu LZ, Song XQ, Lin SJ, Wu JX, Zhang JW, et al: Phase II study of
sorafenib in combination with cisplatin and 5-fluorouracil to treat
recurrent or metastatic nasopharyngeal carcinoma. Ann Oncol.
24:1055–1061. 2013. View Article : Google Scholar
|
|
10
|
Petrini I, Lencioni M, Ricasoli M,
Iannopollo M, Orlandini C, Oliveri F, Bartolozzi C and Ricci S:
Phase II trial of sorafenib in combination with 5-fluorouracil
infusion in advanced hepatocellular carcinoma. Cancer Chemother
Pharmacol. 69:773–780. 2012. View Article : Google Scholar
|
|
11
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seki E, Brenner DA and Karin M: A liver
full of JNK: Signaling in regulation of cell function and disease
pathogenesis, and clinical approaches. Gastroenterology.
143:307–320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Telbisz Á, Hegedüs C, Váradi A, Sarkadi B
and Özvegy-Laczka C: Regulation of the function of the human ABCG2
multidrug transporter by cholesterol and bile acids: Effects of
mutations in potential substrate and steroid binding sites. Drug
Metab Dispos. 42:575–585. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoon CH, Kim MJ, Kim RK, Lim EJ, Choi KS,
An S, Hwang SG, Kang SG, Suh Y, Park MJ and Lee SJ: c-Jun
N-terminal kinase has a pivotal role in the maintenance of
self-renewal and tumorigenicity in glioma stem-like cells.
Oncogene. 31:4655–4666. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jia Q, Zhang X, Deng T and Gao J: Positive
correlation of Oct4 and ABCG2 to chemotherapeutic resistance in
CD90(+) CD133(+) liver cancer stem cells.
Cell Reprogram. 15:143–150. 2013.PubMed/NCBI
|
|
17
|
Sukowati CH, Rosso N, Pascut D, Anfuso B,
Torre G, Francalanci P, Crocè LS and Tiribelli C: Gene and
functional upregulation of the BCRP/ABCG2 transporter in
hepatocellular carcinoma. BMC Gastroenterol. 12:1602012. View Article : Google Scholar
|
|
18
|
Sancho R, Nateri AS, de Vinuesa AG,
Aguilera C, Nye E, Spencer-Dene B and Behrens A: JNK signalling
modulates intestinal homeostasis and tumourigenesis in mice. EMBO
J. 28:1843–1854. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mucha SR, Rizzani A, Gerbes AL, Camaj P,
Thasler WE, Bruns CJ, Eichhorst ST, Gallmeier E, Kolligs FT, Göke B
and De Toni EN: JNK inhibition sensitises hepatocellular carcinoma
cells but not normal hepatocytes to the TNF-related
apoptosis-inducing ligand. Gut. 58:688–698. 2009. View Article : Google Scholar
|
|
20
|
Goodell MA: Multipotential stem cells and
'side population' cells. Cytotherapy. 4:507–508. 2002. View Article : Google Scholar
|
|
21
|
Qiu X, Wang Z, Li Y, Miao Y, Ren Y and
Luan Y: Characterization of sphere-forming cells with stem-like
properties from the small cell lung cancer cell line H446. Cancer
Lett. 323:161–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma S: Biology and clinical implications of
CD133(+) liver cancer stem cells. Exp Cell Res.
319:126–132. 2013. View Article : Google Scholar
|
|
23
|
Lee JH, Shim JW, Choi YJ, Heo K and Yang
K: The combination of sorafenib and radiation preferentially
inhibits breast cancer stem cells by suppressing HIF-1α expression.
Oncol Rep. 29:917–924. 2013.PubMed/NCBI
|
|
24
|
Carra E, Barbieri F, Marubbi D, Pattarozzi
A, Favoni RE, Florio T and Daga A: Sorafenib selectively depletes
human glioblastoma tumor-initiating cells from primary cultures.
Cell Cycle. 12:491–500. 2013. View
Article : Google Scholar : PubMed/NCBI
|