Introduction
Thrombin is one of the key components for the
coagulation cascade and homeostasis (1,2). It
also has a role in the proliferation and migration of vascular
smooth muscle cells (VSMCs), which contributes to atherosclerosis
and re-stenosis (1,2). The proliferation of VSMCs induced by
thrombin is associated with the phosphorylation of signaling
molecules, including extracellular signal-regulated kinase 1/2
(ERK1/2), c-Jun N-terminal kinase (JNK), p38/mitogen-activated
protein kinase (MAPK) and AKT (2),
leading to the activation of their respective pathways. After
stimulation with thrombin, VSMCs undergo G1-to-S-phase cell-cycle
transition, which is tightly regulated by cyclin D1 and p27KIP1
(2,3). In addition, matrix
metalloproteinase-2 (MMP-2) degrades type IV collagen, leading to
VSMC migration and the progression of arterial lesions (4,5). A
previous study has demonstrated that thrombin stimulates the MMP-2
expression via transcription factor nuclear factor (NF)-κB in
chondrosarcoma cells (6).
Ophiopogon japonicas (OJ), a plant
distributed in southeast Asia, has been used as a Traditional
Chinese Medicine (7). Numerous
studies have indicated the pharmaceutical effects of OJ, including
anti-inflammatory, anti-cardiovascular and anti-thrombotic
activities (7–11). Several chemical constituents of OJ,
including ruscogenin and ophiopogonin D, have been identified
(8). Microbial fermentation of
plants has beneficial effects, including the enrichment of
desirable metabolites generated by the beneficial probiotic
bacteria (12,13). The present study reported on the
inhibitory effects of a fermented extract of OJ (FEOJ) on the
proliferation and migration of thrombin-treated VSMCs.
Materials and methods
Materials
Polyclonal antibodies against cyclin E (sc-481),
CDK2 (sc-163), CDK4 (sc-260), p21WAF1 (sc-756), p53 (sc-126), p27
(sc-528) and GAPDH (sc-20357) were obtained from Santa Cruz
Biotechnology Inc. (Santa Cruz, CA, USA). Monoclonal antibodies
against cyclin D1 (04-221) were obtained from Millipore (Millipore,
Temecula, CA, USA). Polyclonal antibodies against ERK (9102),
phospho-ERK (9101), p38 MAP kinase (9212), phospho-p38 MAP kinase
(9211), JNK (9258), phospho-JNK (9251), AKT (9272) and phospho-AKT
(9271) were obtained from Cell Signaling Technology Inc. (Danvers,
MA, USA). Goat anti-rabbit IgG-horseradish peroxidase (HRP)
(sc-2004), goat anti-mouse IgG-HRP (sc-2005) and donkey anti-goat
IgG-HRP (sc-2020) were purchased from Santa Cruz Biotechnology Inc.
Western Lightning Plus-ECL was obtained from PerkinElmer, Inc.
(PerkinElmer, MA, USA). A Nuclear Extract kit and EMSA Gel Shift
kit were obtained from Panomics (Fremont, CA, USA).
Preparation of FEOJ
The roots of OJ (Milyang, Korea), harvested in April
2012, were used for the preparation of an aqueous extract. In
brief, dried OJ (3.5 kg) was extracted with ~6,500 ml water at
105°C for 60 min. The aqueous filtrates of OJ were adjusted to pH
6.5 with NaOH (Youngjin Chemistry, Bucheon, Korea), and autoclaved
(JS Research, Gongju, Korea) for 20 min at 121°C. Subsequently,
Cordyceps militaris was inoculated and fermented by shaking
at 141 × g at 25°C for 10 days. For the second fermentation was
conducted with a combination of 3 types of lactic acid bacteria
(Lactobacillus plantarum, Enterococcus faecium and
Bifidobacterium longum obtained from Mediogen Co., Ltd.,
Seoul, Korea), the culture medium was adjusted to pH 6.5 with NaOH,
sterilized for 40 min at 80°C and then cooled. The concoction was
inoculated with one percent of lactic acid bacteria and fermented
for two days at 37°C. The concoction was inoculated with 1% of each
lactic acid bacteria and fermented for 2 days at 37°C. The
supernatant was filtered, heated at 80°C for 40 min and
freeze-dried. Aqueous extracts were used in the subsequent
experiments.
Cell culture
Enzymatic digestion was used to isolate the rat
aortic smooth muscle cells (VSMCs) as previously reported (3). Briefly, the aortas from two young
male Sprague-Dawley rats (age, 8 weeks; weight, 200–250 g;
DHbiolink, Seoul, Korea) were excised. After elimination of the
adventitia and endothelium, the aortas were sliced, minced and
placed in 5 ml digestion solution (containing 0.125 mg/ml elastase,
0.25 mg/ml soybean trypsin inhibitor, 10 mg/ml collage-nase I, 2.0
mg/ml crystallized bovine albumin, and 15 mM HEPES; all from
Sigma-Aldrich; St Louis, MO, USA) at 37°C. After digestion for 45
min, the cellular digests were filtered using a sterile
100-µM nylon membrane (BD Biosciences, Franklin Lakes, NJ,
USA), centrifuged at 157 × g for 10 min, and maintained in
Dulbecco's modified Eagle's medium (DMEM; Corning, Corning, NY,
USA) supplemented with 10% fetal bovine serum (FBS; Corning). The
characterization of VSMCs was confirmed by immunofluorescence
staining with a monoclonal antibody against SM-α-actin (cat. no.
A7067; Sigma-Aldrich) using a fluorescent microscope (Olympus BX61;
Olympus Corporation, Seoul, Korea). Isolated cells were maintained
in DMEM containing 10% FBS, 2 mM glutamine (Sigma-Aldrich), 50
µg/ml gentamycin (Sigma-Aldrich), and 50 µl/ml
amphotericin-B (Sigma-Aldrich) at 37°C in a humidified 5%
CO2 atmosphere. All experiments were accomplished with
cells at passages five to eight. VSMCs at 80% confluence were
synchronized by incubation for 24 h in DMEM without FBS. The study
was approved by the ethics committee of Chung-Ang University
(Seoul, Korea).
Cell viability assay
Growth-arrested VSMCs (2.0×105 cells per
well) in 24-well plates were pre-treated with FEOJ (0, 1, 5 and 25
µg/ml for 40 min), followed by incubation with thrombin (2
U/ml; Sigma-Aldrich) for various time periods (6, 12 and 24 h).
Cell viability was determined using a modification of the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich), which was based on the conversion of tetrazolium
salt MTT to the formazan product by mitochondrial dehydrogenase
(3). The formazan product was
dissolved in dimethyl sulfoxide (Sigma-Aldrich). Dissolved formazan
was read on a multi-well scanning spectrophotometer (Sclavo, Siena,
Italy) by measuring absorbance at 570 nm.
Immunoblot analysis
The VSMCs (8×106 cells/well), grown to
near confluence in 100-mm tissue culture plates, were synchronized
and pre-treated with FEOJ (0, 1, 5 and 25 µg/ml for 40 min.
Subsequently, cells were incubated with thrombin (2 U/ml) for
various durations (6, 12 and 24 h) at 37°C. The cells were then
washed twice with cold phosphate-buffered saline and freeze-thawed
in 250 µl lysis buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 1
mM EDTA, 2.5 mM ethylene glycol tetraacetic acid, 1 mM
dithiothreitol, 10 mM β-glycerophosphate, 1 mM NaF, 0.1 mM
Na3VO4, 0.1 mM phenylmethylsulfonyl fluoride,
10% glycerol, 0.1% Tween-20, 10 g/ml leupeptin and 2 µg/ml
aprotinin; Sigma-Aldrich). The cells were then scraped into 1.5-ml
tubes. The lysates were placed on ice for 15 min and then
centrifuged at 16,128 × g for 20 min at 4°C. The protein
concentration of the supernatant was determined using a Bradford
reagent method (Bio-Rad Laboratories, Inc., Richmond, CA, USA).
Equal amounts of cellular proteins (30 µg) were resolved by
electrophoresis on a 0.1% SDS-10% polyacrylamide gel under
denaturing conditions. The proteins were transferred
electrophoretically to nitrocellulose membranes (Hybond, GE
Healthcare, Little Chalfont, UK). After blocking with 10 mmol/l
Tris-HCl (pH 8.0), 150 mmol/l NaCl and 5% (wt/vol) non-fat dry milk
(BD Biosciences) for 2 h, the membranes were treated with 1:1,000
dilution of primary antibodies at 4°C for 12 h, followed by
incubation with 1:5,000 dilution of goat anti-rabbit IgG HRP, goat
anti-mouse IgG HRP and donkey anti-goat IgG HRP secondary
antibodies for 2 h. The immunocomplexes were detected using the
Western Lightning Plus-ECL(PerkinElmer, Inc.). The experiments were
repeated at least three times for the immunoblotting studies
(3,14). Gray value analysis of the blots was
measured by ImagePro Plus 6.0 software (Media Cybernetics,
Rockville, MD, USA).
Wound-healing migration assay
The cells (5.0×105 cells per well) were
pre-treated with FEOJ (0, 1, 5 and 25 µg/ml) for 40 min and
a line-shaped incision to the confluent monolayer of
growth-arrested cells (2.0×105 cells per well) was
generated using a 2-mm pipette tip. Cells were then treated with
thrombin (2 U/ml) for 24 h, allowing them to migrate into the
scraped area. Images were captured using an inverted microscope
(magnification, ×40; Optika, Ponteranica, Italy).
Zymography
Growth arrested VSMCs (2×105 cells per
well) in 24-well plates were pre-treated with FEOJ (0, 1, 5 and 25
µg/ml for 40 min), followed by incubation with thrombin (2
U/ml, Sigma-Aldrich) for 24 h. The conditioned medium was
electrophoresed on a polyacrylamide gel containing 1 mg/ml gelatin
(Sigma-Aldrich). The gel was then washed at room temperature for 2
h with 2.5% Triton X-100 and maintained at 37°C overnight in a
buffer containing 10 mM CaCl2, 150 mM NaCl, and 50 mM
Tris-HCl, pH 7.5 (all from Sigma-Aldrich). The gel was stained with
0.2% Coomassie blue (Bio-Rad, Laboratories, Inc.) and images were
captured using a light box (Matin International, Co., Ltd., Seoul,
Korea). Proteolysis was detected as a white zone in a dark blue
field by ImagePro Plus 6.0 software (Media Cybernetics).
Preparation of nuclear extracts and
electrophoretic mobility shift assay (EMSA)
VSMCs (8×106 cells/well), grown to ~80%
confluence in 100 mm tissue culture plates, were synchronized and
pre-treated with EFOJ (0, 1, 5 and 25 µg/ml) for 40 min.
Subsequently, cells were incubated with thrombin (2 U/ml) for 24 h
at 37°C. An EMSA was performed to determine the nuclear factor
(NF)-κB DNA binding activity, in which a labeled double-stranded
DNA sequence (NF-κB; CAG TGG AAT TCC CCA GCC; Bioneer Corporation,
Daejeon, Korea) was used as a DNA probe to bind active NF-κB
protein in nuclear extracts. Nuclear extracts were prepared with a
Nuclear Extract kit (Panomics, Fremont, CA, USA). The EMSA was
accomplished by incubating a biotin-labeled transcription factor
(NF-κB) probe with treated and untreated nuclear extracts. The
labeled oligonucleotides and nuclear extracts were incubated with
or without a 100-fold molar excess of unlabeled NF-κB DNA sequence
as a competitor. Samples were electrophoresed on a non-denaturing
6% polyacrylamide gel. The NF-κB-DNA adduct in the gel were
transferred onto a nylon membrane and detected using horseradish
peroxidase-conjugated streptavidin (cat. no. AY1000; Panomics) and
a chemiluminescent substrate (Panomics). Gray value analysis of the
blots was measured by ImagePro Plus 6.0 software (Media
Cybernetics, Rockville, MD, USA)
Statistical analysis
All data are presented as the mean ± standard error.
Comparisons between two groups were analyzed with factorial
analysis of variance and Fisher's least significant difference
test. P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed using
PASW Statistics 18.0.1 software (SPSS, Inc., Chicago, IL, USA).
Results
FEOJ inhibits thrombin-induced
proliferation of VSMCs
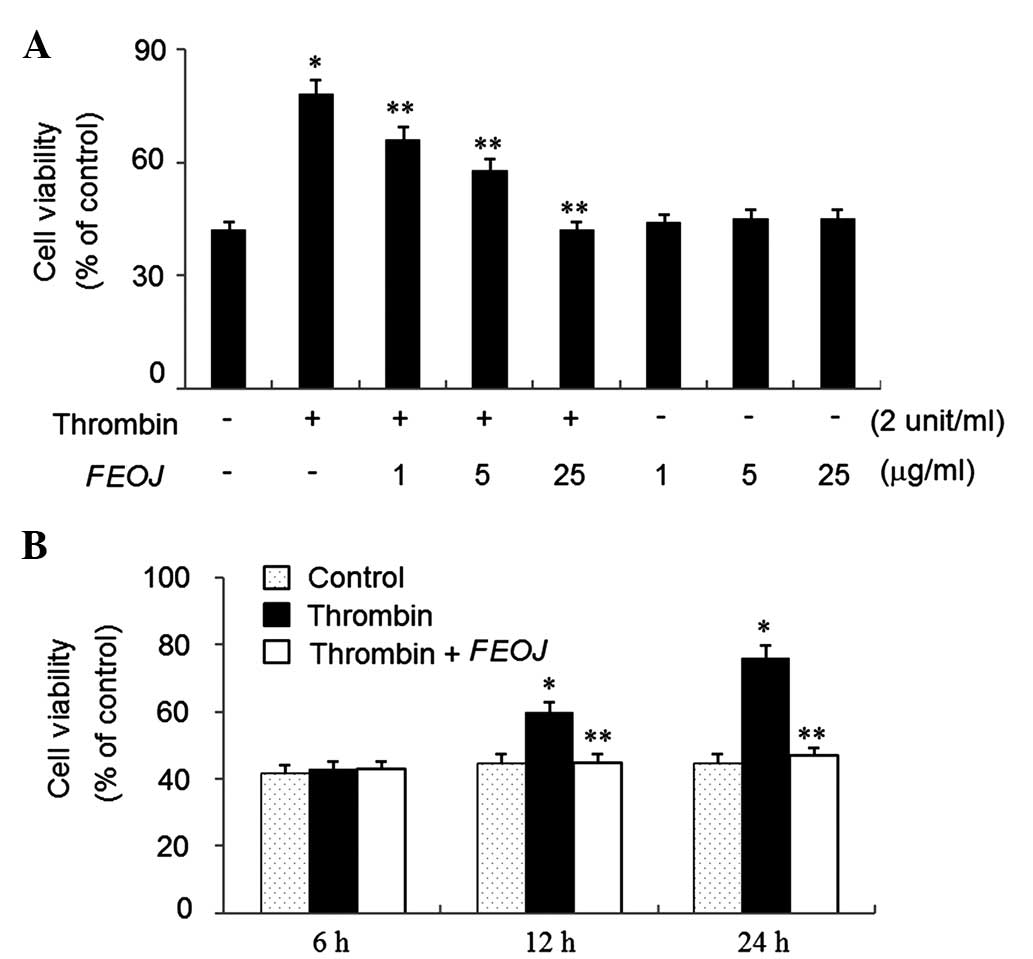
To examine the effects of FEOJ on the viability of
VSMCs, an MTT assay was used. Thrombin treatment for 24 h increased
the cell viability by ~1.8-fold of that of non-treated control
cells (Fig. 1A). However,
thrombin-induced viability of VSSCs was suppressed by FEOJ (1–25
µg/ml) in a concentration-dependent manner (Fig. 1A). Furthermore, as shown in
Fig. 1B, FEOJ suppressed the
viability of VSMCs induced by thrombin in a time-dependent manner,
while FEOJ alone (1–25 µg/ml) did not affect cell viability
(Fig. 1A). These results indicated
that FEOJ suppressed the proliferation of VSMCs induced by
thrombin.
FEOJ suppresses positive cell-cycle
regulator cyclin D1 and induces cell-cycle inhibitor p27KIP1 in
thrombin-treated VSMCs
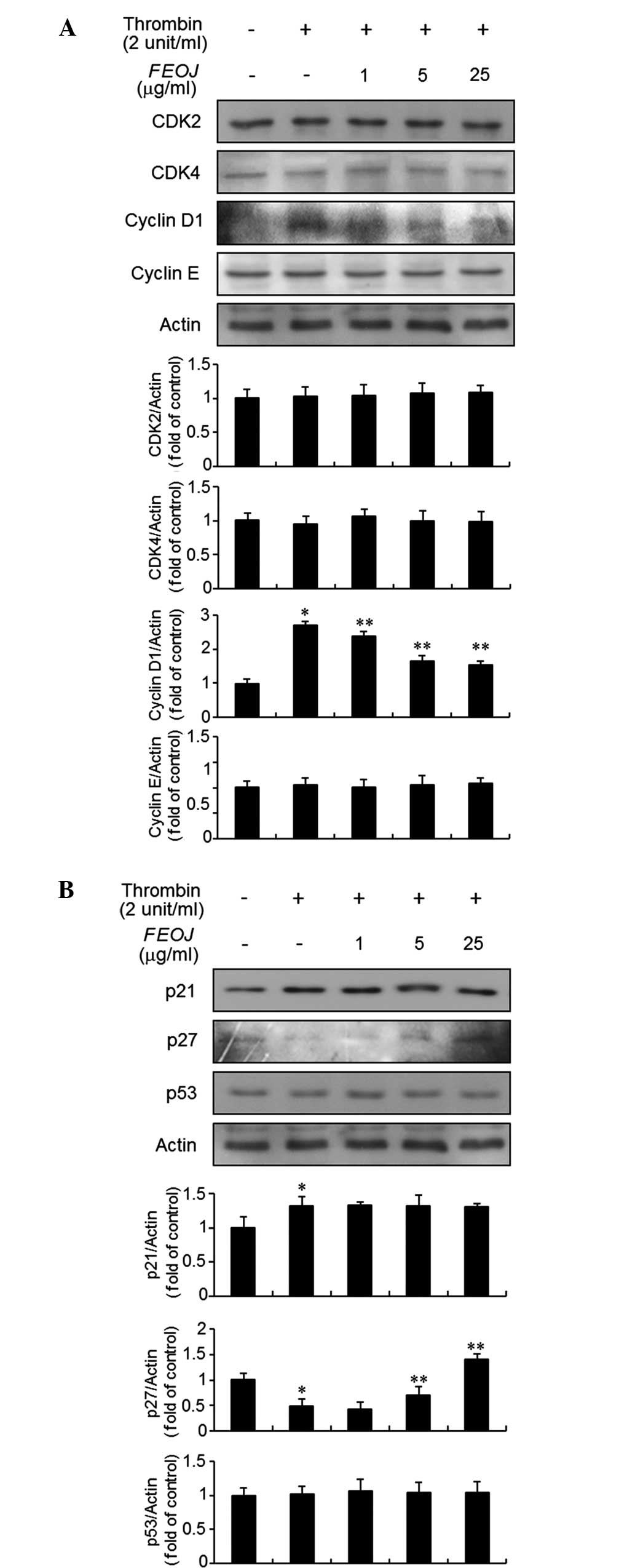
As FEOJ inhibited the proliferation of
thrombin-treated VSMCs, the present study next examined the effects
of FEOJ on G1-phase cell-cycle proteins using immunoblot analysis.
As shown in Fig. 2A, thrombin
treatment resulted in a significant increase of cyclin D1
expression in VSMCs. However, the expression of cyclin E, CDK2, and
CDK4 in VSMCs was not affected by thrombin treatment (Fig. 2A). Of note, Pre-treatment of VSMCs
with FEOJ inhibited the cyclin D1 expression (Fig. 2A). Previous studies demonstrated
that CDKs and cyclins are negatively regulated by cyclin-dependent
kinase inhibitors, including p21WAF1 and p27KIP1 (15,16).
Therefore, the present study examined the effects of FEOJ on
p21WAF1 and p27KIP1 expression in thrombin-treated VSMCs. Treatment
of VSMCs with thrombin induced the expression of p21WAF1, which
was, however, not affected by FEOJ (Fig. 2B). By contrast, p27KIP1 expression
was decreased by thrombin treatment in VSMCs, which was reversed by
FEOJ (Fig. 2B). However, the
expression of p53 in VSMCs was not affected by thrombin with or
without FEOJ pre-treatment (Fig.
2B). These results indicated that FEOJ-induced inhibition of
cell proliferation was mediated via reduction of thrombin-induced
expression of the G1-phase cell-cycle protein cyclin D1 and
reversal of thrombin-induced inhibition of the expression of cell
cycle inhibitor p27KIP1 in VSMCs.
FEOJ inhibits thrombin-induced
phosphorylation of AKT in VSMCs
Previous studies have demonstrated that thrombin
stimulates the phosphorylation of ERK1/2, JNK, p38/MAPK and AKT in
VSMCs (2). Therefore, the present
study examined the effects of FEOJ on the intracellular signaling
pathway induced by thrombin in VSMCs. As expected, after
stimulation with thrombin for 10 min, the expression levels of
ERK1/2, JNK, p38/MAPK and AKT in VSMCs were significantly increased
(Fig. 3A and B). Of note,
pre-treatment of the cells with FEOJ inhibited AKT phosphorylation
induced by thrombin (Fig. 3B).
However, thrombin-induced phosphorylation of ERK1/2, JNK and
p38MAPK was not affected by FEOJ (Fig.
3A). These results suggested that FEOJ blocks thrombin-induced
proliferation of VSMCs via suppressing the AKT signaling
pathway.
 | Figure 3FEOJ inhibits the phosphorylation of
AKT in thrombin-treated VSMCs. (A and B) VSMCs were pre-treated
with various concentrations of FEOJ for 40 min and then stimulated
with thrombin (2 U/ml) for 10 min. Immunoblot analysis was
performed using antibodies specific for p-ERK1/2, ERK1/2, p-p38,
p38, p-JNK, JNK, p-AKT and AKT. Values are expressed as the mean ±
standard error from three triplicate experiments.
*P<0.01 compared with no thrombin treatment.
**P<0.01 compared with no thrombin treatment. p-ERK,
phosphorylated extracellular signal-regulated kinase; JNK, c-Jun
N-terminal kinase. FEOJ, extract of fermented Ophiopogon
japonicas; VSMC, vascular smooth muscle cell. |
FEOJ blocks thrombin-induced migration of
VSMCs
Migration of VSMCs is a main step in atherosclerosis
(1,2) and thrombin has been reported to
induce migration of VSMCs (4,5). To
investigate whether FEOJ inhibits thrombin-induced migration of
VSMCs, a wound-healing assay was used. As shown in Fig. 4, treatment of VSMCs with thrombin
for 24 h caused a significant increase in cell migration. However,
pre-treatment with FEOJ inhibited thrombin-induced VSMC migration
in a concentration-dependent manner (Fig. 4). These results suggested that FEOJ
suppresses thrombin-stimulated migration of VSMCs.
FEOJ abolishes thrombin-stimulated MMP-2
expression via the inhibition of NF-κB binding activity
Accumulating evidence suggested that MMP-2
expression is involved in the migration of VSMCs (4,5). To
investigate the efficacy of FEOJ in blocking the regulation of
MMP-2 expression by thrombin, a gelatin zymographic assay was
employed. Treatment of VSMCs with thrombin resulted in a
significant increase of MMP-2 expression, which was suppressed by
FEOJ (Fig. 5A). To elucidate the
underlying regulatory mechanism of the inhibitory effects of FEOJ
on thrombin-induced MMP-2 expression, an EMSA assay was used. The
results revealed that nuclear extracts of VSMCs treated with
thrombin strongly stimulated the DNA-binding activity of NF-κB
(Fig. 5B). However, FEOJ treatment
abrogated the increased NF-κB binding activity in thrombin-treated
VSMCs (Fig. 5B). These results
demonstrated that FEOJ abolished thrombin-induced MMP-2 expression,
at least in part, by inhibiting the DNA-binding activity of
transcription factor NF-κB.
Discussion
Fermented medicinal herbs have been demonstrated to
be suitable for treating a wide range of diseases, including
cerebral hemodynamics, esophageal cancer, locally prevalent
inflammatory reactions, scapulohumeral periarthritis (of the stasis
type), chronic superficial gastritis and dysuria (13). The present study investigated the
mechanisms of the anti-atherogenic effects of FEOJ on VSMCs
stimulated by thrombin.
The present study showed that FEOJ treatment
inhibited thrombin-induced proliferation of VSMCs without exerting
any cytotoxic effects, as evidenced by an MTT assay. The
suppressive effects of FEOJ were associated with the inhibition of
G1-phase cell-cycle regulatory protein cyclin D1. The results of
the present study demonstrated that FEOJ treatment significantly
attenuated thrombin-induced increases in the expression of cyclin
D1 in VSMCs. The expression of cyclins and CDKs is highly regulated
by the cyclin-dependent kinase inhibitors p21WAF1 and p27KIP1
(15,16). It has been demonstrated that
p27KIP1 expression was reduced during vascular injury (17). In addition, adenovirus-mediated
overexpression of p27 suppressed neointimal lesion formation in
balloon-injured arteries (18).
The present study reported a distinct downregulation of p27KIP1
expression in VSMCs treated with thrombin, which was reversed by
pre-treatment with FEOJ. p21WAF1 was originally identified as an
anti-proliferative factor (16,17).
Several lines of evidence showed that p21WAF1 expression is
involved in the proliferation and migration of VSMCs (19,20).
In the present study, thrombin stimulated p21WAF1 expression, which
was not affected by pre-treatment with FEOJ. These results
demonstrated that inhibition of cell growth by FEOJ is due to
attenuation of thrombin-induced increases of G1-phase cell-cycle
regulatory protein cyclin D1 through reversal of thrombin-induced
reduction of p27KIP1.
Mitogenic stimuli mediate signaling through various
pathways in VSMCs, including the MAPK and AKT pathways (2,3,5,20,21).
The present study therefore investigated the effects of FEOJ on the
early MAPK and AKT signal transduction pathways induced by thrombin
in VSMCs. In previous studies, treatment of VSMCs with thrombin
stimulated the phosphorylation of AKT and MAPKs, including ERK1/2,
JNK and p38MAPK (2,3). Furthermore, in agreement with the
cell proliferation data, FEOJ treatment significantly inhibited the
phosphorylation of AKT in thrombin-treated VSMCs. However, FEOJ
treatment had no effect on the phosphorylation of MAPKs, including
ERK1/2, JNK and p38MAPK in VSMCs treated with thrombin. These
results suggested that FEOJ suppressed thrombin-induced
proliferation of VSMCs via inhibiting AKT phosphorylation. Previous
studies have suggested that the phosphorylation of AKT was
decreased following OJ treatment in lung cancer cells and diabetic
KKAy mouse model (22,23), while the present study was the
first to demonstrate that AKT phosphorylation is implicated in
FEOJ-induced inhibition of VSMCs proliferation.
The migration of VSMCs is one of the crucial
processes in the formation of atherosclerotic lesions (4,5).
Degradation of the extracellular matrix by enzymes including MMP-2
is an essential process in the migration of VSMCs, subsequently
leading to the progression of arterial vascular neointimal lesions
(4,5). In the present study, the
thrombin-induced migratory capacity of VSMCs was reduced by
pre-treatment with FEOJ. In addition, the present study
demonstrated that FEOJ suppressed the expression of MMP-2 in
thrombin-stimulated VSMCs. Several lines of evidence have
demonstrated that transcription factor NF-κB has pivotal roles in
OJ-mediated protection of cerebral ischemic injury and
anti-inflammatory activity (7,24).
Based on these previous studies, an EMSA assay was performed to
gain further insight into the inhibitory regulation of MMP-2
expression by FEOJ in thrombin-induced VSMCs. The present study
showed that FEOJ treatment resulted in a significant downregulation
of the NF-κB DNA-binding activity in thrombin-treated VSMCs. These
results suggested that FEOJ treatment leads to the downregulation
of thrombin-induced MMP-2 expression via the suppression of NF-κB
binding activity in VSMCs, resulting in a halt in extracellular
matrix destruction and the prevention of cell migration.
In conclusion, the present study was the first to
suggest that FEOJ suppressed the proliferation of thrombin-induced
VSMCs via a reduction of AKT phosphorylation, while not showing any
cytotoxicity. In addition, FEOJ-induced inhibition of VSMC
proliferation was due to inhibition of thrombin-induced
upregulation of cyclin D1 through reversal of thrombin-induced
reduction of p27KIP1 expression. Furthermore, pre-treatment with
FEOJ impeded thrombin-stimulated migration of VSMCs. In addition,
FEOJ potently suppressed thrombin-stimulated expression of MMP-2
through downregulating NF-κB binding activity. These results
indicated that FEOJ may be able to prevent cardiovascular diseases
associated with the proliferation and migration of VSMCs. Further
study is required to investigate the efficacy of the FEOJ in
vivo by determining its potential inhibitory effects on the
formation of atherosclerotic lesions.
Acknowledgments
The present study was supported by the 'Food
Functionality Evaluation Program' under the Ministry of
Agriculture, Food and Rural Affairs and in part by the Korea Food
Research Institute (grant no. 20140114). This research was
supported by the Chung-Ang University Research Scholarship Grants
in 2014.
References
|
1
|
Siller-Matula JM, Schwameis M, Blann A,
Mannhalter C and Jilma B: Thrombin as a multi-functional enzyme.
Focus on in vitro and in vivo effects. Thromb Haemost.
106:1020–1033. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patterson C, Stouffer GA, Madamanchi N and
Runge MS: New tricks for old dogs: Nonthrombotic effects of
thrombin in vessel wall biology. Circ Res. 88:987–997. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moon SK, Thompson LJ, Madamanchi N,
Ballinger S, Papaconstantinou J, Horaist C, Runge MS and Patterson
C: Aging, oxidative responses and proliferative capacity in
cultured mouse aortic smooth muscle cells. Am J Physiol Heart Circ
Physiol. 280:H2779–H2788. 2001.PubMed/NCBI
|
|
4
|
Galis ZS, Kranzhöfer R, Fenton JW II and
Libby P: Thrombin promotes activation of matrix metalloproteinase-2
produced by cultured vascular smooth muscle cells. Arterioscler
Thromb Vasc Biol. 17:483–489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smiljanic K, Obradovic M, Jovanovic A,
Djordjevic J, Dobutovic B, Jevremovic D, Marche P and Isenovic ER:
Thrombin stimulates VSMC proliferation through an EGFR-dependent
pathway: Involvement of MMP-2. Mol Cell Biochem. 396:147–160. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen HT, Tsou HK, Tsai CH, Kuo CC, Chiang
YK, Chang CH, Fong YC and Tang CH: Thrombin enhanced migration and
MMPs expression of human chondrosarcoma cells involves PAR receptor
signaling pathway. J Cell Physiol. 223:737–745. 2010.PubMed/NCBI
|
|
7
|
Huang YL, Kou JP, Ma L, Song JX and Yu BY:
Possible mechanism of the anti-inflammatory activity of ruscogenin:
Role of intercellular adhesion molecule-1 and nuclear
factor-kappaB. J Pharmacol Sci. 108:198–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kou J, Sun Y, Lin Y, Cheng Z, Zheng W, Yu
B and Xu Q: Anti-inflammatory activities of aqueous extract from
Radix Ophiopogon japonicus and its two constituents. Biol Pharm
Bull. 28:1234–1238. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kou J, Yu B and Xu Q: Inhibitory effects
of ethanol extract from Radix Ophiopogon japonicus on venous
thrombosis linked with its endothelium-protective and anti-adhesive
activities. Vascul Pharmacol. 43:157–163. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qian J, Jiang F, Wang B, Yu Y, Zhang X,
Yin Z and Liu C: Ophiopogonin D prevents H2O2-induced injury in
primary human umbilical vein endothelial cells. J Ethnopharmacol.
128:438–445. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kou J, Tian Y, Tang Y, Yan J and Yu B:
Antithrombotic activities of aqueous extract from Radix Ophiopogon
japonicus and its two constituents. Biol Pharm Bull. 29:1267–1270.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan L and Kim IH: Effect of dietary grape
pomace fermented by Saccharomyces boulardii on the growth
performance, nutrient digestibility and meat quality in finishing
pigs. Asian Austral J Anim. 24:1763–1770. 2011. View Article : Google Scholar
|
|
13
|
Choi YK, Sul JU, Park SK, Yu SN, Kim SH,
Rhee MS, Ahn SC and Shin MS: Research trends of fermented medicinal
herbs-based on their clinical efficacy and safety assessment. J
Life Science. 22:1729–1739. 2012. View Article : Google Scholar
|
|
14
|
Moon SK, Cha BY and Kim CH: ERK1/2
mediates TNF-alpha-induced matrix metalloproteinase-9 expression in
human vascular smooth muscle cells via the regulation of NF-kappaB
and AP-1: Involvement of the ras dependent pathway. J Cell Physiol.
198:417–427. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiong Y, Hannon GJ, Zhang H, Casso D,
Kobayashi R and Beach D: P21 is a universal inhibitor of cyclin
kinases. Nature. 366:701–704. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Toyoshima H and Hunter T: P27, a novel
inhibitor of G1 cyclin-Cdk protein kinase activity, is related to
p21. Cell. 78:67–74. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanner FC, Yang ZY, Duckers E, Gordon D,
Nabel GJ and Nabel EG: Expression of cyclin-dependent kinase
inhibitors in vascular disease. Circ Res. 82:396–403. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen D, Krasinski K, Sylvester A, Chen J,
Nisen PD and Andrés V: Downregulation of cyclin-dependent kinase 2
activity and cyclin A promoter activity in vascular smooth muscle
cells by p27 (KIP1), an inhibitor of neointima formation in the rat
carotid artery. J Clin Invest. 99:2334–2341. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Besson A, Dowdy SF and Roberts JM: CDK
inhibitors: Cell cycle regulators and beyond. Dev Cell. 14:159–169.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moon SK, Kim HM, Lee YC and Kim CH:
Disialoganglioside (GD3) synthase gene expression suppresses
vascular smooth muscle cell responses via the inhibition of ERK1/2
phosphor-ylation, cell cycle progression and matrix
metalloproteinase-9 expression. J Biol Chem. 279:33063–33070. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhan Y, Kim S, Izumi Y, Izumiya Y, Nakao
T, Miyazaki H and Iwao H: Role of JNK, p38 and ERK in
platelet-derived growth factor-induced vascular proliferation,
migration and gene expression. Arterioscler Thromb Vasc Biol.
23:795–801. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen M, Du Y, Qui M, Wang M, Chen K, Huang
Z, Jiang M, Xiong F, Chen J, Zhou J, et al: Ophiopogonin B-induced
autophagy in non-small cell lung cancer cells via inhibition of the
PI3K/Akt signaling pathway. Oncol Rep. 29:430–436. 2013.
|
|
23
|
Wang LY, Wang Y, Xu DS, Ruan KF, Feng Y
and Wang S: MDG-1, a polysaccharide from Ophiopogon japonicus
exerts hypoglycemic effects through the PI3K/Akt pathway in a
diabetic KKAy mouse model. J Ethnopharmacol. 143:347–354. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guan T, Liu Q, Qian Y, Yang H, Kong J, Kou
J and Yu B: Ruscogenin reduces cerebral ischemic injury via
NF-κB-mediated inflammatory pathway in the mouse model of
experimental stroke. Eur J Pharmacol. 714:303–311. 2013. View Article : Google Scholar : PubMed/NCBI
|