Introduction
One of the most significant changes during pregnancy
is the favorable adjustment of the maternal immune system toward
the semi-allogeneic fetus so that it will not be rejected. It has
been suggested that there is a T helper type 2 (Th-2)-biased shift
in the maternal immune system during pregnancy (1,2).
This shift has been demonstrated in pregnant mice by showing a
stronger antibody (Ab) response but a decreased delayed-type
hypersensitivity against paternal major histocompatibility complex
and other foreign antigens (1,3,4). In
addition, it has been observed that the conditions of certain
autoimmune diseases, including systemic lupus erythematosus, that
are predominantly mediated by Th-2 cells often worsen during
pregnancy whereas the conditions of other autoimmune diseases, such
as rheumatoid arthritis that are predominantly mediated by T helper
type 1 (Th-1) cells often regress (5). Similarly, preferential production of
Th-2 cytokines over Th-1 cytokines in the placenta has been
observed during pregnancy with non-immune cells contributing to the
Th-2 predominance (2,6–8).
This differential induction of cytokines appears to be consistent
with their known functions during pregnancy, whereas Th-1
cytokines, including IFN-γ, IL-2 and TNF, promote fetal loss
(9–12), Th-2 cytokines (IL-10) are
protective against fetal mortality in a murine model of spontaneous
reabsorption (11,13).
The exact causes for these marked local and systemic
immunological alterations occurring during pregnancy remain to be
elucidated. It has been suggested that alterations in endogenous
hormones during pregnancy may contribute to some of the changes. A
number of previous studies have investigated the potential roles of
progesterone, 17β-estradiol (E2), chorionic gonadotropin
and chorionic somatomammotropin in mediating immunosuppression
(14–18). Progesterone, an essential hormone
for maintaining pregnancy, was found to have diverse effects on
different populations of immune cells, including an
immunosuppressive effect in favor of fetal survival (14). Notably, E2 has pro- and
anti-inflammatory effects depending on the dose used (18). During pregnancy, the
quantitatively-predominant estrogen produced in the body is estriol
(E3). However, relatively little is known about its
modulating effect on the immune system during pregnancy. Our
previous study reported that treatment of female BALB/c mice with a
subcutaneous pellet containing 2.5 mg E3 alters the
serum levels of specific Abs against bovine serum albumin (BSA), a
circulating protein, and pneumococcal polysaccharide serotype-14
(PPS-14), a bacterial wall component, in a divergent manner,
whereas E2 (at 0.36 mg E2 per pellet) did not
have the same effect (19). It is
suggested that these alterations elicited by E3 may
increase the ability of a pregnant female to ward off bacterial
infections while decreasing the incidence of autoimmune responses
against circulating components from either the fetus or pregnant
females. The present study aimed to determine the detailed
dose-response relationship of E3 (at selected doses of
0, 0.5, 2.5, 5, 10 or 15 mg E3/pellet) in modulating the
changes of these two specific Abs.
Materials and methods
Chemicals and reagents
Cholesterol, o-phenylenediamine, Tween-20 and BSA
were obtained from Sigma-Aldrich (St. Louis, MO, USA).
E3 was purchased from Steraloids (Newport, RI, USA).
Hydrogen peroxide and gelatin were purchased from Thermo Fisher
Scientific (Pittsburgh, PA, USA). Purified Streptococcus
pneumoniae capsular PPS-14 was obtained from the American Type
Culture Collection (Manassas, VA, USA). Polyclonal goat anti-mouse
immunoglobulin (Ig)G was obtained from Chemicon (Temecula, CA,
USA), and monoclonal rat anti-mouse IgM and rat anti-mouse IgA were
obtained from eBioscience (San Diego, CA, USA). The horseradish
peroxidase (HRP)-conjugated polyclonal goat Abs against mouse IgM,
IgA, IgG, IgG1, IgG2a or IgG2b were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA).
The pellets containing either E3 or
vehicle alone were hand-pressed using the Pellet Presser (PARR
Instrument Co., Moline, IL, USA). Each pellet (weighing 25 mg)
contained 5 mg of sodium chloride and a combined 20 mg of
E3 plus cholesterol. Five different doses of
E3 were used in the present study. In each pellet, the
quantity of E3 was 0.5, 2.5, 5, 10 or 15 mg. Dry
crystals of sodium chloride, cholesterol and estrogen were mixed
thoroughly by grinding the mixtures to fine powders. The pellets
were produced by the same individual by applying approximately the
same quantity of pressing force.
Animal experiments
All animal experimental procedures described in the
present study were approved by the Institutional Animal Care and
Use Committee (IACUC) of the University of Kansas Medical Center at
Kansas City (Kansas, USA). Female 7-8-week-old BALB/c mice (6
animals per treatment group), purchased from Harlan Laboratories
(Indianapolis, IN, USA), were housed in a room with controlled
conditions of temperature (22±2° C) and light (12-h-light and
12-h-dark cycle), and had free access to food and water. Animals
were allowed to acclimate to the new environment for 1 week prior
to the beginning of the experiment.
Each animal was subcutaneously implanted with a
25-mg pellet containing different doses of E3 (0, 0.5,
2.5, 5, 10 or 15 mg/pellet) under the dorsal skin. Our previous
study demonstrated that the average plasma concentration of free
E3 at 5 days after implantation of a 10-mg E3
pellet was 12.5 nM (20). Notably,
the basal circulating levels of E3 in non-pregnant
females were around 50 nM (21).
Thus, the doses of E3 used in the present study were
expected to provide circulating concentrations of E3 in
physiologically-relevant ranges. The animals (5 per group) were
injected with BSA or PPS-14 7 days after implantation of the
estrogen-containing pellet. For BSA immunization, three
intraperitoneal (i.p.) injections of 400 g BSA in 50 µl
phosphate-buffered saline (PBS) were administered to the animals
once every 14 days. For PPS-14 immunization, two i.p. injections of
10 µg PPS-14 in 50 µl PBS were administered once
every 14 days. All the solutions injected into animals were
sterilized.
The animals were sacrificed by CO2
inhalation and the blood samples were collected 1 week after the
last antigen injection. The sera were prepared by centrifugation at
1,500 ×g for 10 min. Aliquots of the serum samples were stored at
−80° C until further assessment. The thymus, spleen and heart were
collected from each animal and the wet weight of the organs was
measured for evaluation of the effect of estrogen.
ELISA
The levels of BSA-specific Abs in mouse sera were
determined by using the standard ELISA method. Briefly, 96-well
plates (Nunc™ Maxisorp™; Nalge Nunc International, Rochester, NY,
USA) were coated with 50 µl BSA (50 µg/ml in 0.1 M
NaHCO3, pH 9.6) overnight at 4° C. After 1 h blocking of
the wells with 150 µl of 0.25% (w/v) gelatin in PBS, a
series of diluted sera were added into each well and incubated for
1 h at room temperature. The HRP-conjugated goat anti-mouse Ig Abs
with specificity for different Ig classes were used as the
secondary Ab at a dilution of 1:1,000 for anti-mouse IgG1 and
1:2,000 for anti-mouse IgG2a, IgG2b and IgM. Following 1 h
incubation with the secondary Ab, freshly prepared substrate (1
mg/ml o-phenylenediamine in 0.1 M citrate buffer + 1.5 µl/ml
hydrogen peroxide) was added and the optical density (OD) value in
each well was measured at 450 nm using a kinetic microplate reader
(Molecular Devices, Sunnyvale, CA, USA). Every well was washed
three times with 200 µl of PBS containing 0.05% (v/v)
Tween-20 following incubation with the primary and secondary
Abs.
Levels of the PPS-14-specific Abs in the mouse sera
were determined as described above. A total of 50 µl of
PPS-14 (5 µg/ml) in PBS was added to each well for coating
for 2 h at 37° C. A BSA solution (10 mg/ml in PBS) was used as the
blocking buffer. Serially diluted serum, secondary Abs and the
substrate solution were added similarly as mentioned above. The OD
value at 450 nm was then measured.
The total Ab levels present in the sera were
determined by using the capture ELISA method. A pre-determined
optimal concentration of the capture Ab was used instead of a
specific antigen. Following coating with the capture Abs, the
plates were developed in the same way as mentioned above. The goat
anti-mouse IgG polyclonal Ab was used as the capturing Ab for IgG1,
IgG2a and IgG2b at 1 µg/ml, and the rat anti-mouse IgM
monoclonal Ab was used at 2 µg/ml to capture IgM.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical significance was analyzed using Student's
t-test with Microsoft Excel (Microsoft, Seattle, WA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
E3 inhibits the production of
BSA-induced specific Abs
Dose-response experiments were conducted to
determine the modulating effect of E3 on the production
of specific Abs against BSA, a circulating protein antigen, using
female BALB/c mice as a model. Animals were initially implanted
with a pellet containing the vehicle alone (no estrogen) or five
different doses of E3 (0.5, 2.5, 5, 10 or 15 mg/pellet),
and then immunized with BSA. As shown in Fig. 1, treatment of animals with
E3 + BSA resulted in dose-dependent inhibition of the
production of BSA-specific IgM, IgA, IgG, IgG1, IgG2a and IgG2b
compared with the control animals treated with the vehicle + BSA.
At relatively lower doses (0.5 and 2.5 mg), E3 had
little or no effect on the production of BSA-specific IgM, IgG,
IgG1 and Ig2b, however, the production of IgA and IgG2a was more
sensitive to inhibition. At higher doses (5, 10 and 15 mg),
E3 decreased serum levels of all classes and subclasses
of specific Igs in BALB/c mice in a dose-dependent manner. The
levels of BSA-specific IgM, IgA, IgG, IgG1, IgG2a and IgG2b in
animals treated with 10 and 15 mg E3 were only 20–30% of
the levels in control animals.
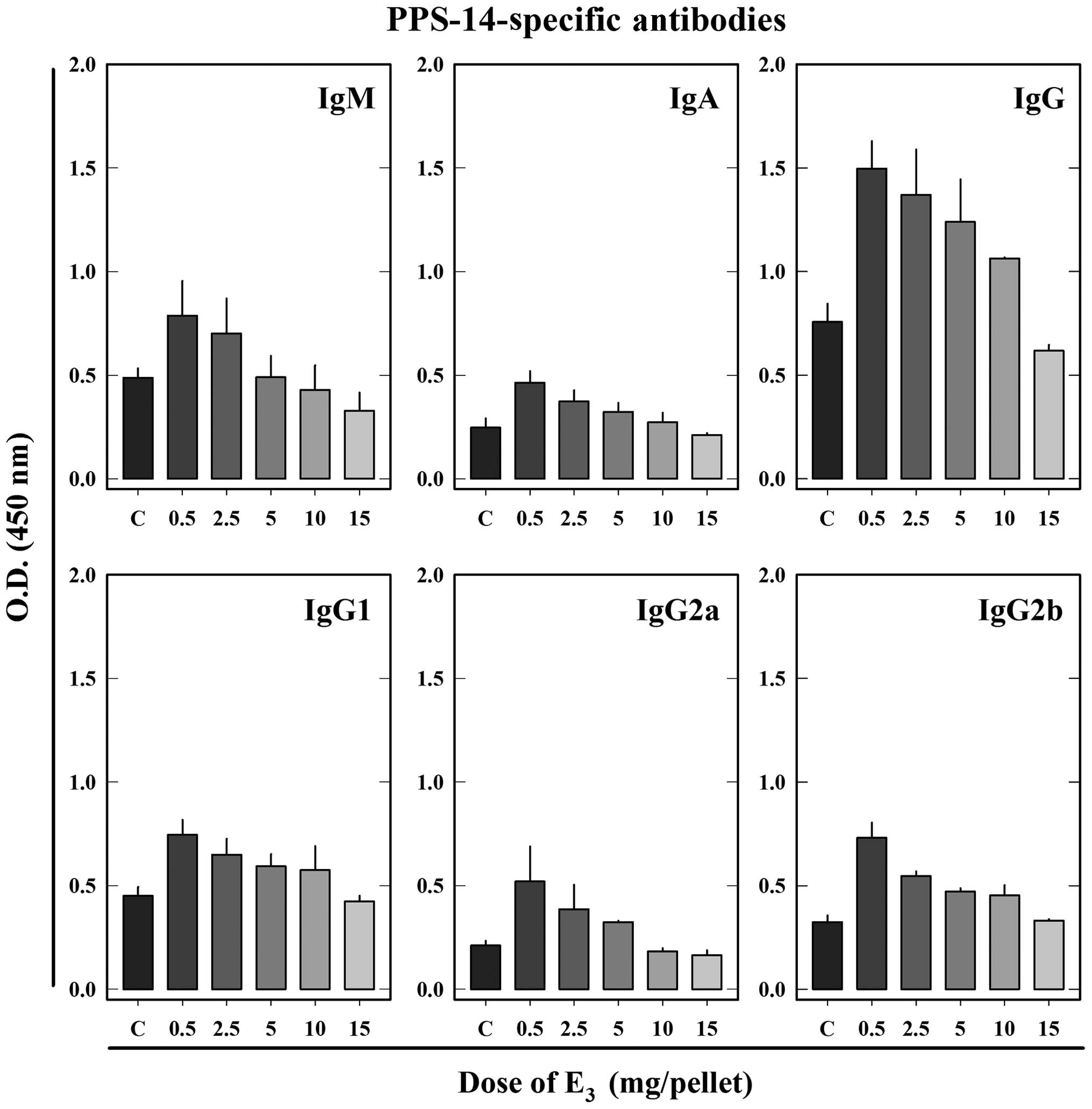
E3 stimulates the production
of PPS-14-induced specific Abs
In order to assess the effect of E3 on
the production of specific Abs against PPS-14, a component of the
bacterial wall, female BALB/c mice were similarly implanted with a
pellet containing different doses of E3 followed by i.p.
injections of PPS-14. As shown in Fig.
2, treatment of animals with a low dose of E3 (0.5
mg) + PPS-14 significantly increased PPS-14-specific Ab levels for
all assessed Ig classes and subclasses (IgM, IgA, IgG, IgG1, IgG2a
and IgG2b) compared with the control animals treated with the
vehicle + PPS-14. IgM, the dominant class among the induced
PPS-14-specific Abs, was increased by ~60% over the control. The
serum levels of PPS-14-specific IgA were also elevated by
E3 treatment at a dose of 0.5 mg and the increase was
increased by 90% compared with the control. Specific total IgG was
increased by 100%, IgG2a by 150%, IgG2b by 130% and IgG1 by
70%.
With increasing doses, the stimulatory effect of
E3 on PPS-14-specific Ab production was gradually
reversed in a dose-dependent manner. Although E3 at a
dose of 2.5 mg also increased PPS-14-specific Ab production, the
extent of the increase was less than that observed at a dose of 0.5
mg. The stimulatory effect of E3 was further weakened
when E3 was increased to 5 and 10 mg. Animals treated
with E3 at 15 mg had comparable levels of
PPS-14-specific IgG1 and IgG2b compared with the control animals
(without E3 treatment), and levels of specific IgM, IgA,
IgG and IgG2a were partially lower than the corresponding
controls.
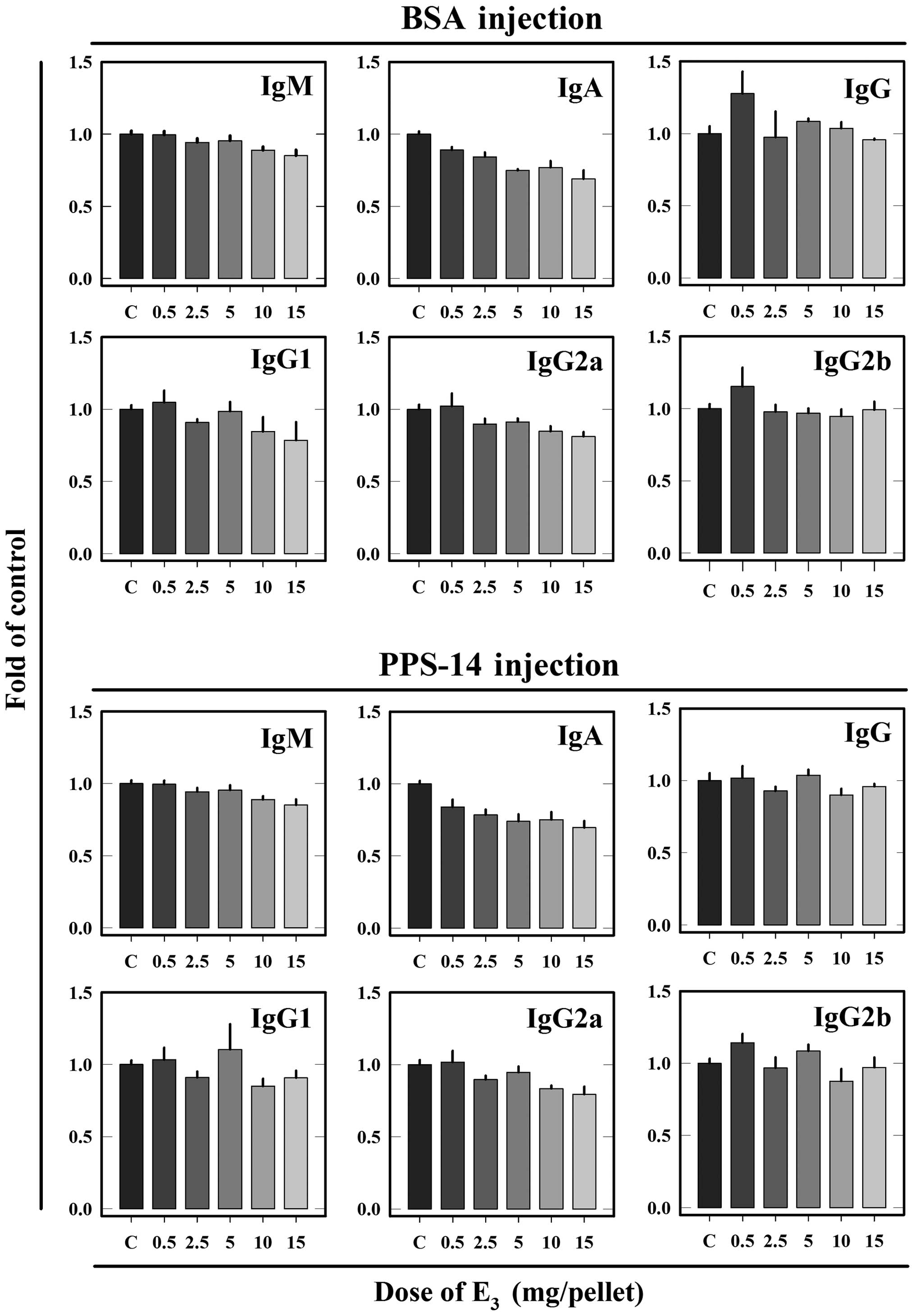
Effect of different doses of
E3 on the static status of the immune system
For comparison, the effect of different doses of
E3 on the total serum Ab levels (specific + nonspecific
Abs) as well as on the weight of central (thymus) and peripheral
(spleen) lymph organs in animals without antigen challenge was also
determined. The total Ab levels in control and
E3-treated animals are summarized in Fig. 3. In the same groups of animals,
alterations in total Ab levels were markedly less pronounced
compared with changes in specific Ab levels. Levels of total IgM,
IgA and IgG2a in animals immunized with BSA or PPS-14 were
partially decreased by E3 treatment in a dose-dependent
manner. By contrast, levels of total IgG, IgG1 and IgG2b in animals
immunized with BSA or PPS-14 were not considerably affected by
E3 treatment.
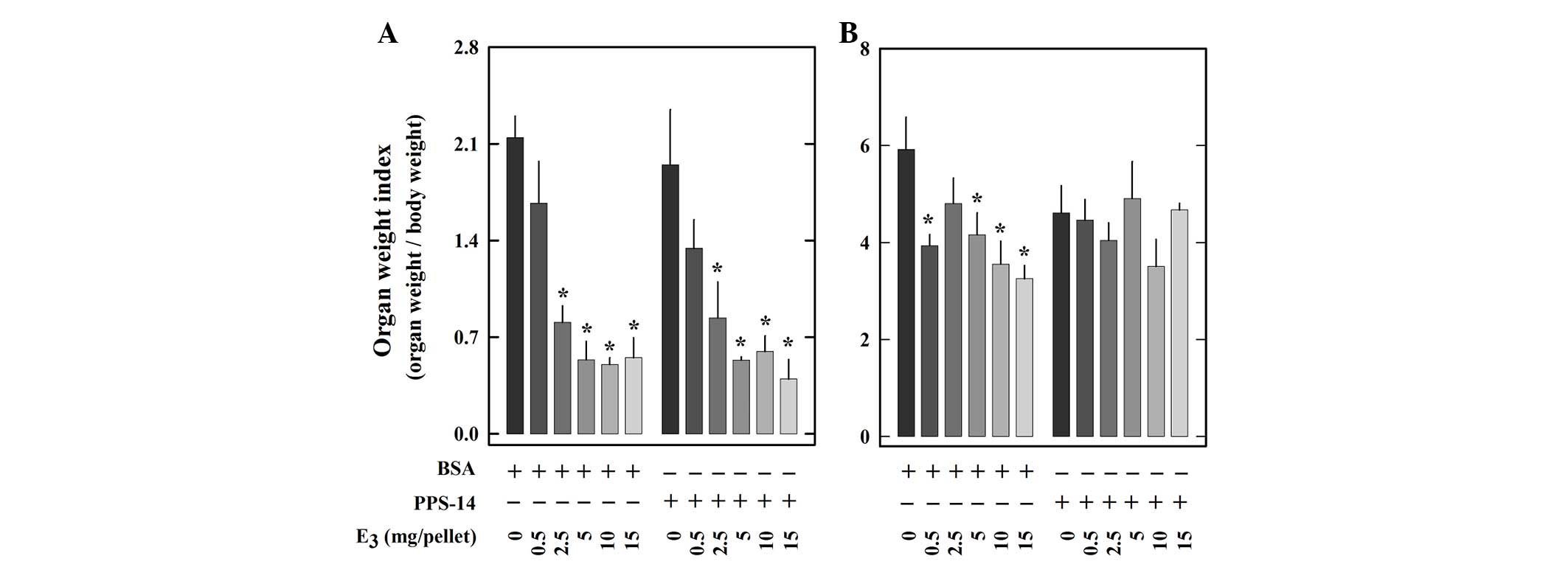
Wet weights of the thymus and spleen of each animal
were determined and the ratios of the organ weights to body weights
(organ weight indices) are summarized in Fig. 4. Treatment with E3
caused a dose-dependent decrease of thymus weight index in animals
either immunized with BSA or PPS-14 (Fig. 4A). By contrast, the spleen weight
was reduced by E3 treatment only in animals immunized
with BSA, but not in animals immunized with PPS-14 (Fig. 4B). In addition, the weight index of
the heart, which is known to be unaffected by estrogen treatment,
was also determined for comparison. As expected, no significant
difference was observed for heart weight indices among animals of
different treatment groups (data not shown).
Discussion
Hormonal alterations during pregnancy have been
suggested to have a contributing role in the changes observed in
the maternal immune system. Progesterone, estrogens (particularly
E2) and chorionic gonadotropin have all been indicated
to be important in the suppression of maternal immunity for the
full acceptance of the allogeneic fetus (14,16).
By contrast, few studies have examined the role of E3,
an estrogen produced in a large quantity only during human
pregnancy, in the induction of immunotolerance during pregnancy.
Jansson et al reported that E3 has a more potent
therapeutic effect on experimental autoimmune encephalomyelitis
than does E2 (22).
However, Bebo et al indicated that an ameliorating effect of
E3, which was observed at circulating levels similar to
pregnancy, was not significantly different from E2
(23). The present study reported
a unique modulating effect of E3 on antigen-induced Ab
responses in vivo. It is apparent that this effect depends
on the dose of E3 used. E3 at doses of 0.5
and 2.5 mg did not have a significant effect on BSA-specific Ab
production. However, at higher doses (>5 mg), it markedly
reduced the serum levels of BSA-specific Abs in a dose-dependent
manner. By contrast, E3 had a stimulatory effect on
PPS-14-induced specific Ab production. The stimulation was
strongest at a low dose (0.5 mg) and weakened at higher doses (up
to 15 mg). Notably, a similar phenomenon was reported by Salazar
et al (24). By injecting
heat-killed Streptococcus pneumoniae to mice, it was
demonstrated that propanil (an herbicide with endocrine-disrupting
activity) significantly increased the number of phosphatidylcholine
(a TI-2 antigen of the bacterium)-specific Ab-secreting B cells in
the spleen whereas the Ab response to pneumococcal surface protein
A (a bacterial TD antigen) was not affected (24). A possible explanation for these
results is that Ab production induced by TI-2 and TD antigens is
regulated differently. While cytokine production is important for
both, direct interaction between T and B cells may have a critical
role in TD antigen-induced Ab response. E3 may function
differently in these two pathways. It is of note that since
E3 is known to promote the production of tolerogenic
dendritic cells in vivo, which would be protective against
autoimmunity (25), the effect of
E3 as observed in the present study may also contribute
to the unique modulating effect of E3 on antigen-induced
Ab response in vivo.
Similar to E2, E3 was found to
have a pronounced effect on the thymus. Atrophy induced by estrogen
treatment was as high as 75% when the in vivo estrogen dose
was ≥5 mg/pellet (regardless of the antigens used). In the spleen,
marked changes were only observed for BSA-injected mice but not for
PPS-14-injected mice. These observations are in line with the
differential modulating effects of E3 on BSA-specific
and PPS-14-specific Ab production. The reduction in spleen weight
following E3 treatment in BSA-immunized mice possibly
indicates a decrease in the total cell population, as flow
cytometric analysis demonstrated that the percentages of splenic
Th, Tc and B cells are hardly affected by E3. A
non-selective suppression of all splenic lymphocytes by
E3 may account for the observed decreases in all
BSA-specific Abs.
In conclusion, the data presented in the present
study suggest that the immunological alterations observed during
pregnancy may be partly associated with the immune-modulating
effect of E3, which is a quantitatively-predominant
estrogen produced during pregnancy. When E3 was used at
a dose of 5 mg/pellet, which would result in in vivo
estrogen levels relevant to those observed during human pregnancy
(20,21), it downregulated the production of
BSA-specific Abs, whereas it upregulated the production of
PPS-14-specific Abs. This is considered to be beneficial for
reducing the risk of developing Ab-mediated autoimmune attacks
against the maternal and fetal components during pregnancy while
enhancing the ability of the maternal body to ward off bacterial
infections. Therefore, it is suggested that E3 may
partially contribute to the development of fetal tolerance,
although other maternal factors, including other hormones, T
regulatory cells and suppressive dendritic cells, may also
contribute to the maintenance of a successful pregnancy.
Acknowledgments
The authors would like to thank the University of
Kansas Medical Center Endowment Fund for supporting the research
described in this study.
References
|
1
|
Wegmann TG, Lin H, Guilbert L and Mosmann
TR: Bidirectional cytokine interactions in the maternal-fetal
relationship: Is successful pregnancy a TH2 phenomenon? Immunol
Today. 14:353–356. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin H, Mosmann TR, Guilbert L,
Tuntipopipat S and Wegmann TG: Synthesis of T helper 2-type
cytokines at the maternal-fetal interface. J Immunol.
151:4562–4573. 1993.PubMed/NCBI
|
|
3
|
Fabris N: Immunological reactivity during
pregnancy in the mouse. Experientia. 29:610–612. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carter J: The effect of progesterone,
oestradiol and HCG on cell-mediated immunity in pregnant mice. J
Reprod Fertil. 46:211–216. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cutolo M, Sulli A, Capellino S, Villaggio
B, Montagna P, Seriolo B and Straub RH: Sex hormones influence on
the immune system: Basic and clinical aspects in autoimmunity.
Lupus. 13:635–638. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chaouat G, Cayol V, Mairovitz V and
Dubanchet S: Localization of the Th2 cytokines IL-3, IL-4, IL-10 at
the fetomaternal interface during human and murine pregnancy and
lack of requirement for Fas/Fas ligand interaction for a successful
allogeneic pregnancy. Am J Reprod Immunol. 42:1–13. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chaouat G: Regulation of T-cell activities
at the feto-placental interface - by placenta? Am J Reprod Immunol.
42:199–204. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mishan-Eisenberg G, Borovsky Z, Weber MC,
Gazit R, Tykocinski ML and Rachmilewitz J: Differential regulation
of Th1/Th2 cytokine responses by placental protein 14. J Immunol.
173:5524–5530. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chaouat G, Menu E, Clark DA, Dy M,
Minkowski M and Wegmann TG: Control of fetal survival in CBA x
DBA/2 mice by lymphokine therapy. J Reprod Fertil. 89:447–458.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gendron RL, Nestel FP, Lapp WS and Baines
MG: Lipopolysaccharide-induced fetal resorption in mice is
associated with the intrauterine production of tumour necrosis
factor-alpha. J Reprod Fertil. 90:395–402. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chaouat G, Assal Meliani A, Martal J,
Raghupathy R, Elliott JF, Mosmann T and Wegmann TG: IL-10 prevents
naturally occurring fetal loss in the CBAxDBA/2 mating combination
and local defect in IL-10 production in this abortion-prone
combination is corrected by in vivo injection of IFN-tau. J
Immunol. 154:4261–4268. 1995.PubMed/NCBI
|
|
12
|
Arck PC, Troutt AB and Clark DA: Soluble
receptors neutralizing TNF-alpha and IL-1 block stress-triggered
murine abortion. Am J Reprod Immunol. 37:262–266. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Murphy SP, Fast LD, Hanna NN and Sharma S:
Uterine NK cells mediate inflammation-induced fetal demise in
IL-10-null mice. J Immunol. 175:4084–4090. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Siiteri PK and Stites DP: Immunologic and
endocrine interrelationships in pregnancy. Biol Reprod. 26:1–14.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Munroe JS: Progesteroids as
immunosuppressive agents. J Reticuloendothelial Soc. 9:361–375.
1971.
|
|
16
|
Lawrence R, Church JA, Richards W and
Borzy M: Immunological mechanisms in the maintenance of pregnancy.
Ann Allergy. 44:166–173. 1980.PubMed/NCBI
|
|
17
|
Jansson L and Holmdahl R:
Estrogen-mediated immunosuppression in autoimmune diseases. Inflamm
Res. 47:290–301. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ahmed SA and Talal N: Sex hormones and the
immune system - Part 2. Animal data Baillieres Clin Rheumatol.
4:13–31. 1990. View Article : Google Scholar
|
|
19
|
Ding J and Zhu BT: Unique effect of the
pregnancy hormone estriol on antigen-induced production of specific
antibodies in female BALB/c mice. Steroids. 73:289–298. 2008.
View Article : Google Scholar
|
|
20
|
Zhou R, Lai Y, Yamabe N, Fukui M and Zhu
BT: Estriol has different effects from 17β-estradiol in modulating
mouse splenocyte function under inflammatory conditions. J
Immunotoxicol. 8:346–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaijser M, Granath F, Jacobsen G,
Cnattingius S and Ekbom A: Maternal pregnancy estriol levels in
relation to anamnestic and fetal anthropometric data. Epidemiology.
11:315–319. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jansson L, Olsson T and Holmdahl R:
Estrogen induces a potent suppression of experimental autoimmune
encephalomyelitis and collagen-induced arthritis in mice. J
Neuroimmunol. 53:203–207. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bebo BF Jr, Fyfe-Johnson A, Adlard K, Beam
AG, Vandenbark AA and Offner H: Low-dose estrogen therapy
ameliorates experimental autoimmune encephalomyelitis in two
different inbred mouse strains. J Immunol. 166:2080–2089. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salazar KD, de la Rosa P, Barnett JB and
Schafer R: The polysaccharide antibody response after Streptococcus
pneumoniae vaccination is differentially enhanced or suppressed by
3,4-dichloropropionanilide and 2,4-dichlorophenoxyacetic acid.
Toxicol Sci. 87:123–133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Papenfuss TL, Powell ND, McClain MA,
Bedarf A, Singh A, Gienapp IE, Shawler T and Whitacre CC: Estriol
generates tolerogenic dendritic cells in vivo that protect against
autoimmunity. J Immunol. 186:3346–3355. 2011. View Article : Google Scholar : PubMed/NCBI
|