Introduction
Diabetic nephropathy (DN) is a chronic microvascular
complication, which affects patients with diabetes. One of the most
common characteristics of DN is diabetic glomerulosclerosis
(1). A previous study demonstrated
that the epithelial-mesenchymal transition (EMT)-like changes
observed in podocytes are associated with podocyte detachment,
which may result in focal glomerulosclerosis (2). Several studies have suggested that
EMT, a process by which differentiated epithelial cells undergo a
phenotypic conversion that gives rise to matrix-producing
fibroblasts and myofibroblasts, may be important in the
pathogenesis of diabetic kidney disease (3,4). The
high glucose (HG)-induced EMT of renal tubular epithelial cells is
a key process in glomerulosclerosis and, mediated by factors,
including E-cadherin and α-smooth muscle actin (α-SMA), epithelial
cells may lose their epithelial characteristics and gain
mesenchymal cell properties (5,6).
However, the process of HG-induced EMT remains to be fully
elucidated.
Procyanidins are the most abundant polyphenols
present in red wine, and they are also present in cereals, fruits,
chocolate and tea (7). Several
epidemiological studies have demonstrated that procyanidin B2 can
inhibit hepatic and cardiac fibrosis (8,9).
Based on the possible anti-fibrotic activity of procyanidins, the
present study investigated whether procyanidins inhibit HG-induced
EMT in the early stage of diabetic kidney glomerulosclerosis.
The transforming growth factor (TGF)-β/small mothers
against decapentaplegic (Smad) and mitogen-activated protein kinase
(MAPK)/P38 signaling pathways are involved in promoting EMT and are
associated with the development of DN (10,11).
The aim of the present study was to determine the role of the
MAPK/P38 and TGF-β/Smad signaling pathways in HG-induced EMT, and
to examine the changes in the two pathways in HK-2 cells cultured
with procyanidin B2. The detailed investigation of this plant
extract may provide a novel therapeutic strategy for the treatment
of DN.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM),
penicillin-streptomycin (5,000 U/ml penicillin; 5,000 U/ml
streptomycin) and fetal bovine serum (FBS) were purchased from
Gibco Life Technologies (Grand Island, NY, USA). D-glucose
(analytical grade) was purchased from Guanghua Chemical Factory
Co., Ltd. (Guangdong, China). Procyanidin B2 and Triton X-100 were
purchased from Sigma-Aldrich (St. Louis, MO, USA). The
anti-β-actin, anti-P38, anti-phosphorylated (p)-P38 and anti-p-Smad
2, 3 and 7 antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). The anti-fibronectin (FN), anti-α-SMA,
anti-E-cadherin and anti-vimentin antibodies were purchased from BD
Biosciences (Franklin Lakes, NJ, USA). An enhanced
chemiluminescence (ECL) kit was purchased from Pierce
Biotechnology, Inc. (Rockford, IL, USA). All reagents used were
trace element analytical grade and all water used was glass
distilled.
Cell culture
The HK-2 human renal proximal tubular epithelial
cell line (CRL-2190; American Type Culture Collection, Danvers, MA,
USA) was cultured at a density of 1×105 cells/ml in DMEM
supplemented with 5.5 mmol/l D-glucose (normal glucose; NG) and 10%
FBS at 37°C in a humidified atmosphere containing 5%
CO2. The cells were subcultured every 3 days using 0.2%
trypsin (Sigma-Aldrich) with 0.02% EDTA (Sigma-Aldrich). The near
confluent HK-2 cells (80%) were subsequently transferred into
serum-free DMEM for overnight starvation prior to each experiment.
In order to induce EMT, the cells were cultured in high glucose
(HG) medium containing 60 mmol/l D-glucose for 48 h at 37°C. The
concentration was selected based on previous studies (12,13).
To study the protective effects of procyanidin B2, HK-2 cells were
incubated with 10 µM procyanidin B2 at 37°C in a humidified
atmosphere for 48 h.
ELISA
The protein expression levels of TGF-β were measured
using a TGF-β ELISA kit (R&D Systems, Minneapolis, MN, USA).
Briefly, the HK-2 cells were seeded at a density of
4×105 cells/well into a 6-well plate and cultured for 24
h at 37°C. The supernatants were collected from the HK-2 cell
cultures for ELISA. The secreted protein concentration of TGF-β per
105 cells was measured and calculated from the standard
curve using the ELISA kit. Briefly, 100 µl samples were
added to sample diluent and incubated for 1 h at 37°C with
agitation, following washing with washing buffer (Sigma-Aldrich).
Enzyme-conjugated secondary antibody was added to the wells and
incubated for 2 h at 37°C prior to addition of the substrate
solution. Absorbance was measured using an ELISA reader (Multiskan
MK3; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at a
wavelength of 450 nm.
Western blot analysis
The cells were collected and washed with
phosphate-buffered saline (PBS) prior to being lysed on ice for 30
min in lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China) containing 50 mM Tris (pH 8.1), 1% SDS, sodium
pyrophosphate, β-glycerophosphate, sodium orthovanadate, sodium
fluoride, EDTA, and leupeptin, and centrifuged at 15,000 x g for 30
min at room temperature. The supernatants were collected, and the
protein concentration was determined using a Bicinchoninic Acid
Protein Assay kit (Pierce Biotechnology, Inc.). The protein were
boiled for 5 min and 10 µg total protein was loaded into the
appropriate well to be separated by 10% SDS-PAGE (Beyotime
Institute of Biotechnology). The proteins on the gel were then
transferred onto a nitrocellulose membranes (EMD Millipore,
Billerica, MA, USA) using a Bio-Rad A101441 apparatus (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) for 2 h at 4°C and 100 V.
The protein-bound membranes were then blocked and washed in
Tris-buffered saline with 20% Tween 20 (Sigma-Aldrich). The
nitrocellulose membranes were cut according to the molecular weight
of the protein, and were incubated with antibodies. The following
primary antibodies were used: Anti-β-actin mouse monoclonal IgG1
(1:400; cat. no. sc-8432; Santa Cruz Biotechnology, Inc.; 24 h
incubation at 4°C), anti-α-SMA goat polyclonal IgG (1:400; cat. no.
sc-324317; Santa Cruz Biotechnology, Inc.; 24 h incubation at 4°C),
anti-E-cadherin mouse monoclonal IgG1 (1:400; cat. no. sc-52327;
Santa Cruz Biotechnology, Inc.; 24 h incubation at 4°C), anti-FN
mouse monoclonal IgG1 (1:400; cat. no. sc-52331; Santa Cruz
Biotechnology, Inc.; 24 h incubation at 4°C), anti-vimentin mouse
monoclonal IgG1 (1:400; cat. no. sc-373717; Santa Cruz
Biotechnology, Inc.; 24 h incubation at 4°C), anti-P38 mouse
monoclonal IgG1 (1:400; cat. no. sc-33688; Santa Cruz
Biotechnology, Inc.; 24 h incubation at 4°C), anti-p-P38 mouse
monoclonal IgG1 (1:400; cat. no. sc-7973; Santa Cruz Biotechnology,
Inc.; 24 h incubation at 4°C), anti-p-Smad 2 mouse monoclonal IgG1
(1:400; cat. no. sc-393312; Santa Cruz Biotechnology, Inc.; 24 h
incubation at 4°C), anti-p-Smad 3 mouse monoclonal IgG1 (1:400;
cat. no. sc-101154; Santa Cruz Biotechnology, Inc.; 24 h incubation
at 4°C) and anti-p-Smad 7 mouse monoclonal IgG1 (1:400; cat. no.
sc-365846; Santa Cruz Biotechnology, Inc.; 24 h incubation at 4°C).
The membranes were then incubated with goat anti-mouse IgG2a-B
peroxidase-conjugated secondary antibodies (1:400; cat. no.
sc-2073; Santa Cruz Biotechnology, Inc.; 24 h incubation at 4°C).
The blots were visualized using an ECL kit (Pierce Biotechnology,
Inc.) and the relative quantities of the proteins were analyzed.
The results were quantified using Quantity One software V4.62
(Bio-Rad Laboratories, Inc.).
Fluorescence microscopy
The cells (1×105 cells/ml) were washed
once with ice-cold PBS and fixed with 4% paraformaldehyde
(Sigma-Aldrich) for 30 min at 4°C. Following being washed three
times with PBS, the cells were incubated with 1% Triton X-100 for
10 min. The cells were blocked at nonspecific antibody binding
sites by incubating in PBS containing 0.3% Triton X-100 and 0.5%
bovine serum albumin (Sigma-Aldrich) for 30 min at room
temperature. The cells were subsequently incubated with an antibody
targeting E-cadherin or α-SMA (1:200) overnight at room
temperature. Subsequently, the cells were incubated with a
fluorescein isothiocyanate- or tetramethylrhodamine-conjugated
immunoglobulin G antibody (1:100 in PBS) for 0.5 h at room
temperature. Hoechst 33342 (10 µg/ml; Sigma-Aldrich) was
then added to the cells for 15 min at room temperature. Following
three washes with PBS, the cells were visualized under a
fluorescence microscope (Olympus SZ51; Olympus Corporation, Tokyo,
Japan).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Variance was homogenous enabling use of the standard
one-way analysis of variance (ANOVA) methodology. On establishment
of statistical significance using ANOVA, individual comparisons
were made using Tukey's multiple comparison test. P<0.05 was
considered to indicate a statistically significant difference.
Statistical analyses were performed using SPSS version 18 (SPSS,
Inc., Chicago, IL, USA).
Results
HG induces HK-2 cells to undergo EMT
To determine whether HG induced EMT, the HK-2 cells
were incubated in NG (5.5 mmol/l D-glucose) or HG (60 mmol/l
D-glucose) conditions. Initially, exposure of the HK-2 cells to HG
for 48 h decreased the protein expression levels of E-cadherin and
increased the expression levels of α-SMA, compared with the NG
group, determined by immunofluorescence (Fig. 1A). In addition, HG-induced EMT was
confirmed by western blotting, which indicated the upregulation of
α-SMA, FN and vimentin and the downregulation of E-cadherin
(Fig. 1B). These results suggested
that an HG environment activated the EMT process in the HK-2
cells.
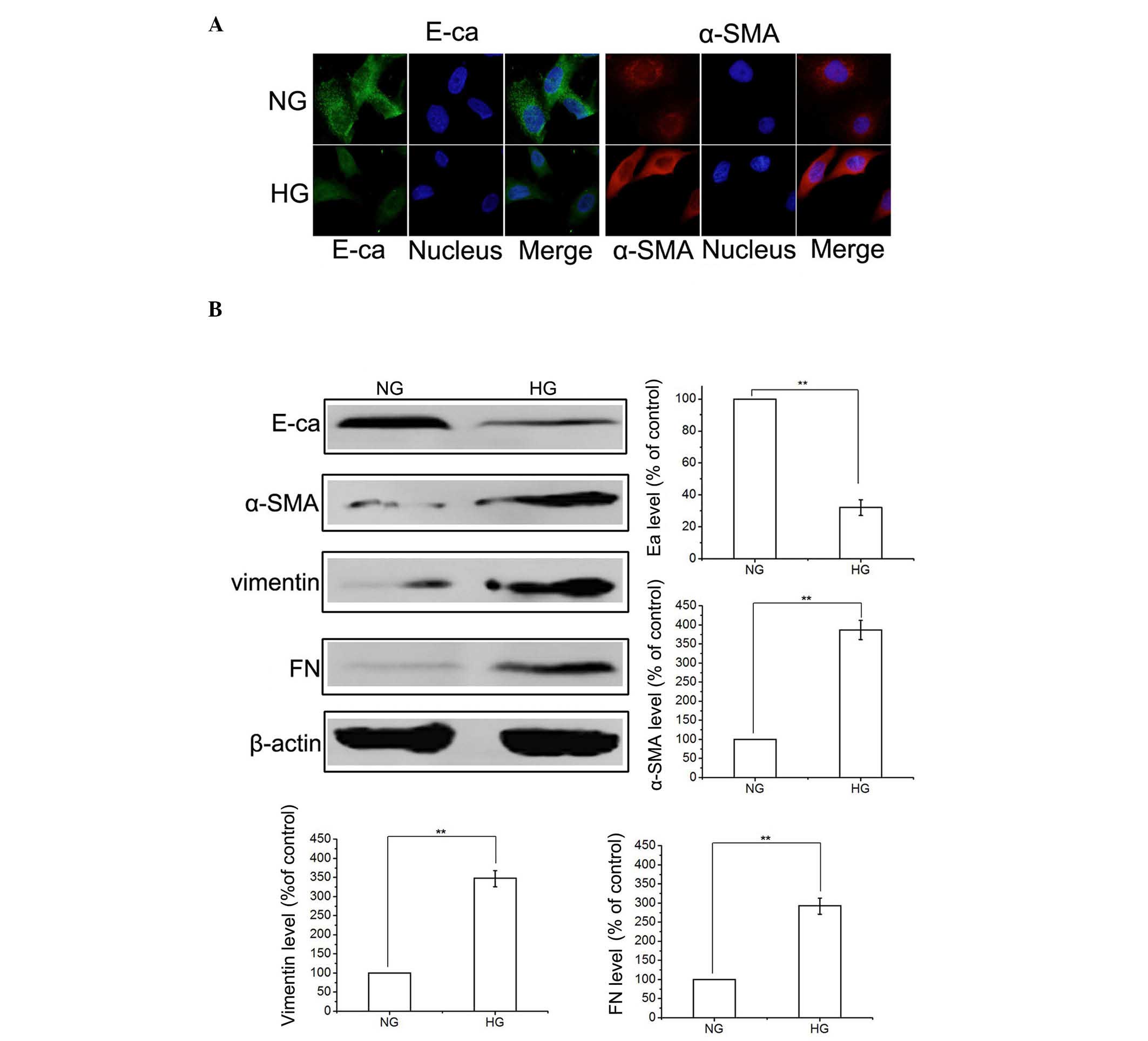 | Figure 1HG promotes epithelial-to-mesenchymal
transition in HK-2 cells. (A) HK-2 cells were incubated in NG or HG
conditions for 48 h, and the expression levels of E-ca and (α-SMA)
were detected using immunofluorescence. (B) Cells were treated, as
described, and the expression levels of E-ca, α-SMA, FN and
vimentin were detected using western blotting. The results are
representative of three independent experiments. β-actin was used
as a loading control. Data are expressed as the mean ± standard
error of the mean. **P<0.01, vs. NG group. HG, high
glucose; NG, normal glucose; E-ca, E-cadherin; α-SMA, α-smooth
muscle actin; FN, fibronectin. |
Effects of procyanidin B2 on the
expression of EMT-associated proteins in HG-induced HK-2 cells
To determine the effects of procyanidin B2 in EMT,
the HK-2 cells were treated with or without 60 mmol/l D-glucose for
48 h, in the presence or absence of procyanidin B2. The expression
levels of FN, E-cadherin, vimentin and α-SMA were detected using
western blotting. As shown in Fig.
2, exposure of the HK-2 cells to HG for 48 h decreased the
protein expression levels of E-cadherin and increased the
expression levels of FN, α-SMA and vimentin, compared with the NG
group. However, HG-induced EMT was attenuated when the HK-2 cells
were pre-treated with 10 µM procyanidin B2, which was
indicated by the reduced expression levels of FN, α-SMA and
vimentin, and the increased expression of E-cadherin (Fig. 2). The concentration of procyanidin
B2 was selected based on a previous study (14). These results suggested that
procyanidin B2 supplementation reversed HG-induced EMT in the HK-2
cells.
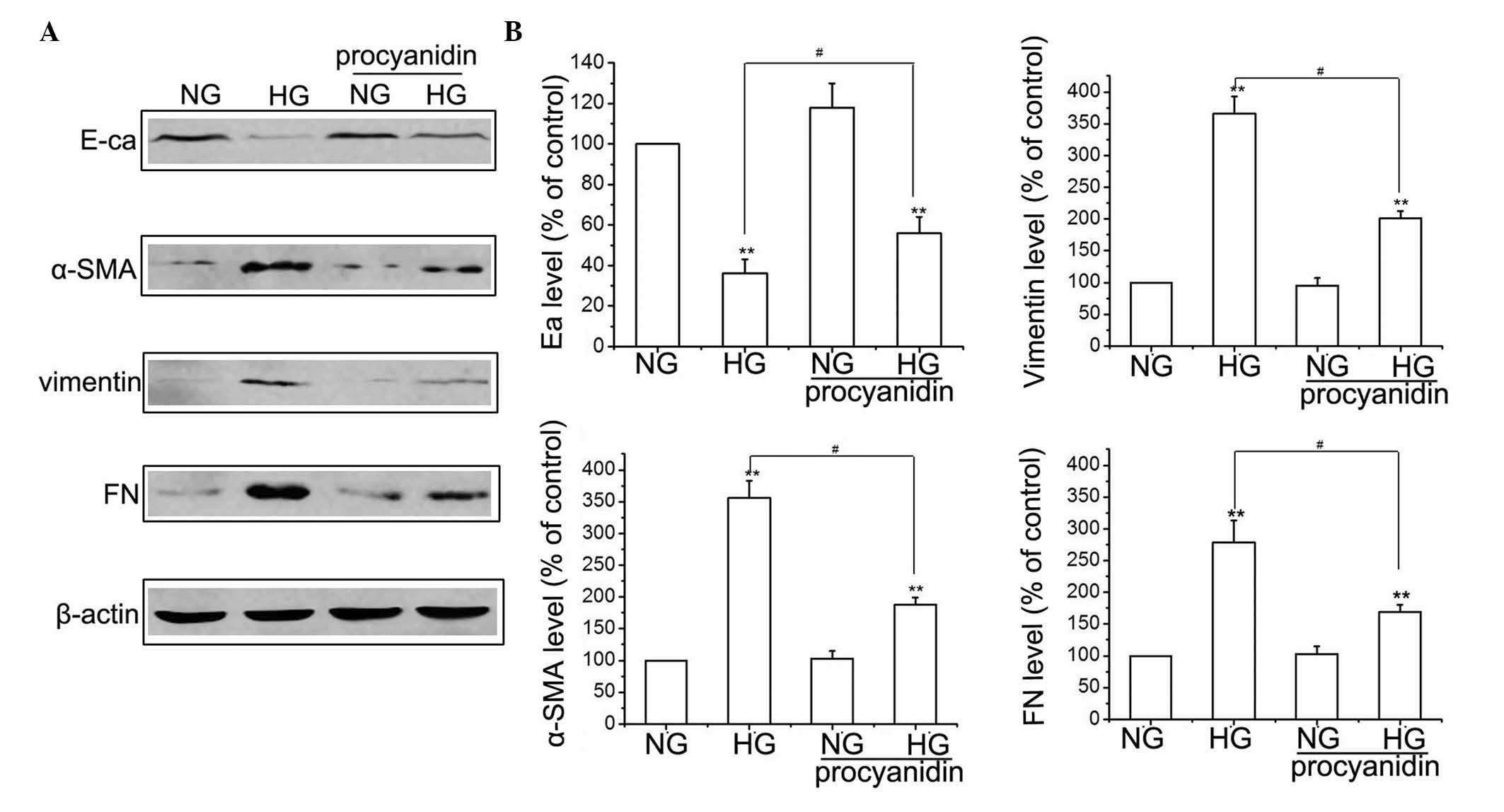 | Figure 2Procyanidin B2 inhibits HG-induced
epithelial-to-mesenchymal transition in HK-2 cells. (A) HK-2 cells
were incubated in NG or HG conditions in the presence or absence of
procyanidin B2 for 48 h, and the expression levels of E-ca, α-SMA,
FN and vimentin were detected using western blotting. (B) Results
are representative of three independent experiments. β-actin was
used as a loading control. Data are expressed as the mean ±
standard error of the mean (**P<0.01, vs. NG group;
#P<0.01, HG vs. HG+procyanidin B2 group). HG, high
glucose; NG, normal glucose; E-ca, E-cadherin; α-SMA, α-smooth
muscle actin; FN, ibronectin. |
Effects of procyanidin B2 on the
expression of TGF-β/Smad in HK-2 cells
The TGF-β/Smads signaling pathway is considered to
contribute to the development of DN by increasing
glomerulosclerosis and inducing EMT (15,16).
To determine the effects of procyanidin B2 on the expression of
TGF-β in HG-treated HK-2 cells, the protein expression of TGF-β was
measured using ELISA (Fig. 3A).
Compared with the NG group, the expression of TGF-β was
significantly higher in the HG-treated group; however, treatment
with procyanidin B2 reduced the HG-induced expression of TGF-β.
Furthermore, the effects of procyanidin B2 on HG-induced expression
of Smads in the HK-2 cells were examined using western blotting. HG
treatment resulted in a substantial increase in the expression
levels of p-Smad2 and p-Smad3, and decreased the protein expression
of p-Smad7 in the HK-2 cells. However, procyanidin B2 pre-treatment
significantly attenuated HG-induced expression of p-Smad2 and
p-Smad3, and increased the expression of p-Smad7 in the HK-2 cells
(Fig. 3B–E). These results
indicated that procyanidin B2 inhibited HG-induced EMT via the
TGF-β/Smads signaling pathway.
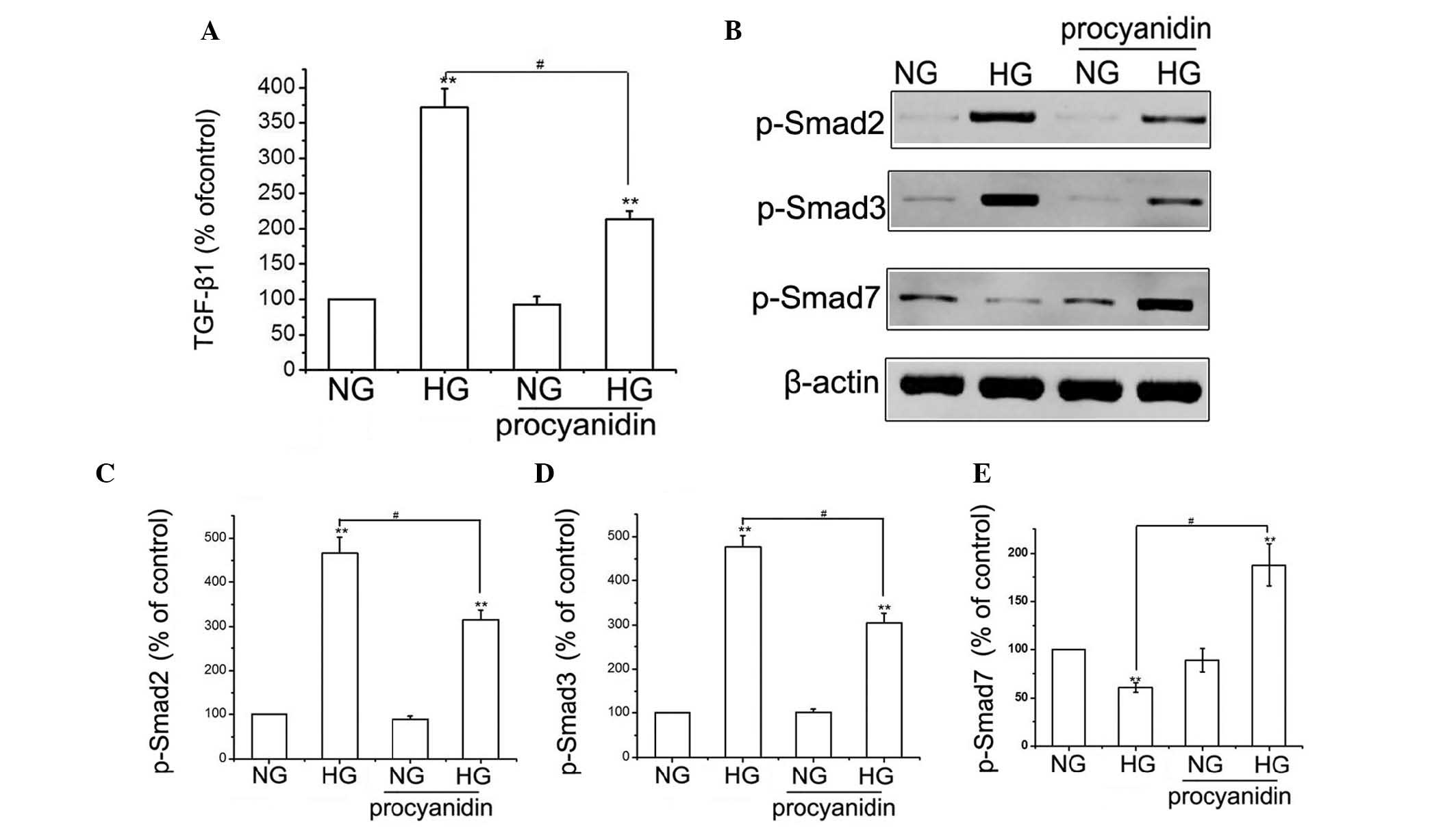 | Figure 3Procyanidin B2 inhibits HG-induced
epithelial-to-mesenchymal transition via the TGF-β/Smads signaling
pathway in HK-2 cells. (A) HK-2 cells were incubated in NG or HG
conditions in the presence or absence of procyanidin B2 for 48 h,
and the protein expression levels of TGF-β were measured using
ELISA. The results are representative of three independent
experiments. **P<0.01, vs. NG group;
#P<0.01, HG vs. HG+procyanidin B2 group. (B) Cells
were treated, as described, and the expression levels of p-Smad2, 3
and 7 were detected using western blotting. (C–E) Results are
representative of three independent experiments. β-actin was used
as a loading control. **P<0.01, vs. NG group;
#P<0.01, HG vs. HG+procyanidin B2 group. Data are
expressed as the mean ± standard error of the mean. HG, high
glucose; NG, normal glucose; TGF, transforming growth factor; Smad,
small mothers against decapentaplegic; p-, phosphorylated. |
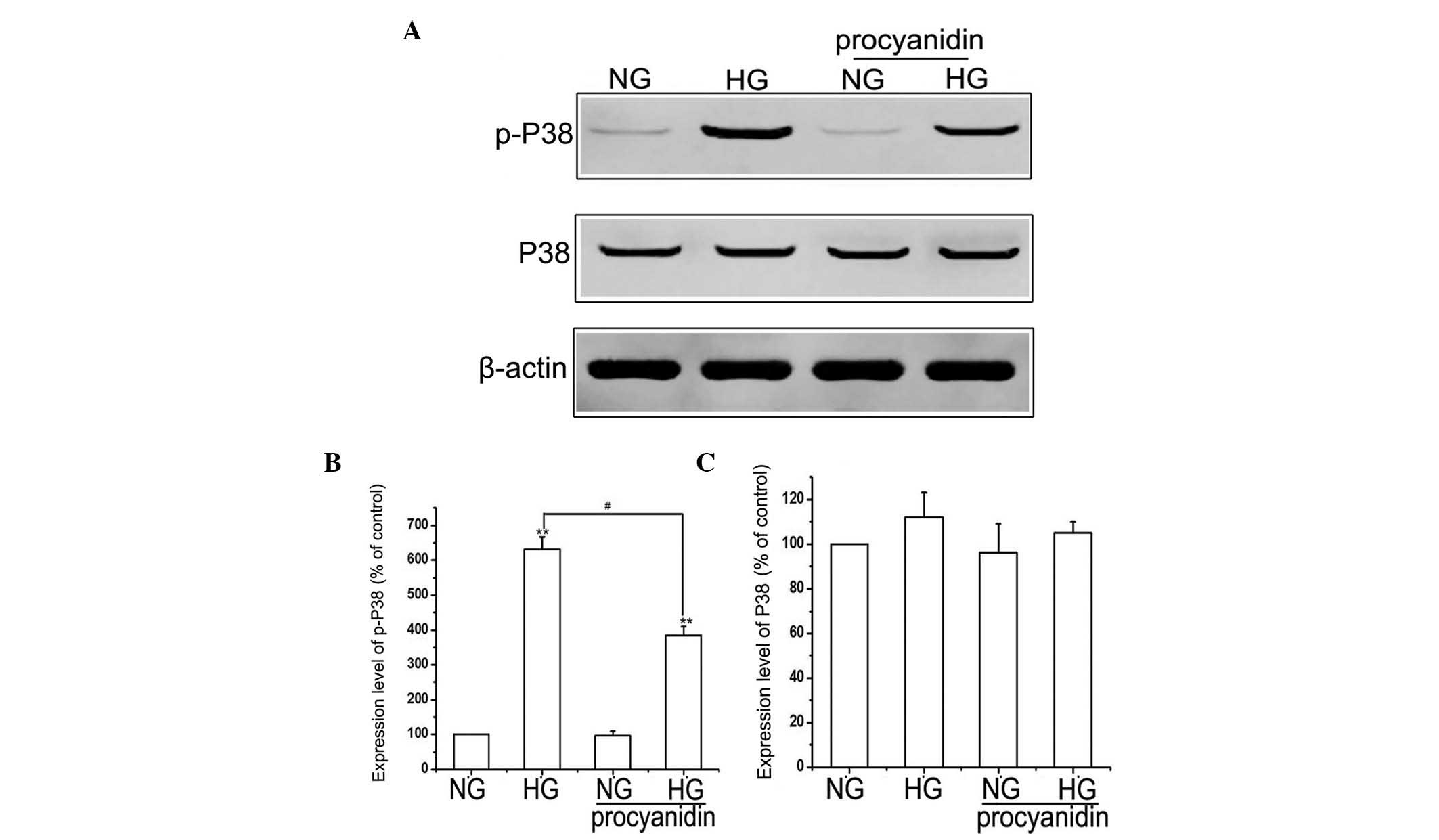
Effects of procyanidin B2 on the
expression of MAPK/P38 in HK-2 cells
The MAPK/P38 signaling pathway has previously been
reported to be involved in EMT (17); however, whether procyanidin B2
exerts its effect on EMT in HK-2 cells via this pathway remains to
be elucidated. Therefore, the present study examined the effect of
procyanidin B2 supplementation on the MAPK/P38 pathway. Compared
with the NG group, the expression of p-P38 was upregulated by HG,
whereas the expression levels of total P38 were not significantly
altered. Notably, pre-treatment of the HK-2 cells with procyanidin
B2 significantly inhibited the HG-induced expression of p-P38.
However, no significant difference was observed in the expression
of total P38 in the HG+procyanidin B2 group, compared with the HG
group (Fig. 4). These results
suggested that procyanidin B2 may be involved in HG-induced EMT via
regulation of the MAPK/P38 pathway.
Discussion
The pathogenesis of DN is the result of several
factors, and insulin metabolism disorder as a result of long-term
high blood sugar is considered the predominant cause of DN
(18). Renal hemodynamic changes
caused by high blood sugar, and the series of consequences of
abnormal glucose metabolism are the basis of kidney disease
(19). HG-induced EMT is an
important process, leading to glomerulosclerosis and
tubulointerstitial fibrosis (5).
EMT is described as the loss of epithelial characteristics from
epithelial cells, and the acquisition of properties typical of
mesenchymal cells. EMT facilitates cell movement and the generation
of novel tissue types during development, and also contributes to
the pathogenesis of disease (20,21).
The characteristics of EMT essential to wound healing also link EMT
to organ fibrosis, including pulmonary, renal and hepatic fibrosis
(20). A previous study
demonstrated that tubular epithelium can transdifferentiate into
fibroblasts via the process of EMT, which is regarded as an
important event in the pathogenesis of tubulointerstitial fibrosis
(22). The findings of the present
study were consistent with these previous results. HG-induced EMT
was confirmed using western blotting, which detected the
upregulation of α-SMA, FN and vimentin, and the downregulation of
E-cadherin, associated with a transition from the epithelial
phenotype of HK-2 cells to a myofibroblastic phenotype.
Proanthocyanidins are a class of substances, which
are linked by catechin, epicatechin and gallate (23). Procyanidin B2 is a biologically
active component of grape seeds, which has been reported to possess
various pharmacological and biochemical effects (24). The effects of procyanidin B2 on
various types of cancer have been reported (25,26);
however, the effects of procyanidin B2 on EMT in human renal
proximal tubular epithelial cells remain to be fully elucidated.
The present study investigated the effects of procyanidin B2 on
HG-induced EMT, and examined the underlying mechanisms. The
occurrence of oxidative stress is key in EMT (27), and procyanidin B2, which is present
in grape seeds, apples and cacao beans, has antioxidant properties
(28). Therefore, the present
study hypothesized that procyanidin B2 may have an inhibitory
effect on EMT. The results of the present study demonstrated that
procyanidin B2 treatment provided effective protection against
HG-induced EMT, evidenced by a decrease in the upregulation of
vimentin and α-SMA, and the amelioration of E-cadherin in the human
renal proximal tubular epithelial cell line (Fig. 2).
TGF-β has been described as a ʻmaster switchʼ
regulating EMT, and has been demonstrated to signal primarily via
the Smad 2/3 pathway (29). Smad7
is crucial in antagonizing EMT induced by TGF-β signaling (30). The present study demonstrated that
procyanidin B2 pre-treatment significantly attenuated HG-induced
EMT by directly downregulating p-Smad2 and 3, and indirectly
upregulating p-smad7; accompanied by a decrease in the upregulation
of FN, α-SMA and vimentin, and an increase in the downregulation of
E-cadherin. The downstream effects of TGF-β may not only be as a
consequence of the Smads signaling pathway, but may be the result
of multiple signaling pathways, acting to modulate the effects of
TGF-β signaling. Activation of the MAPK/P38 signaling pathway is
not exclusive to TGF-β and may be activated to induce EMT (31). The present study also demonstrated
that procyanidin B2 significantly inhibited the HG-induced
expression of p-P38 and weakened HG-induced EMT.
In conclusion, the results of the present study
demonstrated that procyanidin B2 inhibited HG-induced EMT, most
likely via inhibition of the expression of TGF-β, p-Smad2 and 3, as
well as the P38/MAPK signaling pathway, in HK-2 cells. The
identification of procyanidin B2 as an inhibitor of HG-induced EMT
represents an important finding. Further investigations may examine
this target for limiting EMT and for the treatment of DN.
Abbreviations:
|
HG
|
high glucose
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FITC
|
fluorescein isothiocyanate
|
|
RIPA
|
radioimmunoprecipitation assay
|
|
TGF
|
transforming growth factor
|
|
EMT
|
epithelial-mesenchymal transition
|
|
SMA
|
smooth muscle cell actin, ROS,
reactive oxygen species
|
|
MAPK
|
mitogen-activated protein kinase
|
|
DN
|
diabetic nephropathy
|
|
PBS
|
phosphate-buffered saline
|
Acknowledgments
The present study was supported by the Military
Logistics Research Programs of China (grant no. CJN10L075) and by
the China Postdoctoral Science Foundation (grant no.
2012M512175).
References
|
1
|
Zhao HL, Lai FM, Tong PC, Tomlinson B and
Chan JC: Clinicopathologic characteristics of nodular
glomerulosclerosis in Chinese patients with type 2 diabetes. Am J
Kidney Dis. 44:1039–1049. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Samejima K, Nakatani K, Suzuki D, Asai O,
Sakan H, Yoshimoto S, Yamaguchi Y, Matsui M, Akai Y, Toyoda M, et
al: Clinical significance of fibroblast-specific protein-1
expression on podocytes in patients with focal segmental
glomerulosclerosis. Nephron Clin Pract. 120:c1–c7. 2012. View Article : Google Scholar
|
|
3
|
Liu Y: New insights into
epithelial-mesenchymal transition in kidney fibrosis. J Am Soc
Nephrol. 21:212–222. 2010. View Article : Google Scholar
|
|
4
|
Carew RM, Wang B and Kantharidis P: The
role of EMT in renal fibrosis. Cell Tissue Res. 347:103–116. 2012.
View Article : Google Scholar
|
|
5
|
Li J, Kang SW, Kim JL, Sung HY, Kwun IS
and Kang YH: Isoliquiritigenin entails blockade of TGF-beta1-SMAD
signaling for retarding high glucose-induced mesangial matrix
accumulation. J Agric Food Chem. 58:3205–3212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang L, Li H, Gou R, Cheng G, Guo Y, Fang
Y and Chen F: Endothelin-1 mediated high glucose-induced
epithelial-mesenchymal transition in renal tubular cells. Diabetes
Res Clin Pract. 104:176–182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quesada IM, Del Bas JM, Bladé C, Ardèvol
A, Blay M, Salvadó MJ, Pujadas G, Fernández-Larrea J and Arola L:
Grape seed procyanidins inhibit the expression of metallothione in
genes in human HepG2 cells. Genes Nutr. 2:105–109. 2007. View Article : Google Scholar
|
|
8
|
Palfi A, Bartha E, Copf L, Mark L, Gallyas
F Jr, Veres B, Kalman E, Pajor L, Toth K, Ohmacht R and Sumegi B:
Alcohol-free red wine inhibits isoproterenol-induced cardiac
remodeling in rats by the regulation of Akt1 and protein kinase C
alpha/beta II. J Nutr Biochem. 20:418–425. 2009. View Article : Google Scholar
|
|
9
|
Choi JH, Hwang YP, Choi CY, Chung YC and
Jeong HG: Anti-fibrotic effects of the anthocyanins isolated from
the purple-fleshed sweet potato on hepatic fibrosis induced by
dimethylnitrosamine administration in rats. Food Chem Toxicol.
48:3137–3143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodrigues-Diez R, Rodrigues-Diez RR, Lavoz
C, Carvajal G, Droguett A, Garcia-Redondo AB, Rodriguez I, Ortiz A,
Egido J, Mezzano S and Ruiz-Ortega M: Gremlin activates the Smad
pathway linked to epithelial mesenchymal transdifferentiation in
cultured tubular epithelial cells. Biomed Res Int. 2014:8028412014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Antoon JW, Nitzchke AM, Martin EC, Rhodes
LV, Nam S, Wadsworth S, Salvo VA, Elliott S, Collins-Burow B,
Nephew KP and Burow ME: Inhibition of p38 mitogen-activated protein
kinase alters microRNA expression and reverses
epithelial-to-mesenchymal transition. Int J Oncol. 42:1139–1150.
2013.PubMed/NCBI
|
|
12
|
Rodrigues-Díez R, Carvajal-González G,
Sánchez-López E, Rodríguez-Vita J, Rodrigues Díez R, Selgas R,
Ortiz A, Egido J, Mezzano S and Ruiz-Ortega M: Pharmacological
modulation of epithelial mesenchymal transition caused by
angiotensin II. Role of ROCK and MAPK pathways. Pharm Res.
25:2447–2461. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu L, Gao Q, Ni L, Wang M and Shen F:
Fasudil inhibits epithelial-myofibroblast transdifferentiation of
human renal tubular epithelial HK-2 cells induced by high glucose.
Chem Pharm Bull (Tokyo). 61:688–694. 2013. View Article : Google Scholar
|
|
14
|
Cai Q, Li BY, Gao HQ, Zhang JH, Wang JF,
Yu F, Yin M and Zhang Z: Grape seed procyanidin b2 inhibits human
aortic smooth muscle cell proliferation and migration induced by
advanced glycation end products. Biosci Biotechnol Biochem.
75:1692–1697. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
O'Kane D, Jackson MV, Kissenpfennig A,
Spence S, Damkat-Thomas L, Tolland JP, Smyth AE, Denton CP, Stuart
Elborn J, McAuley DF and O'Kane CM: SMAD inhibition attenuates
epithelial to mesenchymal transition by primary keratinocytes in
vitro. Exp Dermatol. 23:497–503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Islam SS, Mokhtari RB, El Hout Y, Azadi
MA, Alauddin M, Yeger H and Farhat WA: TGF-β1 induces EMT
reprogramming of porcine bladder urothelial cells into collagen
producing fibroblasts-like cells in a Smad2/Smad3-dependent manner.
J Cell Commun Signal. 8:39–58. 2014. View Article : Google Scholar :
|
|
17
|
Antoon JW, Nitzchke AM, Martin EC, Rhodes
LV, Nam S, Wadsworth S, Salvo VA, Elliott S, Collins-Burow B,
Nephew KP and Burow ME: Inhibition of p38 mitogen-activated protein
kinase alters microRNA expression and reverses
epithelial-to-mesenchymal transition. Int J Oncol. 42:1139–1150.
2013.PubMed/NCBI
|
|
18
|
Yeh WJ, Yang HY and Chen JR: Soy
β-conglycinin retards progression of diabetic nephropathy via
modulating the insulin sensitivity and angiotensin-converting
enzyme activity in rats fed with high salt diet. Food Funct.
5:2898–2904. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Filippone EJ, Gupta A and Farber JL:
Normoglycemic diabetic nephropathy: The role of insulin resistance.
Case Rep Nephrol Urol. 4:137–143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kothari AN, Mi Z, Zapf M and Kuo PC: Novel
clinical therapeutics targeting the epithelial to mesenchymal
transition. Clin Transl Med. 3:352014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Srivastava SP, Koya D and Kanasaki K:
MicroRNAs in kidney fibrosis and diabetic nephropathy: Roles on EMT
and EndMT. Biomed Res Int. 2013:1254692013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fintha A, Gasparics Á, Fang L, Erdei Z,
Hamar P, Mózes MM, Kökény G, Rosivall L and Sebe A:
Characterization and role of SCAI during renal fibrosis and
epithelial-to-mesenchymal transition. Am J Pathol. 182:388–400.
2013. View Article : Google Scholar
|
|
23
|
Rinaldi A, Jourdes M, Teissedre PL and
Moio L: A preliminary characterization of Aglianico (Vitis vinifera
L. cv) grape proanthocyanidins and evaluation of their reactivity
towards salivary proteins. Food Chem. 164:142–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Agarwal C, Veluri R, Kaur M, Chou SC,
Thompson JA and Agarwal R: Fractionation of high molecular weight
tannins in grape seed extract and identification of procyanidin
B2-3,3′-di-O-gallate as a major active constituent causing growth
inhibition and apoptotic death of DU145 human prostate carcinoma
cells. Carcinogenesis. 28:1478–1484. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chou SC, Kaur M, Thompson JA, Agarwal R
and Agarwal C: Influence of gallate esterification on the activity
of procyanidin B2 in androgen-dependent human prostate carcinoma
LNCaP cells. Pharm Res. 27:619–627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Avelar MM and Gouvêa CM: Procyanidin b2
cytotoxicity to mcf-7 human breast adenocarcinoma cells. Indian J
Pharm Sci. 74:351–355. 2012. View Article : Google Scholar
|
|
27
|
Liu J, Zeng L, Zhao Y, Zhu B, Ren W and Wu
C: Selenium suppresses lipopolysaccharide-induced fibrosis in
peritoneal mesothelial cells through inhibition of
epithelial-to-mesenchymal transition. Biol Trace Elem Res.
161:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sakano K, Mizutani M, Murata M, Oikawa S,
Hiraku Y and Kawanishi S: Procyanidin B2 has anti- and pro-oxidant
effects on metal-mediated DNA damage. Free Radic Biol Med.
39:1041–1049. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Borthwick LA, Gardner A, De Soyza A, Mann
DA and Fisher AJ: Transforming growth factor-β1 (TGF-β1) driven
epithelial to mesenchymal transition (EMT) is accentuated by tumour
necrosis factor α (TNFα) via crosstalk between the SMAD and NF-κB
pathways. Cancer Microenviron. 5:45–57. 2012. View Article : Google Scholar
|
|
30
|
Tsai TH, Sun MH, Ho TC, Ma HI, Liu MY and
Tsao YP: Notch prevents transforming growth factor-beta-assisted
epithelial-mesenchymal transition in cultured limbal progenitor
cells through the induction of Smad7. Mol Vis. 20:522–534.
2014.PubMed/NCBI
|
|
31
|
Chen HH, Zhou XL, Shi YL and Yang J: Roles
of p38 MAPK and JNK in TGF-β1-induced human alveolar epithelial to
mesenchymal transition. Arch Med Res. 44:93–98. 2013. View Article : Google Scholar : PubMed/NCBI
|