Introduction
Increased differentiation of fat precursor cells,
preadipocytes, into mature adipocytes leads to fat tissue
hypertrophy and obesity (1).
However, impaired differentiation of preadipocytes in the abdominal
adipose tissue positively correlates with insulin resistance in
obese patients (2). Thus,
modulation of preadipocyte differentiation into adipocytes may be
an attractive approach in the therapy of obesity and
obesity-associated metabolic disorders. A previous study
demonstrated that appetite-regulating peptides such as orexin A,
neuropeptide B and W, and fibroblast growth factor-21 modulate the
various functions of mature adipocytes in addition to their
precursor cells, preadipocytes (3–6). In
addition, previous studies have demonstrated that the metabolic and
endocrine functions of adipose tissue are influenced by obestatin,
an appetite-regulating peptide hormone (7,8).
Obestatin, a 23-amino acid peptide, is the product of the
proteolytic cleavage of preproghrelin (9). Preproghrelin is predominantly
expressed in the stomach (9), with
lower expression observed in other tissues, such as adipocytes
(7) and the pancreas (10). Obestatin was first identified as a
hormone that maintains energy homeostasis by inhibiting food
intake, delaying gastric emptying and facilitating body weight loss
(9). Furthermore, there is growing
evidence indicating that obestatin modulates the lipid and glucose
metabolism by acting directly on adipocytes. Obestatin activates
protein kinase B signaling and stimulates glucose uptake in 3T3-L1
adipocytes (7). In addition,
obestatin was reported to influence the lipid metabolism by
stimulating lipid accumulation and inhibiting lipolysis in 3T3-L1
cells and human adipocytes (8,11).
By contrast, a previous study demonstrated that obestatin
suppresses glucose uptake and lipogenesis, whereas it potentiates
adrenalin-induced lipolysis in rat primary adipocytes (12). Studies investigating the influence
of obestatin on adipogenesis have produced contradictory results.
Although it was initially observed that obestatin inhibits
differentiation and proliferation of 3T3-L1 preadipocytes (13), later studies indicated that
obestatin promotes the proliferation and differentiation of 3T3-L1
preadipocytes into mature adipocytes (7,8,11).
However, alternative studies have demonstrated that obestatin
failed to influence the differentiation of 3T3-L1 preadipocytes
(14). Thus, the role of obestatin
in controlling preadipocyte differentiation remains to be fully
understood. Therefore, the aim of the current study was to
investigate the role of obestatin in regulating rat primary
preadipocyte differentiation, lipid metabolism and leptin
secretion.
The present study investigated whether obestatin
enhanced differentiation of rat preadipocytes by measuring lipid
accumulation and the expression of adipogenic transcription
factors. Furthermore, the differential effects of obestatin on
lipolysis at the early and late stages of differentiation were
investigated.
Materials and methods
Chemicals
Obestatin was purchased from Sigma-Aldrich (St.
Louis, MO, USA). Gibco Dulbecco's modified Eagle's medium
(DMEM/F12) media for cell culture was obtained from Thermo Fisher
Scientific, Inc., (Waltham, MA, USA). Unless otherwise stated, all
additional reagents were obtained from Sigma-Aldrich.
Animals
For independent isolations of preadipocytes, eight
rats were used. Male Wistar rats, weighing 80–100 g (age, 5–6
weeks) were maintained on a 12:12 light-dark cycle at 21°C and fed
ad libitum with standard chow. The rats were sacrificed by
decapitation according to the approval of the Local Ethics
Commission for Investigations on Animals (National Ethics
Commission for Investigations on Animals, Ministry of Science and
Higher Education, Poznań, Poland).
Preadipocyte isolation and culture
Stromal-vascular cells (preadipocytes) were isolated
from epididymal adipose fat pads, as previously described (15) with modifications. Following the
removal of blood vessels, the pooled tissue was washed three times
in sterile Krebs-Ringer buffer [118 mM NaCl, 4.8 mM KCl, 1.3 mM
CaCl2, 1.2 mM KH2PO4, 1.2 mM
MgSO4, 24.8 mM NaHCO3, 10 mM
4-(2-hydroxyethyl)-1-pipera-zineethanesulfonic acid] supplemented
with 3% bovine serum albumin, 5 mM glucose and antibiotics (100
U/ml penicillin and 0.1 mg/ml streptomycin). Tissue was minced
using scissors and digested with collagenase type II at 3 mg/ml for
45 min at 37°C, with gentle agitation. The cell suspension was
filtered through 100 µm nylon mesh to discard the remaining
undigested tissue debris and centrifuged at 450 × g for 10 min at
room temperature (RT). The infranatant containing mature adipocytes
was discarded and Red Blood Cell Lysing buffer (Sigma-Aldrich) was
added to lyse the erythrocytes. Subsequently, cells were filtered
through a 45 µm mesh and centrifuged (450 × g, 10 min at
RT). The cell pellet was resuspended in DMEM/F12 containing 10%
fetal bovine serum and antibiotics. Following cell counting with
0.4% trypan blue (cell viability >95%) cells were seeded in
multi-well plates and cultured for 24 h. Cell culture was performed
at 37°C in a humidified atmosphere of 95% air with 5%
CO2. Subsequently, preadipocytes were differentiated in
serum-free DMEM/F12 containing adipogenesis-promoting agents (30 nM
dexamethasone, 167 nM insulin and 2 nM triiodothyronine) in the
absence or presence of obestatin (1, 10 or 100 nM). Preadipocytes
were differentiated for 1, 2, 4 and 5 days. The media was then
collected and stored at −20°C for determination of leptin and
glycerol concentrations. Simultaneously, cells were harvested and
stored at −80°C in Tripure reagent (Roche Diagnostics, Basel,
Switzerland) for RNA extraction. Separate experiments were
performed for oil red O (ORO) staining.
ORO staining
Preadipocytes were washed with phosphate-buffered
saline (PBS) and fixed with 10% formaldehyde in PBS for 5 min.
Following this, the formaldehyde solution was replaced with fresh
formaldehyde and fixed for 1 h at RT. Working ORO solution was
prepared prior to each experiment by mixing 6 parts ORO stock
solution (0.7 g/200 ml isopropanol) with 4 parts distilled water
and incubating for 20 min at RT, followed by filtration through a
0.2 µm syringe filter. Fixed cells were washed with 60%
isopropanol, completely dried and stained with ORO working solution
for 10 min at RT. Subsequently, cells were washed four times with
distilled water and photographed using an LSM 510 inverted
microscope and AxioVision Rel. software, version 4.6 (Carl Zeiss,
Oberkochen, Germany). For quantification, cells were dried and ORO
was eluted by adding 100% isopropanol. Eluates were then
transferred to 96-well plates and the absorbance was measured at a
wavelength of 520 nm using a Synergy 2 Multi-Mode Microplate Reader
(BioTek Instruments, Inc., Winooski, VT, USA).
Reverse transcription-quantitative
polymerase chain reaction
Total RNA was extracted using TriPure Isolation
Reagent (Roche Diagnostics) according to the manufacturer's
instructions. First strand cDNA was generated using 0.5 µg
total RNA and a Transcriptor First Strand cDNA Synthesis kit (Roche
Diagnostics). Gene specific primers and probes were designed using
Roche software (http://qpcr.probefinder.com/organism.jsp), and are
presented in Table I. Multiplex
real-time expression was conducted using Light Cycler TaqMan Master
kit in a Light Cycler 2.0 (Roche Diagnostics) using the following
thermocy-cling conditions: cDNA was initially pre-denaturated at
95°C for 10 min, followed by 40 cycles of denaturation at 95°C for
10 sec, annealing at 58°C for 30 sec and extension at 72°C for 8
sec. Analysis of gene expressions was conducted using Light Cycler
software, version 4.5 (Roche Diagnostics). The results are based on
the relative quantification method with efficiency correction
(standard curve method) (16).
Results are presented as a ratio of the detected gene expression to
the expression of the glyceraldehyde 3-phosphate dehydrogenase
gene.
 | Table IPrimer sequences used for measurement
of PPARγ, C/EBPα and C/EBPβ expression using reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primer sequences used for measurement
of PPARγ, C/EBPα and C/EBPβ expression using reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence | Product size
(nt) |
|---|
| PPARγ | Forward
5′-CAGGAAAGACAACAGACAAATCA-3′ | |
| (NM_013124.3) | Reverse
5′-GGGGGTGATATGTTTGAACTTG-3′ | 95 |
| C/EBPα | Forward
5′-ATAAAGCCAAACAGCGCAAC-3′ | |
| (NM_012524.2) | Reverse
5′-CGGTCATTGTCACTGGTCAA-3′ | 67 |
| C/EBPβ | Forward
5′-CTTCAGCCCCTACCTGGAG-3′ | |
| (NM_024125.4) | Reverse
5′-GAGGTCGGAAAGGAAGTCGT-3′ | 88 |
| GAPDH | Forward
5′-CTGCACCACCAACTGCTTAG-3′ | |
|
(ENSRNOT00000025351) | Reverse
5′-TGATGGCATGGACTGTGG-3′ | 92 |
Lipolysis assay
Lipolysis was analyzed by the determination of
glycerol release. The glycerol concentration in the medium was
measured using a Free Glycerol Determination kit (Sigma-Aldrich),
according to the manufacturer's instructions. The absorbance
reading was performed at 540 nm using a Synergy 2 Multi-Mode
Microplate Reader.
Leptin secretion assay
The leptin concentration in the culture media was
measured using a Rat Leptin Radio Immuno Assay kit (Merck
Millipore, Darmstad, Germany), according to the manufacturer's
instructions. The assay's sensitivity was 0.639 ng/ml.
Statistical analysis
Analysis of variance followed by the Bonferroni post
hoc test were used to determine statistical significance. P<0.05
was considered to indicate a statistically significant difference.
Data are presented as the mean ± standard error, and are derived
from experiments conducted a minimum of four times. GraphPad Prism
5.0 (GraphPad Software, Inc., La Jolla, CA, USA) was used for all
statistical analyses.
Results
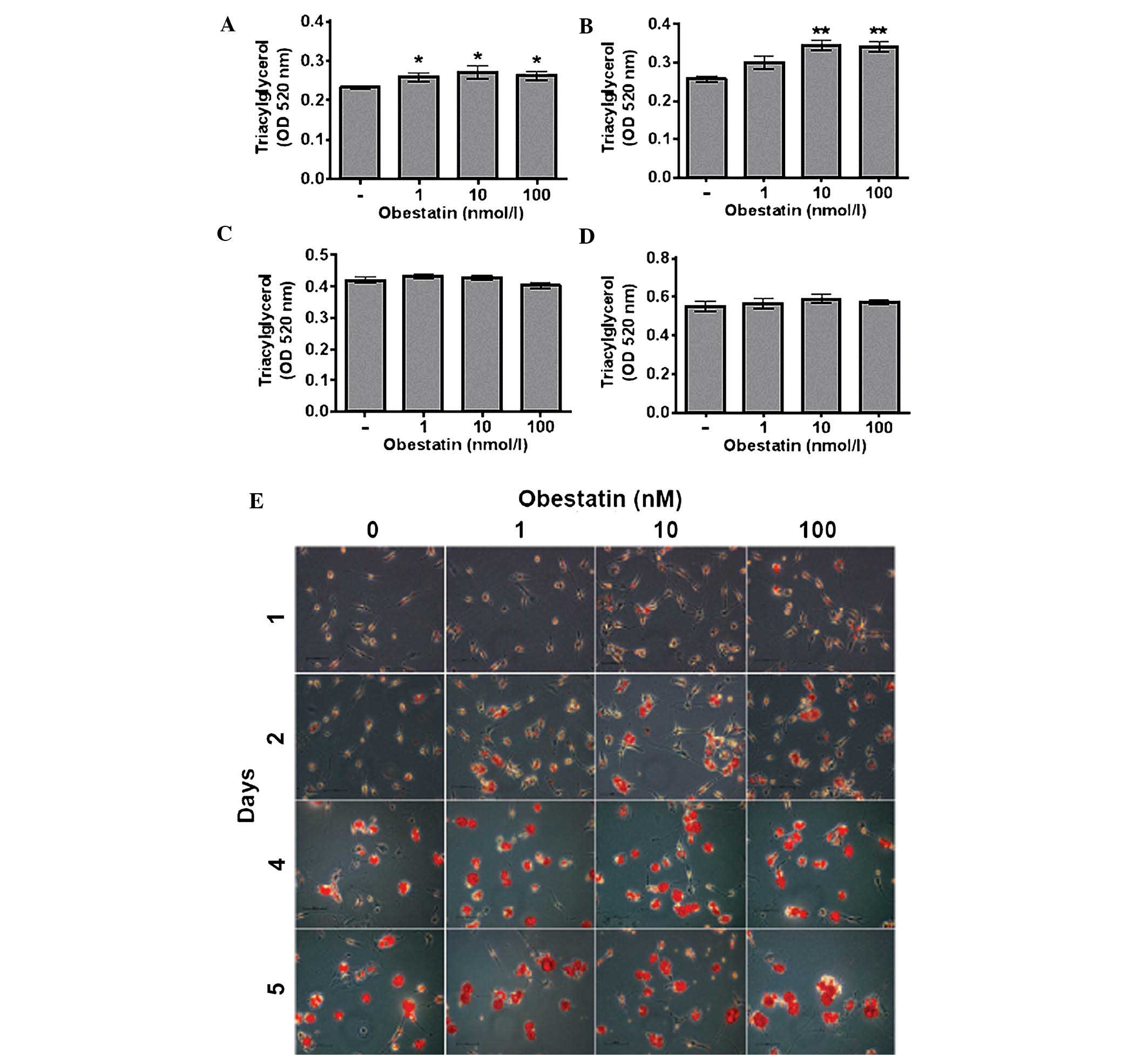
Obestatin increases triacylglycerol
accumulation in rat adipocytes
The effect of obestatin on rat preadipocytes
differentiation was investigated by measuring the lipid
accumulation and evaluating the morphology of the cells. Obestatin
(1–100 nM) increased intracellular triacylglycerol content at 24 h
following the initiation of differentiation (Fig. 1A). Increased intracellular
triacylglycerol content was additionally observed in cells exposed
to 10 and 100 nM obestatin for 48 h (Fig. 1B). The obestatin-mediated increase
in lipid content was accompanied by the presence of larger lipid
droplets (Fig. 1E). Obestatin
failed to significantly affect lipid content and preadipocyte
morphology at 4 and 5 days following the onset of differentiation
(Fig. 1C–E). These results
indicate that obestatin enhances triacylglycerol accumulation in
rat preadipocytes at an earlier stage of differentiation.
Obestatin stimulates peroxisome
proliferator-activated receptor γ (PPARγ), CCAAT-enhancer-binding
protein α (C/EBPα) and C/EBPβ expression in rat preadipocytes
The effects of obestatin on the expression of PPARγ,
C/EBPα and C/EBPβ were investigated (Table II). Obestatin at 100 nM
significantly increased PPARγ expression at 2 days following the
onset of differentiation. Furthermore, obestatin at all tested
concentrations increased PPARγ expression at 4 days following the
induction of differentiation. The increase in PPARγ expression was
additionally observed in preadipocytes differentiated in the
presence of 10 or 100 nM obestatin for 5 days. By contrast, no
increase was observed at 24 h following the onset of
differentiation. Obestatin (1 nM) increased C/EBPα expression at 24
h of differentiation. At 2 days of differentiation, obestatin (at 1
and 10 nM) enhanced C/EBPα expression. In addition, the increase in
C/EBPα expression was observed in preadipocytes exposed to 1 and
100 nM obestatin for 4 days and in cells treated with 10 and 100 nM
obestatin for 5 days. C/EBPβ mRNA expression was upregulated by 1
and 10 nM obestatin following 24 h of incubation. Thus, obestatin
increases adipogenic gene expression in a dose- and time-dependent
manner.
 | Table IIEffects of obestatin on PPARγ, C/EBPα
and C/EBPβ mRNA expression in rat preadipocytes. |
Table II
Effects of obestatin on PPARγ, C/EBPα
and C/EBPβ mRNA expression in rat preadipocytes.
| Day | Obestatin (nM) | Gene
|
|---|
| PPARγ | C/EBPα | C/EBPβ |
|---|
| 1 | 0 | 1.000±0.010 | 1.000±0.028 | 1.000±0.039 |
| 1 | 0.952±0.011 | 1.250±0.020b | 1.133±0.034a |
| 10 | 0.932±0.010 | 1.048±0.041 | 1.108±0.019a |
| 100 | 0.969±0.012 | 1.139±0.046 | 1.075±0.013 |
| 2 | 0 | 1.000±0.002 | 1.000±0.015 | 1.000±0.011 |
| 1 | 0.967±0.010 | 1.307±0.068a | 1.013±0.026 |
| 10 | 1.018±0.005 | 1.571±0.096b | 1.041±0.018 |
| 100 | 1.046±0.010b | 1.250±0.016 | 0.988±0.018 |
| 4 | 0 | 1.000±0.013 | 1.000±0.032 | 1.000±0.033 |
| 1 | 1.245±0.017b | 1.144±0.038a | 1.128±0.043 |
| 10 | 1.123±0.016b | 1.045±0.020 | 1.078±0.039 |
| 100 | 1.069±0.012a | 1.173±0.039a | 1.104±0.026 |
| 5 | 0 | 1.000±0.008 | 1.000±0.118 | 1.000±0.043 |
| 1 | 0.992±0.012 | 1.646±0.066 | 1.140±0.011 |
| 10 | 1.168±0.018b | 2.215±0.409a | 1.083±0.046 |
| 100 | 1.116±0.014b | 2.748±0.168b | 1.067±0.026 |
Obestatin regulates lipolysis in rat
preadipocytes
Glycerol release was analyzed in preadipocytes
differentiated in the absence or presence of obestatin for 2, 4 or
5 days. At 100 nM, obestatin reduced glycerol release from
preadipocytes at 2 days following the onset of differentiation
(Fig. 2A). Obestatin failed to
influence glycerol release following 4 days of differentiation
(Fig. 2B). By contrast, glycerol
secretion increased in preadipocytes exposed to 10 and 100 nM
obestatin for 5 days (Fig. 2C).
These results suggest that obestatin suppresses lipolysis during
the first stages of differentiation, whereas it stimulates
lipolysis in well-differentiated preadipocytes.
Obestatin stimulates leptin secretion
from rat preadipocytes
The effect of obestatin on leptin secretion from rat
preadipocytes was investigated during the different stages of the
differentiation process. At 10 or 100 nM, obestatin increased
leptin secretion from preadipocytes at 24 h following the
initiation of differentiation (Fig.
3A). In addition, at 1, 10 and 100 nM, obestatin increased
leptin secretion from rat preadipocytes following 4 days of
differentiation (Fig. 3C).
Obestatin at all tested doses failed to significantly modulate
leptin secretion from preadipocytes differentiated for 2 (Fig. 3B) and 5 days (Fig. 3D). These results indicate that
obestatin is able to enhance leptin secretion from rat
preadipocytes.
Discussion
A previous study reported that obestatin inhibits
lipogenesis and glucose uptake in differentiated rat adipocytes
(12). The current study extends
these observations by demonstrating that obestatin increases lipid
accumulation, enhances preadipocyte differentiation and modulates
lipolysis and leptin secretion in rat primary preadipocytes.
Furthermore, the results demonstrate that obestatin increases
leptin secretion in rat preadipocytes.
Differentiation of preadipocytes into mature
adipocytes is accompanied by enhanced triacylglycerol accumulation
(17). The current study observed
that obestatin increased early intracellular triacylglycerol
accumulation in preadipocytes. As a hallmark of adipogenesis
(18) the size of lipid droplets
in obestatin-treated cells increased, and notably, these effects
were detected at 1 or 2 however not 4 or 5 days following the onset
of differentiation. These results correspond to previous reports
regarding the inability of a 9-day treatment of 3T3-L1
preadipocytes with obestatin to influence lipid content (14). In contrast to these observations,
it has been reported that obestatin is able to potentiate lipid
accumulation even following 8 and 14 days of differentiation in
3T3-L1 preadipocytes and in human primary subcutaneous
preadipocytes (8). Notably, in the
same study obestatin failed to affect lipid accumulation in omental
preadipocytes obtained from lean humans, whereas it enhanced lipid
accumulation in preadipocytes isolated from obese individuals
(8). These conflicting data may
result either from different experimental methodology or the health
conditions of the preadipocytes donors.
Although differentiation of fat precursor cells into
mature adipocytes is modulated by a variety of transcription
factors (17), there is convincing
evidence that the expression of numerous genes which induce and
maintain adipognesis is coordinated by PPARγ and the C/EBPs
(17,19,20).
In the current study, obestatin increased PPARγ, C/EBPα and C/EBPβ
expression in rat preadipocytes. It should be emphasized that these
effects were time- and dose-dependent. Gene expression was more
potently stimulated by the lower doses of obestatin. This
observation is in line with a previous report indicating that
obestatin enhances 3T3-L1 preadipocyte proliferation only at lower
concentrations (14). It cannot be
ruled out that the dynamic and dose-dependent effects of obestatin
on preadipocytes may result from obestatin receptor
desensitization.
However, as obestatin was observed to stimulate
lipid accumulation in addition to potentiating the expression of
genes which regulate adipogenesis, it is suggested that obestatin
promotes the differentiation of rat preadipocytes. Of note, the
adipogenic role of this peptide is supported by studies
demonstrating that obestatin promotes adipogenesis in 3T3-L1 cells
(7) and in porcine preadipocytes
(21).
A previous study indicated that obestatin promotes
lipolysis and suppresses lipogenesis in fully differentiated rat
adipocytes (12). In the current
study, the consequences of obestatin treatment on glycerol release
during the differentiation of preadipocytes was investigated. These
data demonstrated that obestatin suppresses glycerol release
following 2 days of differentiation. By contrast, obestatin
enhances glycerol release from cells that were continually exposed
to obestatin for 5 days. These data suggest that the effects of
obestatin on lipid metabolism in rat adipocytes are biphasic.
Obestatin appears to enhance lipid accumulation at an early stage
of differentiation, which may result from enhanced adipogenesis,
whereas it inhibits lipogenesis in mature adipocytes. Furthermore,
this suggests that obestatin may only induce lipolysis in
well-differentiated fat cells. This suggestion is supported by a
previous study demonstrating that obestatin promotes free fatty
acid release from 3T3-L1 adipocytes (14). The same study indicated that
cell-permeable TAT-obestatin is able to evoke free fatty acid and
glycerol release from 3T3-L1 adipocytes (14). However, conflicting studies have
reported that obestatin suppresses lipolysis in 3T3-L1 adipocytes
and in human adipocytes (8,11).
Overall, the influence of obestatin on adipocytes lipid metabolism
is complex and requires further investigation.
Furthermore, the current study observed that
obestatin increased leptin secretion from rat preadipocytes.
Notably, leptin enhanced rat preadipocyte differentiation in
vitro (22). Leptin inhibits
glucose transport and triggers lipolysis in mature rat white
adipocytes (23,24). These observations support the
hypothesis that the effects of obestatin on rat preadipocytes
(enhanced differentiation) and adipocytes (lipolysis) may be
contributed to by enhanced leptin secretion. However, in contrast
with the results of the current study, others have observed
obestatin to inhibit leptin secretion in human preadipocytes
(8). Why obestatin selectively
stimulates leptin secretion in rat adipocytes whilst suppressing
secretion from human preadipocytes remains unclear. However, it
cannot be ruled out that this is due to the different receptors
conferring the effects of obestatin in rat and human adipo-cytes.
It remains to be fully elucidated whether obestatin binds to G
protein-coupled receptor 37 (GPR37) or/and to glucagon-like
peptide-1 receptor (GLP1R), however GPR37 and GLP1R have been
previously reported as obestatin receptors in adipocytes (7,8,12).
Furthermore, it has been suggested that the presence of
intracellular receptors conferring the metabolic effects of
obestatin cannot be excluded (14,25).
In conclusion, the current study demonstrates that
obestatin stimulates early lipid accumulation and the expression of
adipogenic genes, and provides evidence that obestatin stimulates
leptin secretion from preadipocytes.
Acknowledgments
The current study was supported by a grant from the
Polish Ministry of Science and Higher Education to Dr Tatiana
Wojciechowicz (grant no. N N303 319337).
References
|
1
|
Stephens JM: The fat controller: Adipocyte
development. PLoS Biol. 10:e10014362012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Isakson P, Hammarstedt A, Gustafson B and
Smith U: Impaired preadipocyte differentiation in human abdominal
obesity: Role of Wnt, tumor necrosis factor-alpha, and
inflammation. Diabetes. 58:1550–1557. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Skrzypski M, T Le T, Kaczmarek P,
Pruszynska-Oszmalek E, Pietrzak P, Szczepankiewicz D, Kolodziejski
PA, Sassek M, Arafat A, Wiedenmann B, et al: Orexin A stimulates
glucose uptake, lipid accumulation and adiponectin secretion from
3T3-L1 adipocytes and isolated primary rat adipocytes.
Diabetologia. 54:1841–1852. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Skrzypski M, Pruszyńska-Oszmałek E,
Ruciński M, Szczepankiewicz D, Sassek M, Wojciechowicz T, Kaczmarek
P, Kołodziejski PA, Strowski MZ, Malendowicz LK and Nowak KW:
Neuropeptide B and W regulate leptin and resistin secretion, and
stimulate lipolysis in isolated rat adipocytes. Regul Pept.
176:51–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Skrzypski M, Kaczmarek P, Le TT,
Wojciechowicz T, Pruszyńska-Oszmalek E, Szczepankiewicz D, Sassek
M, Arafat A, Wiedenmann B, Nowak KW and Strowski MZ: Effects of
orexin A on proliferation, survival, apoptosis and differentiation
of 3T3-L1 preadipocytes into mature adipocytes. FEBS Lett.
586:4157–4164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arafat AM, Kaczmarek P, Skrzypski M,
Pruszyńska-Oszmalek E, Kołodziejski P, Szczepankiewicz D, Sassek M,
Wojciechowicz T, Wiedenmann B, Pfeiffer AF, et al: Glucagon
increases circulating fibroblast growth factor 21 independently of
endogenous insulin levels: A novel mechanism of glucagon-stimulated
lipolysis? Diabetologia. 56:588–597. 2013. View Article : Google Scholar
|
|
7
|
Gurriarán-Rodríguez U, Al-Massadi O,
Roca-Rivada A, Crujeiras AB, Gallego R, Pardo M, Seoane LM, Pazos
Y, Casanueva FF and Camiña JP: Obestatin as a regulator of
adipocyte metabolism and adipogenesis. J Cell Mol Med.
15:1927–1940. 2011. View Article : Google Scholar
|
|
8
|
Granata R, Gallo D, Luque RM, Baragli A,
Scarlatti F, Grande C, Gesmundo I, Córdoba-Chacón J, Bergandi L,
Settanni F, et al: Obestatin regulates adipocyte function and
protects against diet-induced insulin resistance and inflammation.
FASEB J. 26:3393–3411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang JV, Ren PG, Avsian-Kretchmer O, Luo
CW, Rauch R, Klein C and Hsueh AJ: Obestatin, a peptide encoded by
the ghrelin gene, opposes ghrelin's effects on food intake.
Science. 310:996–999. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chanoine JP, Wong AC and Barrios V:
Obestatin, acylated and total ghrelin concentrations in the
perinatal rat pancreas. Horm Res. 66:81–88. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miegueu P, St Pierre D, Broglio F and
Cianflone K: Effect of desacyl ghrelin, obestatin and related
peptides on triglyceride storage, metabolism and GHSR signaling in
3T3-L1 adipocytes. J Cell Biochem. 112:704–714. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pruszynska-Oszmalek E, Szczepankiewicz D,
Hertig I, Skrzypski M, Sassek M, Kaczmarek P, Kolodziejski PA,
Mackowiak P, Nowak KW, Strowski MZ and Wojciechowicz T: Obestatin
inhibits lipogenesis and glucose uptake in isolated primary rat
adipocytes. J Biol Regul Homeost Agents. 27:23–33. 2013.PubMed/NCBI
|
|
13
|
Zhang Z, Zou D, Chen Y, Wang M, Wu J and
Guo Z: Obestatin inhibits proliferation and differentiation of
3T3-L1 preadipocytes. Acad J Second Mil Med Univ. 28:929–932.
2007.In Chinese.
|
|
14
|
Ren G, He Z, Cong P, Yu J, Qin Y, Chen Y
and Liu X: Effect of TAT-obestatin on proliferation,
differentiation, apoptosis and lipolysis in 3T3-L1 preadipocytes. J
Pept Sci. 19:684–691. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shillabeer G, Li ZH, Hatch G, Kumar V and
Lau DC: A novel method for studying preadipocyte differentiation in
vitro. Int J Obes Relat Metab Disord. 20(Suppl 3): S77–83.
1996.PubMed/NCBI
|
|
16
|
Larionov A, Krause A and Miller W: A
standard curve based method for relative real time PCR data
processing. BMC Bioinformatics. 6:622005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gregoire FM, Smas CM and Sul HS:
Understanding adipocyte differentiation. Physiol Rev. 78:783–809.
1998.PubMed/NCBI
|
|
18
|
Fu Y, Luo N, Klein RL and Garvey WT:
Adiponectin promotes adipocyte differentiation, insulin
sensitivity, and lipid accumulation. J Lipid Res. 46:1369–1379.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rosen ED, Sarraf P, Troy AE, Bradwin G,
Moore K, Milstone DS, Spiegelman BM and Mortensen RM: PPAR gamma is
required for the differentiation of adipose tissue in vivo and in
vitro. Mol Cell. 4:611–617. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Farmer SR: Regulation of PPARgamma
activity during adipogenesis. Int J Obes. 29(Suppl 1): S13–S16.
2005. View Article : Google Scholar
|
|
21
|
Tang S, Dong X and Zhang W: Obestatin
changes proliferation, differentiation and apoptosis of porcine
preadipocytes. Ann Endocrinol (Paris). 75:1–9. 2014. View Article : Google Scholar
|
|
22
|
Machinal-Quélin F, Dieudonné MN, Leneveu
MC, Pecquery R and Giudicelli Y: Proadipogenic effect of leptin on
rat preadipocytes in vitro: Activation of MAPK and STAT3 signaling
pathways. Am J Physiol Cell Physiol. 282:C853–C863. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Müller G, Ertl J, Gerl M and Preibisch G:
Leptin impairs metabolic actions of insulin in isolated rat
adipocytes. J Biol Chem. 272:10585–10593. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rodríguez VM, Macarulla MT, Echevarría E
and Portillo MP: Lipolysis induced by leptin in rat adipose tissue
from different anatomical locations. Eur J Nutr. 42:149–153.
2003.PubMed/NCBI
|
|
25
|
Ren G, He Z, Cong P, Chen H, Guo Y, Yu J,
Liu Z, Ji Q, Song Z and Chen Y: Peripheral administration of
TAT-obestatin can influence the expression of liporegulatory genes
but fails to affect food intake in mice. Peptides. 42:8–14. 2013.
View Article : Google Scholar : PubMed/NCBI
|