Introduction
Paraquat (PQ) is a widely used non-selective
herbicide. It is highly toxic upon uptake into the human body, with
the lungs being its major target organ (1–3).
Paraquat poisoning has a high mortality rate due to the lack of
effective treatments and antidotes.
The lung is the main organ of PQ accumulation
(4). PQ-induced pulmonary toxicity
causes pulmonary edema, hemorrhage, interstitial inflammation and
damage to the alveolar epithelium, which may progress to severe
fibrosis (5,6). The most common cause of mortality
from PQ poisoning is respiratory failure due to pulmonary fibrosis
(7).
Although PQ is known to induce pulmonary fibrosis,
the pathogenic mechanism of PQ-induced pulmonary fibrosis has
remained to be fully elucidated. Recent studies have demonstrated
the role of the epithelial-to-mesenchymal transition (EMT) in the
pathogenesis of pulmonary fibrosis (8–10).
However, whether EMT occurs in PQ-induced pulmonary fibrosis has
remained elusive. Therefore, the present study was designed to
investigate the involvement of EMT in pulmonary fibrosis caused by
paraquat. Furthermore, the participation of the transforming growth
factor (TGF)-β/Smad pathway, which is known to induce EMT and
fibrosis, was examined to clarify the underlying mechanisms of
PQ-induced EMT in vitro.
Materials and methods
Animal model of PQ-induced pulmonary
fibrosis
A total of 32 adult male Sprague-Dawley rats
weighing 200–250 g, aged 6–8 weeks were provided by the Laboratory
Animal Center, Academy of Military Medical Sciences (Beijing,
China). The rats were housed in individual cages with free access
to food and fresh water, and under conditions of a 12 h/12 h
light/dark cycle and a controlled temperature (22–24°C). The
experimental animal protocol of the present study was approved by
the Ethics Committee of Tianjin First Center Hospital (Tianjin,
China) and followed the national and institutional guidelines for
animal experiments. Paraquat was purchased from Sigma-Aldrich (St.
Louis, MO, USA). The rats in the control group (n=8) were
administered with saline. Rats in the treatment group (n=24) were
intraperitoneally injected with PQ at a dose of 15 mg/kg per rat.
On days 7, 14 and 21 following PQ treatment, the rats were
anesthetized by intraperitoneal injection of pentobarbital (20
mg/kg). The lungs were excised and immediately frozen in liquid
nitrogen.
Cell culture
The A549 lung cancer cell line were purchased from
the American Type Culture Collection (Manassas, VA, USA). Cells
were cultured in Dulbecco's modified Eagle's medium (Invitrogen;
Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal
bovine serum (Invitrogen) at 37°C in a humidified atmosphere with
5% CO2. PQ was diluted with saline solution to obtain
various final concentrations (5, 10, 25, 50 and 100 μM),
with which A549 cells were treated for 24 h.
Transfection
To knockdown the expression of TGF-β1, small hairpin
(sh)RNA specific for TGF-β1 was constructed and transfected into
A549 cells using Lipofectamine™ 2000 (Invitrogen) according to the
manufacturer's instructions. The sequences of
shRNAwereasfollows:Sense5′-CACCCAGCACGTGGAGCTG
TACCAGAAATTTCAAGAGAATTTCTGGTACAGCT CCACGTGCTGTTTTTTG-3′ and
antisense 5′-GATCCAAAAAACAGCACGTGGAGCTGTACCAGAAA
TTCTCTTGAAAGGGCTGGTACAG CTCCACGTGCTG-3′. The shRNA were synthesized
by GenePharma Co., Ltd. (Shanghai, China). Prior to transfection,
cells were seeded into six-well plates and allowed to attach for 24
h. 5 μl Lipofectamine™ 2000 and 4 μg TGF-β1-specific
shRNA were diluted in 250 μl Opti-Mimimum Essential Medium I
(Invitrogen) each. The solutions were mixed and incubated at room
temperature for 20 min to form lipid-DNA complexes. Cells were
incubated with the lipid-DNA complexes at 37°C for 6 h.
Subsequently, the supernatant was replaced with fresh medium and
cells were maintained in culture for 24 h.
Hematoxylin and eosin (HE) staining
The left lungs were fixed in 10% paraformaldehyde
solution (Beijing Dinguo Chansheng Biotechnology Co., Ltd., Beijing
China) de-hydrated and then embedded in paraffin. 4-μm
sections were cut and mounted on slides. To remove the paraffin,
the slides were immersed in xylene (Guangzhou Xinchenghuagong,
Inc., Guangzhou, China) and a graded series of ethanol (Guangzhou
Xinchenghuagong, Inc.). Subsequently, the tissue samples were
stained with HE (Maixin, Fuzhou, China). The sections were observed
and images were captured using a light microscope (E200; Nikon,
Tokyo, Japan).
Western blot analysis
Total protein was extracted from the rat lung
tissues and A549 cells using a total protein extraction kit (cat.
no. E211-02; Vazyme, Inc., Nanjing, China). Protein concentrations
were determined using a bicinchoninic acid kit (cat. no. P0010;
Beyotime Institute of Biotechnology, Haimen, China). Protein
samples (50 μg) were separated by 10% SDS-PAGE and then
transferred onto a nitrocellulose membrane (EMD Millipore Corp.,
Billerica, MA, USA). The nitrocellulose membrane was blocked with
3% bovine serum albumin (Amresco Inc., Solon, OH, USA) in
Tris-buffered saline overnight at 4°C. After washing with
phosphate-buffered saline, the membrane was incubated with the
following primary antibodies: rabbit polyclonal anti-E-cadherin
(cat. no. sc-7870; 1:800), mouse monoclonal anti-vimentin (cat. no.
sc-373717; 1:400), rabbit polyclonal anti-TGF-β1 (cat. no. sc-146;
1:400), mouse monoclonal anti-Smad2 (cat. no. sc-101153; 1:200),
all from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA), mouse
monoclonal anti-α-smooth muscle actin (cat. no. BM0002; 1:500) or
rabbit polyclonal anti-GAPDH (cat. no. BA2913; 1:2,000), both from
Boster Biological Technology Ltd., (Wuhan, China), overnight at
4°C. The membrane was then incubated with the horseradish
peroxidase (HRP)-labeled secondary antibody [goat anti-mouse
immunoglobulin (Ig)G-HRP; sc-2005; 1:4,000) or goat anti-rabbit
IgG-HRP (cat. no. sc-2004; 1:4,000; Santa Cruz Biotechnology, Inc.)
for 2 h at room temperature. The immunoreactive proteins were
detected using an enhanced chemiluminescence kit (cat. no. 32209;
Pierce Biotechnolgy, Inc., Rockford, IL, USA). GAPDH was used as a
loading control. Images of the blots were captured using a ScanJet
4C Flatbed Scanner (Hewlett-Packard, Palo Alto, CA, USA).
Densitometric quantification was performed using Image-Pro Plus
software (version 6.0; Media Cybernetics, Inc., Rockville, MD,
USA).
Statistical analysis
Values are expressed as the mean ± standard
deviation. Two-tailed Student's t-test was used to analyze
differences between two groups. SPSS 19.0 statistical software was
used (IBM SPSS, Armonk, NY, USA). Data are representative of at
least three independent experiments. P<0.05 was considered to
indicate a statistically significant difference between values.
Results
PQ induces pulmonary fibrosis in
rats
In order to identify PQ-induced pulmonary fibrosis,
HE staining of lung tissues from PQ-treated rats was performed. As
shown in Fig. 1, the lung
structure in PQ-treated animals was distorted with severe
epithelial degeneration, alveolar disruption, inflammatory cell
infiltration and fibrotic invasion at day 7 following PQ treatment,
which was further aggravated with increasing time until the end of
the experiment on day 21.
PQ treatment enhances the expression of
EMT markers in rat lung tissues
To examine the effects of PQ treatment on the EMT in
rat lung tissues, the expression of molecular EMT markers,
including E-cadherin, α-SMA and vimentin, was detected by western
blot analysis. As shown in Fig. 2,
the epithelial-cell marker E-cadherin was decreased following PQ
treatment as compared with that in the control group, and
progressively decreased until the end of the experiment on day 21.
By contrast, from day 7 onwards, an increase in the expression of
mesenchymal cell markers α-SMA and vimentin was observed.
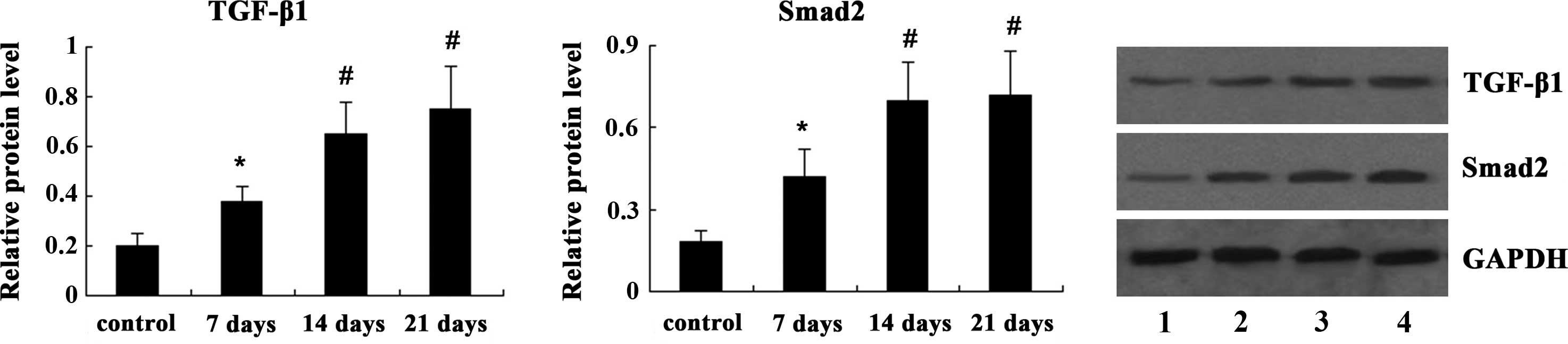
PQ treatment upregulates TGF-β/Smad
signaling in rat lung tissues
Next, the present study examined the effects of PQ
treatment on the TGF-β/Smad signaling pathway in rat lung tissues.
As shown in Fig. 3, western blot
analysis indicated that the expression of TGF-β1 and Smad2 was
significantly increased on day 7 following PQ treatment (P<0.05)
and was further increased on days 14 and 21 (P<0.01).
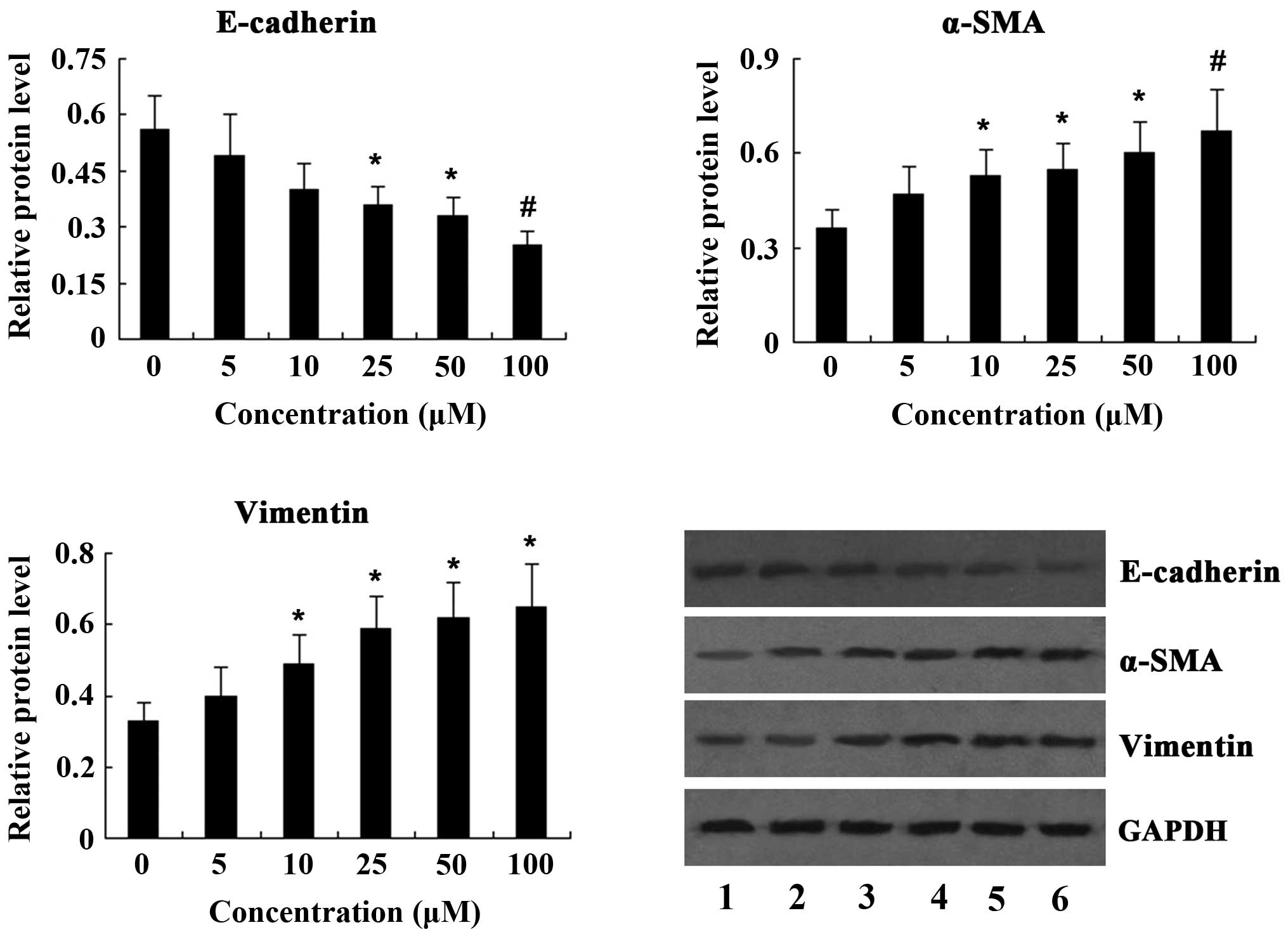
PQ treatment enhances the expression of
EMT markers in vitro
To investigate the induction of the EMT by PQ in
vitro, A549 lung cancer cells were incubated with various
concentrations of PQ (5, 10, 25, 50 and 100 μM) for 24 h and
the expression of EMT markers was determined by western blot
analysis. The results revealed that treatment of A549 cells with PQ
resulted in a dose-dependent decrease of E-cadherin expression,
while the expression of α-SMA and vimentin was significantly
increased in a dose-dependent manner following PQ treatment
(Fig. 4).
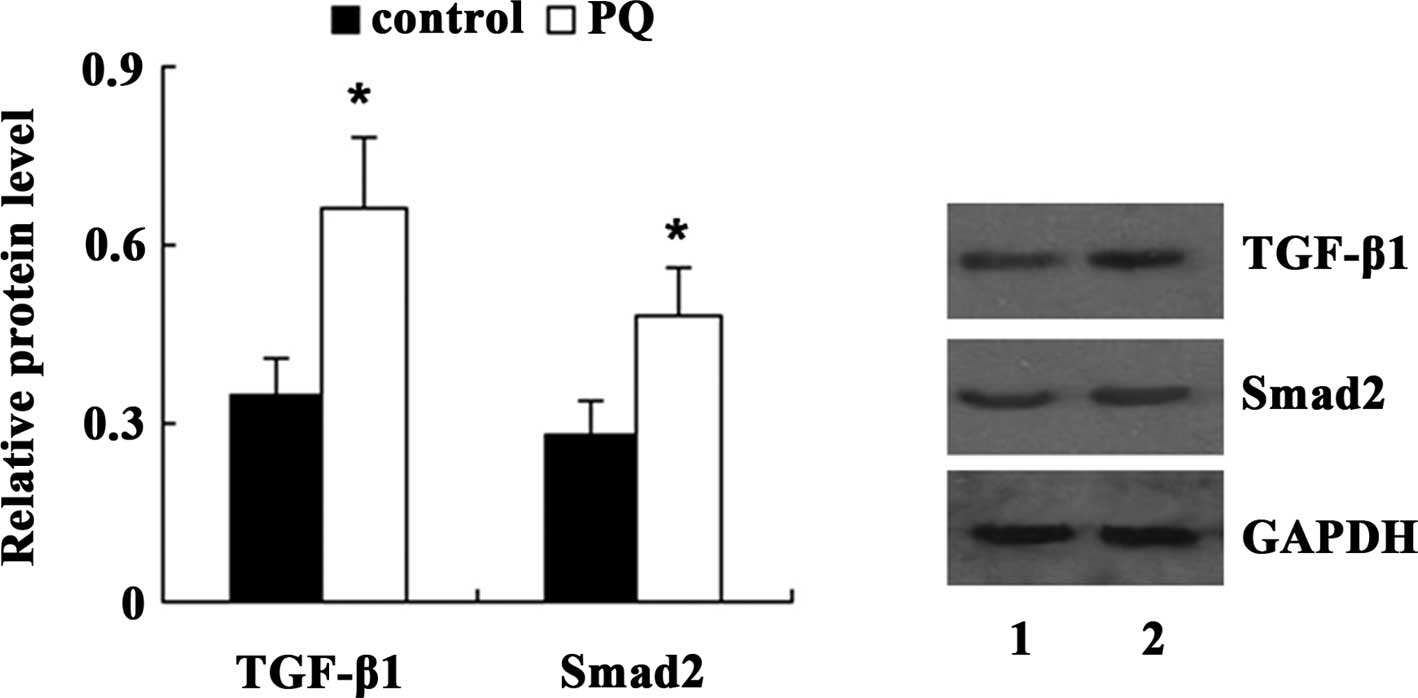
TGF-β/Smad pathway mediates the
PQ-induced EMT in A549 cells
To clarify the underlying mechanisms of PQ-induced
EMT in A549 cells, the present study examined the expression of
TGF-β1 and Smad2 in A549 cells following treatment with 100
μM PQ for 24 h. Western blot analysis demonstrated that
compared with the control, the expression of TGF-β1 and Smad2 was
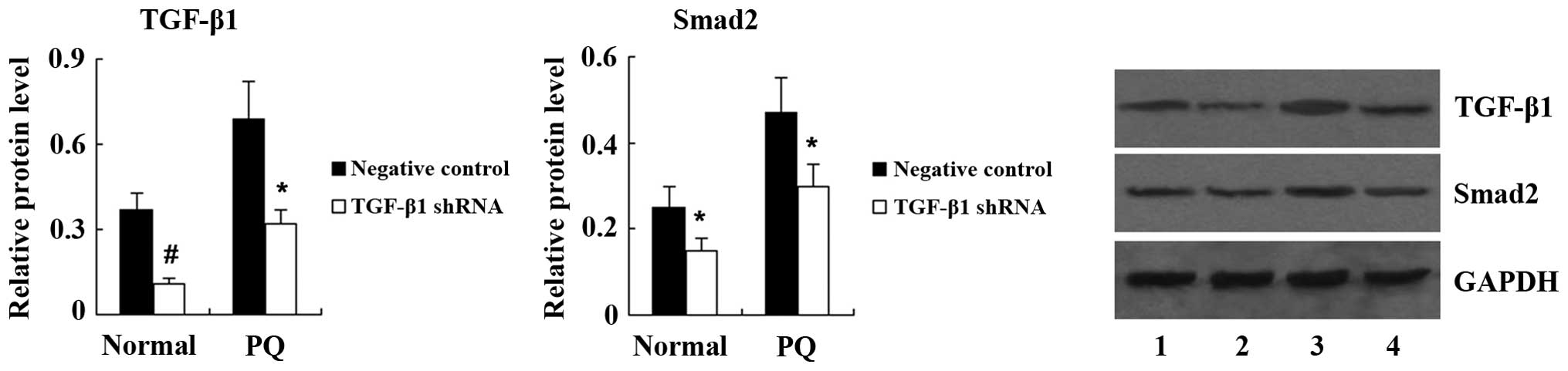
significantly increased in A549 cells treated with PQ (Fig. 5). Next, TGF-β1 shRNA was
transfected into A549 cells to knockdown TGF-β1 expression. Western
blot analysis showed that the expression of TGF-β1 and Smad2 was
significantly decreased in PQ-untreated A549 cells following TGF-β1
shRNA transfection. PQ-induced upregulation of TGF-β1 and Smad2 was
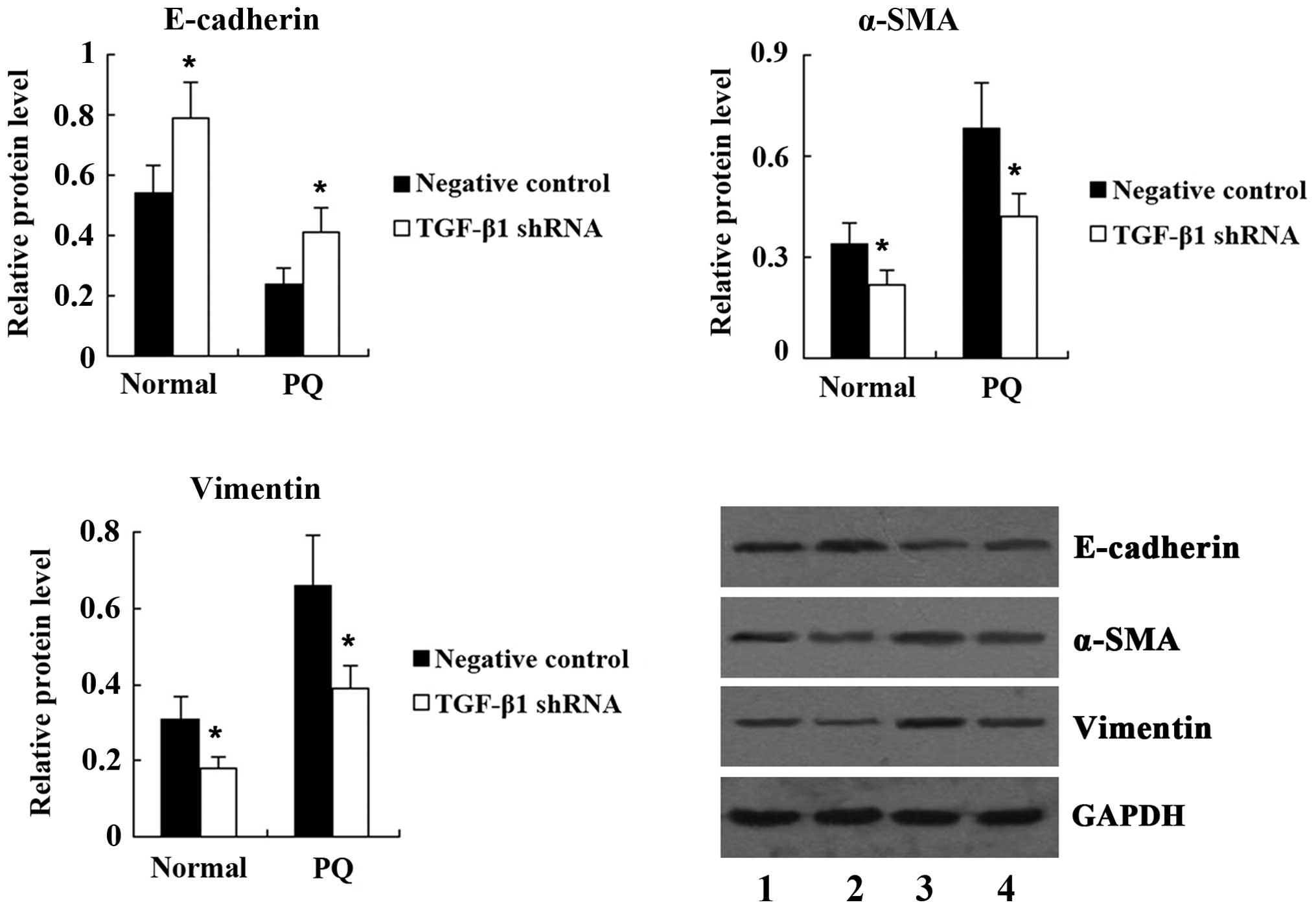
also significantly suppressed by TGF-β1 shRNA (Fig. 6). As shown in Fig. 7, transfection with TGF-β1 shRNA led
to an increase in E-cadherin, and a decrease in α-SMA and vimentin
expression in PQ-untreated A549 cells. Furthermore, compared with
the PQ group, the expression of E-cadherin was significantly
increased, while the expression of α-SMA and vimentin was
significantly decreased in the PQ + TGF-β1 shRNA group.
Discussion
Paraquat is a highly toxic herbicide which induces
pulmonary fibrosis in humans and animals. In the present study,
adult male Sprague-Dawley rats were treated by intraperitoneal
injection with PQ and the time-dependent development of pulmonary
fibrosis was confirmed by HE staining at days 7, 14 and 21. Next,
the present study aimed to elucidate the mechanisms involved in
PQ-induced in vivo and in vitro.
Lung tissues from patients with pulmonary fibrosis
are known to contain myofibroblasts (11–14),
which are responsible for the deposition of collagen and other
extracellular matrix components during the development and
progression of pulmonary fibrosis (15). It has been suggested that
myofibroblasts are derived from at least three sources (16–19):
Proliferation and activation of resident tissue fibroblasts,
transition of alveolar epithelial cells into mesenchymal cells and
differentiation of circulating bone marrow-derived progenitor
cells. An accumulating number of studies supported the notion that
EMT transition has a central role in pulmonary fibrosis (8–10).
EMT is a process during which epithelial cells lose
their epithelial cell characteristics and acquire mesenchymal
characteristics. During the EMT, the expression of the epithelial
marker E-cadherin is lost, while the expression of mesenchymal
markers, including α-SMA, vimentin and matrix metalloproteinase, is
increased, leading to the loss of cell-cell contacts, cytoskeletal
rearrangement, increased cell-migratory behavior and excess
extracellular matrix production (20). The present study investigated the
involvement of the EMT in PQ-induced pulmonary fibrosis. The in
vivo study showed that the expression of epithelial marker
E-cadherin was significantly decreased, while the expression of
mesenchymal markers α-SMA and vimentin was significantly increased
in the lung tissues of PQ-treated rats. These results suggested
that the EMT was involved in PQ-induced pulmonary fibrosis in rats.
Next, the present study investigated the effects of PQ on EMT
markers in vitro. Western blot analysis demonstrated a
decrease of E-cadherin and an increase of α-SMA and vimentin in
PQ-treated A549 cells in a dose-dependent manner. Therefore, the
in vitro study confirmed that PQ induced EMT in lung
epithelial cells.
TGF-β1 is a pivotal mediator of fibrosis (21–23)
and the TGF-β1 signal is transduced through the activation of its
downstream effectors, the Smad proteins. TGF-β/Smad signaling
induces EMT and fibrosis in a variety of organs (24–26).
It has been demonstrated that TGF-β1 was able to induce alveolar
epithelial cells to undergo EMT in vivo and in vitro
via Smad2 activation (27). The
in vivo experiment of the present study revealed that the
expression of TGF-β1 and Smad2 was increased in the rat model of
PQ-induced pulmonary fibrosis. Furthermore, an in vitro
experiment was performed to clarify whether TGF-β/Smad signaling
was involved in PQ-induced EMT in A549 cells. The results
demonstrated that TGF-β/Smad signaling was activated and the EMT
was induced in PQ-treated A549 cells; however, these effects were
inhibited when PQ-treated A549 cells were transfected with TGF-β1
shRNA. Therefore, TGF-β/Smad signaling was confirmed to be involved
in the PQ-induced EMT in A549 cells.
In conclusion, the present study provided the
underlying molecular mechanism of PQ-induced pulmonary fibrosis. It
was demonstrated that PQ induced EMT in vivo and in
vitro, and the EMT was indicated to be an important process in
the development of PQ-induced pulmonary fibrosis. In addition,
TGF-β/Smad signaling was demonstrated to be involved in PQ-induced
EMT.
Acknowledgments
The present study was supported by the Science and
Technology Foundation of Tianjin Municipal Health Bureau (no.
2013KY06).
References
|
1
|
Lee SK, Ameno K, In SW, Yang JY, Kim KU,
Koo KS, Yoo YC, Ameno S and Ijiri I: Levels of paraquat in fatal
intoxications. Int J Legal Med. 112:198–200. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin JL, Lin-Tan DT, Chen KH, Huang WH, Hsu
CW, Hsu HH and Yen TH: Improved survival in severe paraquat
poisoning with repeated pulse therapy of cyclophosphamide and
steroids. Intensive Care Med. 37:1006–1013. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang RL, Tang X, Wu X, Xu R, Yu KL and Xu
K: The relationship between HIF-1a expression and the early lung
fibrosis in rats with acute paraquat poisoning. Chinese Journal of
Industrial Hygiene and Occupational Diseases. 30:273–277. 2012.In
Chinese.
|
|
4
|
Dinis-Oliveira RJ, Duarte JA,
Sánchez-Navarro A, Remião F, Bastos ML and Carvalho F: Paraquat
poisonings: Mechanisms of lung toxicity, clinical features and
treatment. Crit Rev Toxicol. 38:13–71. 2008. View Article : Google Scholar
|
|
5
|
Venkatesan N: Pulmonary protective effects
of curcumin against paraquat toxicity. Life Sci. 66:PL21–PL28.
2000.PubMed/NCBI
|
|
6
|
Tomita M, Okuyama T, Katsuyama H, Miura Y,
Nishimura Y, Hidaka K, Otsuki T and Ishikawa T: Mouse model of
paraquat-poisoned lungs and its gene expression profile.
Toxicology. 231:200–209. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hagiwara S, Iwasaka H, Matsumoto S and
Noguchi T: An antisense oligonucleotide to HSP47 inhibits
paraquat-induced pulmonary fibrosis in rats. Toxicology.
236:199–207. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Willis BC, Liebler JM, Luby-Phelps K,
Nicholson AG, Crandall ED, du Bois RM and Borok Z: Induction of
epithelial-mesenchymal transition in alveolar epithelial cells by
transforming growth factor-beta1: Potential role in idiopathic
pulmonary fibrosis. Am J Pathol. 166:1321–1332. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim KK, Kugler MC, Wolters PJ, Robillard
L, Galvez MG, Brumwell AN, Sheppard D and Chapman HA: Alveolar
epithelial cell mesenchymal transition develops in vivo during
pulmonary fibrosis and is regulated by the extracellular matrix.
Proc Natl Acad Sci USA. 103:13180–13185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim KK, Wei Y, Szekeres C, Kugler MC,
Wolters PJ, Hill ML, Frank JA, Brumwell AN, Wheeler SE, Kreidberg
JA and Chapman HA: Epithelial cell alpha3beta1 integrin links
beta-catenin and Smad signaling to promote myofibroblast formation
and pulmonary fibrosis. J Clin Invest. 119:213–224. 2009.
|
|
11
|
Adler KB, Low RB, Leslie KO, Mitchell J
and Evans JN: Contractile cells in normal and fibrotic lung. Lab
Invest. 60:4773–485. 1989.
|
|
12
|
Mitchell J, Woodcock-Mitchell J, Reynolds
S, Low R, Leslie K, Adler K, Gabbiani G and Skalli O: Alpha-smooth
muscle actin in parenchymal cells of bleomycin-injured rat lung.
Lab Invest. 60:643–650. 1989.PubMed/NCBI
|
|
13
|
Kuhn C and McDonald JA: The roles of the
myofibroblast in idiopathic pulmonary fibrosis. Ultrastructural and
immunohisto-chemical features of sites of active extracellular
matrix synthesis. Am J Pathol. 138:1257–1265. 1991.PubMed/NCBI
|
|
14
|
Pache JC, Christakos PG, Gannon DE,
Mitchell JJ, Low RB and Leslie KO: Myofibroblasts in diffuse
alveolar damage of the lung. Mod Pathol. 11:1064–1070.
1998.PubMed/NCBI
|
|
15
|
Phan SH: The myofibroblast in pulmonary
fibrosis. Chest. 122(Suppl 6): S286–S289. 2002. View Article : Google Scholar
|
|
16
|
Epperly MW, Guo H, Gretton JE and
Greenberger JS: Bone marrow origin of myofibroblasts in irradiation
pulmonary fibrosis. Am J Respir Cell Mol Biol. 29:213–224. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Willis BC, duBois RM and Borok Z:
Epithelial origin of myofibroblasts during fibrosis in the lung.
Proc Am Thorac Soc. 3:377–382. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scotton CJ and Chambers RC: Molecular
targets in pulmonary fibrosis: The myofibroblast in focus. Chest.
132:1311–1321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kasai H, Allen JT, Mason RM, Kamimura T
and Zhang Z: TGF-beta1 induces human alveolar epithelial to
mesenchymal cell transition (EMT). Respir Res. 6(56)2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesen-chymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leask A and Abraham DJ: TGF-beta signaling
and the fibrotic response. FASEB J. 18:816–827. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Steen V: Targeted therapy for systemic
sclerosis. Autoimmun Rev. 5:122–124. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bergeron A, Soler P, Kambouchner M,
Loiseau P, Milleron B, Valeyre D, Hance AJ and Tazi A: Cytokine
profiles in idiopathic pulmonary fibrosis suggest an important role
for TGF-beta and IL-10. Eur Respir J. 22:69–76. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chapman HA: Epithelial-mesenchymal
interactions in pulmonary fibrosis. Annu Rev Physiol. 73:413–435.
2011. View Article : Google Scholar
|
|
26
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Willis BC and Borok Z: TGF-beta-induced
EMT: Mechanisms and implications for fibrotic lung disease. Am J
Physiol Lung Cell Mol Physiol. 293:L525–L534. 2007. View Article : Google Scholar : PubMed/NCBI
|