Introduction
Gastric cancer is a significant worldwide health
problem, which has high rates of morbidity and mortality and is the
fifth most common type of cancer and the third leading cause of
annual cancer-associated mortality worldwide (1). In China, gastric cancer morbidity and
mortality rates are double the global mean (2). Helicobacter pylori is the most
significant risk factor for gastric cancer, contributing to between
60 and 70% of gastric cancer cases globally (3). Other risk factors include chronic
gastritis, consumption of smoked or salted fish and other meat,
consumption of pickled vegetables, obesity, tobacco use, type A
blood and geographic location, including China, Japan, Southern and
Eastern Europe and South and Central America (3,4). The
incidence and mortality rates of gastric cancer are decreasing due
to the eradication of H. pylori and advances in early
endoscopic detection; however, the treatment of gastric cancer
remains a challenge, as surgery is the only effective cure
available to patients (3). Gastric
cancer is not particularly sensitive to chemotherapy and
non-surgical interventions are usually only used palliatively to
reduce tumor size or relieve symptoms of the disease (5). Thus, it is important to improve
current understanding of the molecular mechanisms of gastric
carcinogenesis, to enable the identification and development of
novel strategies for the prevention and treatment of gastric
cancer.
A substantial number of studies have demonstrated
that the development of gastric cancer, as with the majority of
types of human cancer, involves gene alterations, including p53 or
mTOR, or the process of epithelial-mesenchymal transition (6,7). The
present study aimed to focus on the role of serine/threonine kinase
11 (STK11), also termed liver kinase B1 (LKB1), in
gastric cancer.
LKB1 is ubiquitously expressed in human cells and is
a necessary element in cell metabolism, which is required to
maintain energy homeostasis. LKB1 can regulate cell polarity and
can function as a tumor suppressor by inhibiting the growth and
migration of cells, inducing cell cycle arrest, and promoting tumor
cell apoptosis (8–11). The majority of these effects are
mediated through the activation of adenine monophosphate-activated
protein kinase (AMPK) and AMPK-associated kinases (12,13).
At the molecular level, LKB1 can also inhibit the mammalian target
of rapamycin (mTOR) signaling pathway to regulate cell autophagy
following the activation of AMPK (14), and can inhibit tumor cell
glycolysis and lipid synthesis to reduce the rate of tumor cell
growth and migration (15–17). A reduction in the expression of
LKB1 alters cell polarity and affects cell adhesion, promoting the
transformation of normal cells and tumor metastasis (10). LKB1 loss-of-function
mutations are an etiological factor in Peutz-Jeghers syndrome, an
autosomal dominant genetic disorder (18,19).
LKB1 is mutated in a several different sporadic cancer
types, including lung adenocarcinoma and breast cancer (20) and restoration of the expression of
LKB1 suppresses lung cancer cell invasion and metastasis (21). In the present study, the expression
of LKB1 was examined in human gastric cancer tissue specimens, and
the effects of the expression of LKB1 in gastric cancer cells were
assessed in vitro to understand the role of LKB1 in gastric
cancer. The results of the present study may assist in the future
development of novel gene therapies against gastric cancer.
Materials and methods
Tissue specimens
A total of 63 gastric cancer tissue samples were
obtained from 63 patients as unique cases from the Department of
Pathology, The First Hospital of Nanchang University (Nanchang,
China). The patients underwent gastrostomy in the Surgery
Department at the First Hospital of Nanchang University between
March 2010 and April 2012, and all patients were diagnosed
histologically with gastric adenocarcinoma (Table I). The present study was approved
by the ethical committee of The First Affiliated Hospital of
Nanchang University (Nanchang, China). The tissue specimens were
collected by surgical resection and deposited to the pathological
specimen library of The First Affiliated Hospital of Nanchang
University.
 | Table ICorrelation between the expression of
LKB1 and clinicopathological parameters in patients with gastric
cancer. |
Table I
Correlation between the expression of
LKB1 and clinicopathological parameters in patients with gastric
cancer.
| Parameter | Expression of LKB1
|
|---|
| n | Negative | Positive | P-value |
|---|
| Gender | | | | 0.42 |
| Male | 48 | 41 | 7 | |
| Female | 15 | 14 | 1 | |
| Borrmann | | | | 0.75 |
| I and II | 36 | 31 | 5 | |
| III and IV | 27 | 24 | 3 | |
| TNM | | | | 0.75 |
| I and II | 27 | 24 | 3 | |
| III and IV | 36 | 31 | 5 | |
Immunohistochemistry
Immunohistochemistry was performed using a ZSGB-bio
kit (ZSGB-BIO, Beijing, China) according to the manufacturer's
instructions. Briefly, citrate buffer (cat. no. ZLI-9064; ZSGB-BIO)
was used for antigen retrieval, and the tissue sections (2 × 1 cm
and 2 × 3 cm) were incubated at 4°C with monoclonal goat anti-human
LKB1 antibody at a dilution of 1:250 (cat. no. sc-32245; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) overnight. The secondary
antibody was rabbit anti-mouse IgG (cat. no. PV9000; ZSGB-BIO),
which was incubated with the tissue sections for 4 h at 37°C. The
reaction was developed using 3,3′-diaminobenzidine (DAB; ZSGB-BIO)
and counterstained with hematoxylin (Yulu Experimental Equipment
Co., Ltd., Nanchang, China). The immunostained tissue sections were
evaluated semi-quantitatively under a light-microscope (Eclipse Ni;
Nikon Corporation, Tokyo, Japan), according to the immunoreactive
score (IRS), which evaluates the staining intensity and the
percentage of positive staining (22).
Cell culture and treatment
HEK-293T cells were obtained from the Research
Institute of Digestive Diseases (Nanchang, China). The SGC-7901
cell line was obtained from the Research Institute of Digestive
Diseases and cultured in high glucose Dulbecco's modified Eagle's
medium (DMEM) containing 10% fetal bovine serum (FBS) and 100 U/ml
penicillin/100 g/ml streptomycin solution (all GE Healthcare Life
Sciences, Logan, UT, USA) at 37°C with 5% CO2. For
anticancer drug treatment, SGC-7901 cells and stably expressing
LKB1 cells were seeded into 96-well plates at a density of
3×103 cells/well, and treated with various
concentrations of anticancer drugs for 2 days in a cell incubator
at 37°C. The anti-cancer drugs and the concentrations used were as
follows: 10, 20, 40 and 80 µM 5-fluorouracil (5-FU;
Sigma-Aldrich, St. Louis, MO, USA) 25, 50 and 100 µM
oxaliplatin (Sanofi S.A., Paris, France) and 5, 10, 20 and 40
µM irinotecan (Pfizer, Inc. New York, NY, USA).
mRNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total cellular mRNA was isolated using a Labserv
Universal RNA kit (KFR0-803096 IX96) from Thermo Fisher Scientific
(Waltham, MA, USA), according to the manufacturer's instructions,
and then reverse transcribed into cDNA using a QuantScript RT kit
(TianGen Biotech, Beijing, China). qPCR was performed using a
GoTaq® qPCR Master mix kit from Promega Corporation
(Madison, WI, USA), according to the manufacturer's instructions. A
total of 2 µl cDNA, 2X 10 µl GoTaq® qPCR
Master Mix, 100X 0.2 µl CXR Reference Dye, 0.4 µl
forward primer, 0.4 µl reverse primer, and 7 µl
nuclease-free water were used for the reaction. The LKB1 primers
[Generay Biotech (Shanghai) Co., Ltd., Shanghai, China] were
forward 5′-CGC TCT CTG ACC TGC TGA AA-3′ and reverse 5′-CAC CGT GAA
GTC CTG AGT GT-3′, which produced a 260 bp PCR product. The
internal control GAPDH primers were forward 5′-CAG GGC TGC TTT TAA
CTC TGGT-3′ and reverse 5′-GAT TTT GGA GGG ATC TCG CT-3′, which
produced a 199 bp PCR product. The qPCR amplification was at 95°C
for 2 min, followed by 40 cycles of 95°C for 15 sec and 58°C for 30
sec. The mRNA expression levels of LKB1 were quantified and
compared with GAPDH mRNA using the 2−∆∆CT method
(23).
Protein extraction and western blot
analysis
Total cellular protein was extracted using protein
lysis buffer, as previously described (24). The protein concentration was then
measured using the Bradford method (25). Subsequently, the protein samples
were separated by 10% sodium dodecylsulphate-polyacrylamide gel
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) electrophoresis at 60 V for 3–4 h. The proteins were then
transferred onto a nitrocellulose membrane (Whatman International
Ltd., Maidstone, UK) by wet electroblotting with a constant current
of 200 mA for 2 h at room temperature. The membrane was
subsequently blocked for 1 h in 5% skim milk-Tris-buffered saline
(TBS; Beijing Solarbio Science & Technology Co., Ltd.). The
membrane was then incubated with primary antibody at 4°C overnight.
β-actin antibody (ZSGB-BIO) was used as an internal control. The
following day, the membrane was washed with Tween 20 three times
(15 min each), followed by incubation with horseradish
peroxidase-labeled goat anti-mouse IgG (ZSGB-BIO) at 4°C for 4 h.
Following incubation, the blot was developed using a SuperSignal
West Pico Chemiluminescence kit (Thermo Fisher Scientific) and
detected and quantified using a ChemiDoc XRS+ system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Packaging of the lentivirus and cell
infection
A lentivirus vector carrying LKB1 cDNA was obtained
from the Dr Zhijun Luo of the School of Medicine, Boston University
(Boston, NY, USA). To package the lentivirus, HEK-293T cells were
passaged in 10 cm culture dishes (1×106 cells) and
transfected with either the LKB1 vector or control vector (10 ng of
each target plasmid and a packaging plasmid) using a calcium
phosphate transfection method. Briefly, all the plasmids were mixed
with 62 µl 2M CaCl2 (Beijing Solarbio Science
& Technology Co., Ltd.) and ddH2O, forming a total
volume of 500 µl, and the solution was subsequently added
into 2X Hanks' Balanced Salts (Beijing Solarbio Science &
Technology Co., Ltd.), incubated at room temperature for 20 min,
and finally incubated with the cells for 12 h. When the cells
reached 70 to 80% confluency, the cell culture supernatant
containing the lentivirus was collected using a PEG-8000 method to
concentrate and purify the virus particles. Briefly, the virus
supernatant was filtered with a 0.45 µm microfiltration
membrane, then added to 5X PEG-8000 NaCl solution (Beijing Solarbio
Science & Technology Co., Ltd.), and then used to treat the
cells prior to incubation at 4°C overnight. After 24 h, the
solution was centrifuged at 4,000 × g for 20 min at 4°C, and the
supernatant was discarded. Multiplicity of infection (MOI) was
assessed by diluting the concentrated lentivirus in a series of
gradients, which were then used to infect the 293T cells. Following
incubation for 48 h in a cell incubator, the medium of the cells
was changed, and after 4 days, RNA was extracted from the cells to
assess the MOI by comparing the Ct values of the control compared
with the experimental group.
To infect the gastric cancer cells with the
lentivirus, 1×105 SGC-7901 cells were grown in 6 cm
culture dishes and infected with 200 µl viral supernatant
using polybrene (GeneChem Co., Ltd., Shanghai, China) for 12 h at
37°C, following which the growth medium was replaced with medium
containing 1 µg/ml puromycin (Beijing Solarbio Science &
Technology Co., Ltd.) to screen for stable cells (stable cells
remain live following puromycin treatment).
Cell viability MTT assay
The cells were inoculated into three 96-well plates
at a density of 3×103 cells per well and grown for up to
72 h. At the end of each experiment, 20 µl MTT (5
µg/µl; Beijing Solarbio Science & Technology Co.,
Ltd.) was added to the cell culture medium and the cells were
incubated at 37°C for another 4 h. The medium was then aspirated
and 150 µl dimethyl sulfoxide (DMSO) was added to each well.
Subsequently, the optical density value was measured at a
wavelength of 490 nm using a SpectraMax M Series Multi-Mode
microplate reader (Molecular Devices, Sunnyvale, CA, USA). The
experiments were performed in nine replicates and repeated at least
three times.
Tumor cell wound-healing assay
The stable cells were trypsinized (Gibco-BRL,Grand
Island, NY, USA) and plated into a six-well plate at a density of
5×106 cells per well. Following overnight incubation at
37°C, two parallel wounds, ~400 µm wide, were made in the
cell layer using a 10 µl pipette tip. Following rinsing
three times with phosphate buffered saline (PBS), the cells were
cultured in 2 ml DMEM without FBS, supplemented with
penicillin/streptomycin, for up to 48 h at 37°C. Images were
captured of each plate at 0, 24 and 48 h under an inverted
microscope (magnification, ×100; Eclipse TS100; Nikon, Tokyo,
Japan). The cell migration distance was determined by measuring the
width of the wound, divided this value by two, and subtracting this
value from the initial width of the wound.
Flow cytometric analysis of the
expression of CD44
The stable cells were inoculated into a six-well
plate at a density of 1×105 cells per well, and
trypsinized and resuspended the following day at room temperature.
The resuspended solution was transferred into two 1.5 ml conical
centrifuge tubes and washed twice with PBS following centrifugation
of the cells at 94 × g at 4°C for 5 min. Subsequently, either
anti-CD44 antibody (Cell Signaling Technology, Inc., Danvers,
Massachusetts, USA), at a dilution of 1:200, or PBS, as a negative
control, were added to the solution and incubated on ice for 30 min
in the dark. The cells were then centrifuged at 845 × g at 4°C for
5 min, washed with PBS twice at 94 × g at 4°C for 5 min and
suspended in 1 ml PBS for flow cytometric analysis (BD Biosciences,
San Jose, CA, USA).
Flow cytometric analysis of cell cycle
distribution
The stable cells were inoculated into six-well
plates at a density of 1×105 cells per well, and
trypsinized and resuspended the following day at room temperature.
The solution was transferred into two 1.5 ml conical centrifuge
tubes and stained using 1 ml propidium iodide (PI; Beijing Solarbio
Science & Technology Co., Ltd.) for 30 min at 4°C in the dark,
followed by centrifugation at 845 × g at 4°C for 5 min, washing
with PBS twice at 94 × g at 4°C for 5 min, and resuspending in 1 ml
PBS for flow cytometric analysis (BD Accuri™ C6; BD
Biosciences).
Statistical analysis
All data are presented as the mean ± standard error
of the mean and were statistically analyzed using SPSS 17 software
(SPSS, Inc., Chicago, IL, USA). An unpaired t-test, one-way
analysis of variance was used to determine the statistical
significance of data. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of LKB1 is lost in gastric
cancer tissue
The present study examined the expression of LKB1 in
63 cases of gastric cancer and found that the expression of LKB1
was significantly lower in the tumor tissue, compared with the
adjacent healthy tissues (P<0.01; Fig. 1). Potential associations between
the reduction in the expression of LKB1 with the
clinicopathological data from the 63 patients were then examined.
The expression of LKB1 was not associated with
tumor-node-metastasis classification, gender or other
clinicopathological factors (Table
I).
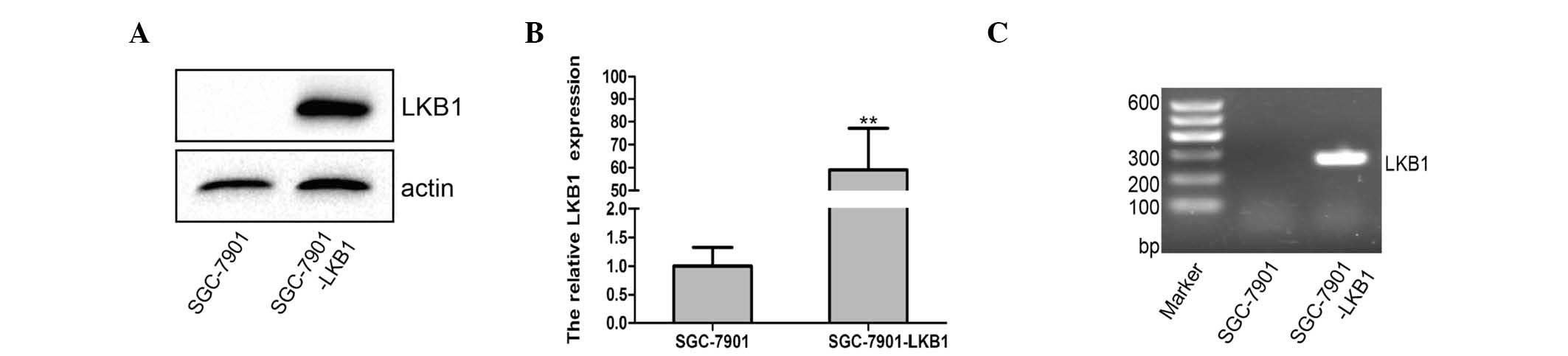
Establishment of stable LKB1-expressing
gastric cancer cells
The present study established the stable LKB1
protein-expressing SGC-7901 gastric cancer cell line using a
lentivirus carrying LKB1 cDNA. Data from the subsequent
RT-qPCR and western blot analyses revealed that the SGC-7901-LKB1
cells exhibited higher levels of LKB1 mRNA and LKB1 protein,
compared with the SGC-7901-wild-type cells (P<0.01; Fig. 2).
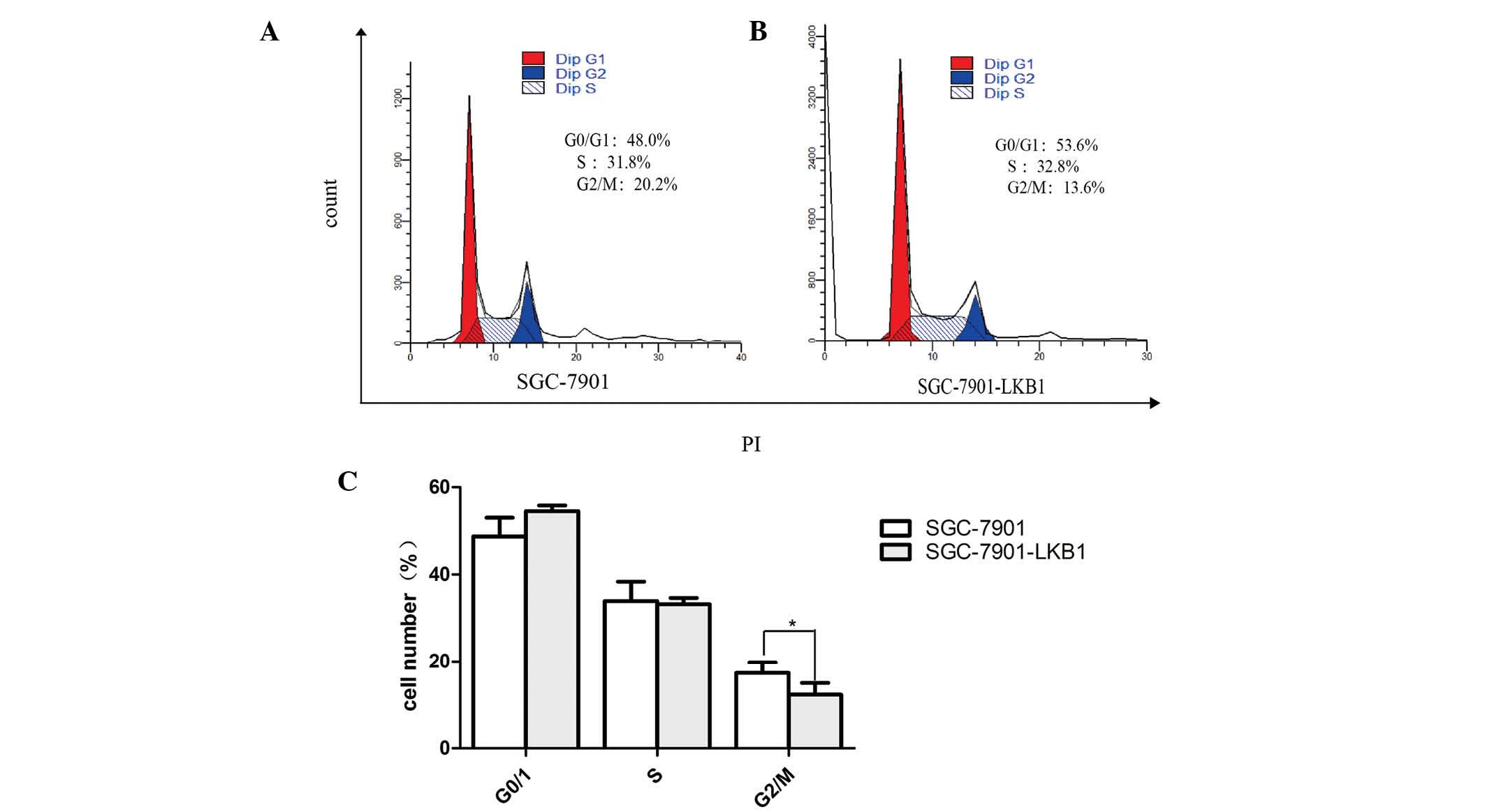
Effect of the expression of LKB1 on the
regulation of gastric cancer cell phenotypes
Restoration of the expression of LKB1 reduced tumor
cell viability and migration rate, and induced cell cycle arrest at
the G2 phase of the cell cycle (Figs.
3 and 4). It is known that
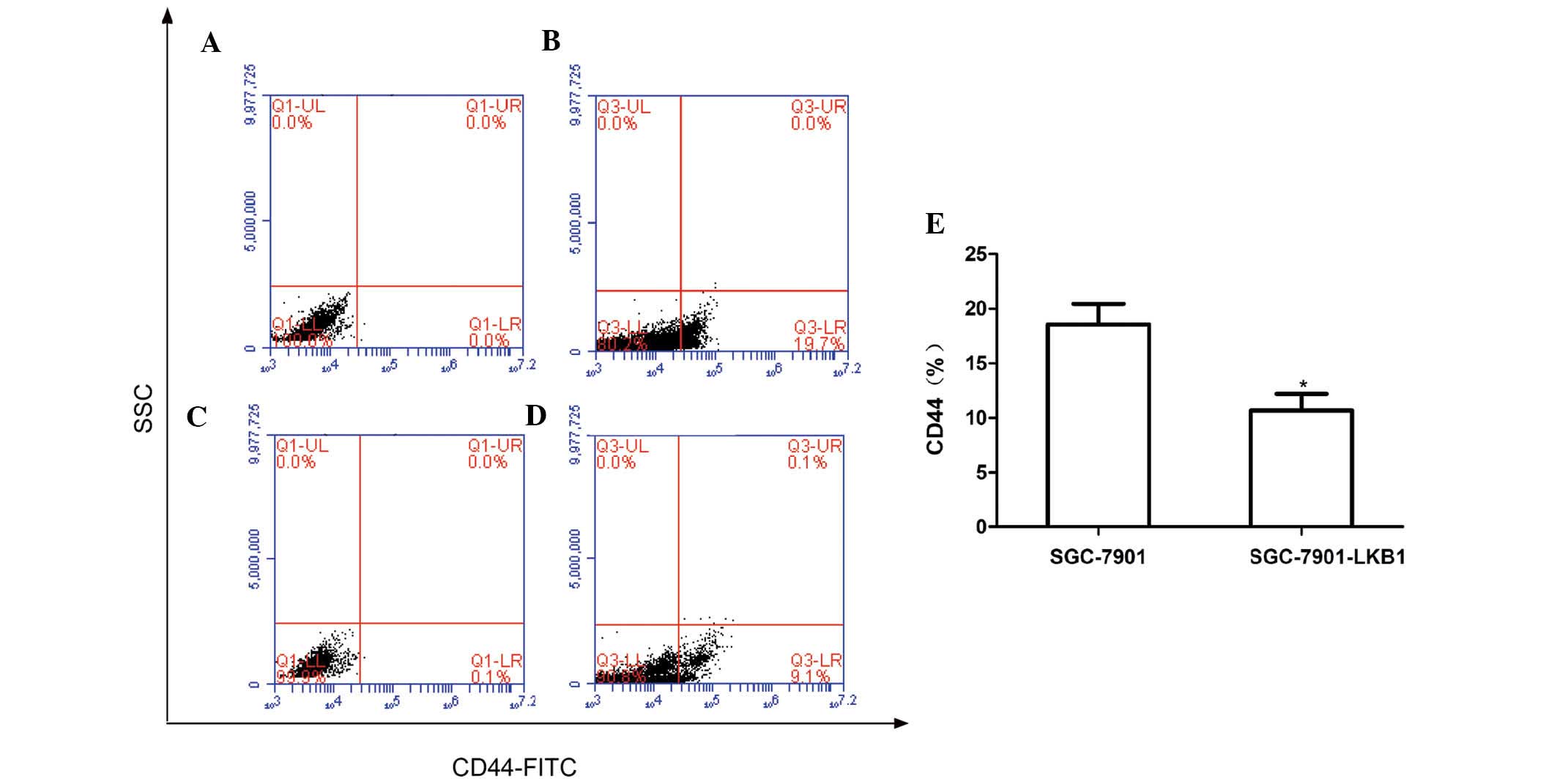
LKB1 is an important regulatory factor in determining cell polarity
(26), and that CD44 protein is an
important cell adhesion molecule, which is expressed on the cell
surface (27). To understand the
effect of LKB1 on the expression of CD44 in SGC-7901 cells, the
present study examined the expression of CD44 in the SGC-7901 and
SGC-7901-LKB1 cell lines. As shown in Fig. 5, the number of CD44-expressing
cells was significantly lower in the SGC-7901-LKB1 cells, compared
with the SGC-7901 cells (P<0.05), indicating that the inhibition
of tumor cell migration and adhesion by LKB1 may occur via
suppression of the expression of CD44.
LKB1 increases gastric cancer cell
sensitivity to anticancer drug treatment
The present study also assessed the effects of the
expression of LKB1 on the sensitivity of gastric cancer cells to
the chemotherapeutic drugs oxaliplatin, fluorouracil 5-FU and
irinotecan. The results demonstrated that the SGC-7901-LKB1 gastric
cancer cells stably expressing LKB1 were significantly more
sensitive to treatment with these anticancer drugs, compared with
the SGC-7901 cells (P<0.05; Fig.
5). This suggested that the expression of LKB1 may be important
in enhancing the sensitivity of gastric cancer cells to anticancer
drugs.
Discussion
The present study examined the expression of LKB1 in
63 gastric cancer tissue specimens and found that, although the
expression of LKB1 was reduced in gastric cancer tissues, there was
no association between the reduction in the expression of LKB1 and
the available clinicopathological data for the patients. The
present study also assessed the effects of the expression of LKB1
in gastric cancer cells on the regulation of cell viability, cell
cycle, migration, expression of CD44 and sensitivity to
oxaliplatin, 5-FU and irinotecan chemotherapeutic drugs. The
results revealed that the expression of LKB1 reduced tumor cell
viability and migration rate, and induced the cell cycle arrest of
the tumor cells at the G2 phase of the cell cycle. The expression
of LKB1 also inhibited the expression of CD44 in gastric cancer
cells, and the gastric cancer cells stably expressing LKB1 were
more sensitive to treatment with the anticancer drugs, compared
with the control cells.
LKB1 acts as a tumor suppressor in lung and breast
cancer and is able to suppress tumor progression (21,23).
In the present study, the protein expression of LKB1 was lost in
gastric cancer tissues, whereas expression of the protein was
observed in the normal mucosae adjacent to the tumor. However, an
earlier study failed to identify LKB1 mutations in sporadic
gastric cancer (22). Therefore,
the mechanism underlying the reduction in the expression of LKB1 in
gastric cancer remains to be elucidated. Although mutations in LKB1
may not be common in gastric cancer, a previous study demonstrated
that LKB1 protein affected the development and progression of
gastric cancer in patients with Peutz-Jeghers syndrome, a disease
that is characterized by gastrointestinal polyps and cancer of
different organs. In contrast with the previous study, which
reported no association between LKB1 mutation and cancer,
the Peutz-Jeghers investigation revealed an association between an
LKB1 mutation and the development of gastric cancer. Thus,
further investigation is required to assess the mechanism
underlying the reduction in the expression of LKB1 in gastric
cancer. In addition, a previous study have demonstrated that
LKB1 mutations are the major cause of the loss of expression
of LKB1 in various other human types of cancer, including lung
adenocarcinoma, cervical, breast, intestinal, testicular,
pancreatic and skin cancer (28).
The in vitro data of the present study
demonstrated a novel finding, which, to the best of our knowledge,
has not been previously reported. This was that restoration of the
expression of LKB1 reduces tumor cell viability and migration rate,
and induces cell cycle arrest at the G2 phase of the cell cycle in
tumor cells. Supporting these results, a previous study
demonstrated that, in breast cancer, LKB1 overexpression led to
significant inhibition of tumor cell invasion, reduced tumor growth
in the mammary fat pad and microvessel density, and suppressed
tumor metastasis to the lung (21). This LKB1 overexpression was
associated with the downregulation of matrix metalloproteinase -2
and 9, vascular endothelial growth factor and basic fibroblast
growth factor (21). In lung
cancer, the expression of LKB1 inhibits the invasion capacity of
lung cancer cells by suppressing the expression levels of tissue
factor and vascular endothelial growth factor (23). These data support the hypothesis
that LKB1 is a tumor suppressor gene in lung and breast
cancer, and the results of the present study suggested that
LKB1 is also a tumor suppressor gene in gastric cancer.
Further investigation is required to confirm these results.
The results of the present study also demonstrated
that the expression of LKB1 sensitized the gastric cancer cells to
treatment with anticancer drugs. Further evaluation of this result
may lead to the discovery of novel therapies for gastric cancer. In
addition, the expression of LKB1 reduced the levels of CD44 in
gastric cancer cells. CD44 is a cell-surface glycoprotein, which is
involved in cell-cell interactions, cell adhesion and cell
migration (29). The CD44 protein
is involved in a variety of cellular functions, including
lymphocyte activation, recirculation and homing, hematopoiesis and
tumor metastasis (30). In human
cancer, CD44 protein is a cell surface marker for breast and
prostate cancer stem cells, is associated with the progression of
head and neck cancer and is involved in the migration of ovarian
cancer cells (31,32).
The inhibition of gastric cancer cell migration by
LKB1 may, therefore, occur through suppression of the expression of
CD44 by LKB1. However, the preliminary results reported in the
present study only partially explain the mechanism by which the
reduced expression of LKB1 leads to the development of cancer, and
further investigation is required to fully understand the role of
LKB1 in the regulation of gastric cancer development and
progression. In conclusion, the present study demonstrated that the
LKB1 protein has tumor-suppressive activity in gastric cancer, and
further investigation may lead to the development of novel
therapies for the treatment of gastric cancer.
Acknowledgments
This study was supported, in part, by grants from
the National Science Foundation of China (grant. nos. 81171952,
81272926, 31460304 and 81460374) and Jiangxi Provincial Department
of Science and Technology, Technical Support Project (grant. no.
20133BBG70061, 2008). The authors would like to thank Dr Zhijun Luo
of the School of Medicine, Boston University (Boston, MA, USA) and
Dr Yong Xie of the Research Institute of Digestive Diseases, The
First Affiliated Hospital of Nanchang University (Nanchang, China)
for providing technical support.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
He J, Gu D, Wu X, Reynolds K, Duan X, Yao
C, Wang J, Chen CS, Chen J, Wildman RP, et al: Major causes of
death among men and women in china. N Engl J Med. 353:1134. 2005.
View Article : Google Scholar
|
|
3
|
Fock KM: Review article: The epidemiology
and prevention of gastric cancer. Aliment Pharmacol Ther.
40:250–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Øverby A, Zhao CM and Chen D: Plant
phytochemicals: Potential anticancer agents against gastric cancer.
Curr Opin Pharmacol. 19:6–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scartozzi M, Galizia E, Verdecchia L,
Berardi R, Antognoli S, Chiorrini S and Cascinu S: Chemotherapy for
advanced gastric cancer: Across the years for a standard of care.
Expert Opin Pharmacother. 8:797–808. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lordick F, Allum W, Carneiro F, Mitry E,
Tabernero J, Tan P, Van Cutsem E, van de Velde C and Cervantes A:
Unmet needs and challenges in gastric cancer: The way forward.
Cancer Treat Rev. 40:692–700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kubota E, Williamson CT, Ye R, Elegbede A,
Peterson L, Lees-Miller SP and Bebb DG: Low ATM protein expression
and depletion of p53 correlates with olaparib sensitivity in
gastric cancer cell lines. Cell Cycle. 13:2129–2137. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang S, Schafer-Hales K, Khuri FR, Zhou
W, Vertino PM and Marcus AI: The tumor suppressor LKB1 regulates
lung cancer cell polarity by mediating cdc42 recruitment and
activity. Cancer Res. 68:740–748. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
MacIver NJ, Blagih J, Saucillo DC, Tonelli
L, Griss T, Rathmell JC and Jones RG: The liver kinase B1 is a
central regulator of T cell development, activation and metabolism.
J Immunol. 187:4187–4198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakano A and Takashima S: LKB1 and
AMP-activated protein kinase: Regulators of cell polarity. Genes
Cells. 17:737–747. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hezel AF and Bardeesy N: LKB1; linking
cell structure and tumor suppression. Oncogene. 27:6908–6919. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sebbagh M, Olschwang S, Santoni MJ and
Borg JP: The LKB1 complex-AMPK pathway: The tree that hides the
forest. Fam Cancer. 10:415–424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kyriakis JM: At the crossroads:
AMP-activated kinase and the LKB1 tumor suppressor link cell
proliferation to metabolic regulation. J Biology. 2:262003.
View Article : Google Scholar
|
|
14
|
Shaw RJ: LKB1 and AMP-activated protein
kinase control of mTOR signalling and growth. Acta Physiol (Oxf).
196:65–80. 2009. View Article : Google Scholar
|
|
15
|
Shaw RJ, Bardeesy N, Manning BD, Lopez L,
Kosmatka M, DePinho RA and Cantley LC: The LKB1 tumor suppressor
negatively regulates mTOR signaling. Cancer Cell. 6:91–99. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li T, Leong MH, Harms B, Kennedy G and
Chen L: MicroRNA-21 as a potential colon and rectal cancer
biomarker. World J Gastroenterol. 19:5615–5621. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miller RA, Chu Q, Le Lay J, Scherer PE,
Ahima RS, Kaestner KH, Foretz M, Viollet B and Birnbaum MJ:
Adiponectin suppresses gluconeogenic gene expression in mouse
hepatocytes independent of LKB1-AMPK signaling. J Clin Invest.
121:2518–2528. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo Z, Zang M and Guo W: AMPK as a
metabolic tumor suppressor: Control of metabolism and cell growth.
Future Oncol. 6:457–470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hemminki A, Markie D, Tomlinson I,
Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M,
Höglund P, et al: A serine/threonine kinase gene defective in
Peutz-Jeghers syndrome. Nature. 391:184–187. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhuang ZG, Di GH, Shen ZZ, Ding J and Shao
ZM: Enhanced expression of LKB1 in breast cancer cells attenuates
angiogenesis, invasion and metastatic potential. Mol Cancer Res.
4:843–849. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang X, Li ZL, Jiang LL, Guo QQ, Liu MJ
and Nan KJ: Suppression of lung cancer cell invasion by LKB1 is due
to the downregulation of tissue factor and vascular endothelial
growth factor, partly dependent on SP1. Int J Oncol. 44:1989–1997.
2014.PubMed/NCBI
|
|
22
|
Park WS, Moon YW, Yang YM, Kim YS, Kim YD,
Fuller BG, Vortmeyer AO, Fogt F, Lubensky IA and Zhuang Z:
Mutations of the STK11 gene in sporadic gastric carcinoma. Int J
Oncol. 13:601–604. 1998.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(T) (−Delta Delta C) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Luo LY, Huang W, Tao R, Hu N, Xiao ZX and
Luo Z: ATM and LKB1 dependent activation of AMPK sensitizes cancer
cells to etoposide-induced apoptosis. Cancer Lett. 328:114–119.
2013. View Article : Google Scholar
|
|
25
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Granot Z, Swisa A, Magenheim J,
Stolovich-Rain M, Fujimoto W, Manduchi E, Miki T, Lennerz JK,
Stoeckert CJ Jr, Meyuhas O, et al: LKB1 regulates pancreatic beta
cell size, polarity and function. Cell Metab. 10:296–308. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hopkins AM: A novel mechanism of
regulating breast cancer. Breast Cancer Res. 162014.
|
|
28
|
Sanchez-Cespedes M, Parrella P, Esteller
M, Nomoto S, Trink B, Engles JM, Westra WH, Herman JG and Sidransky
D: Inactivation of LKB1/STK11 is a common event in adenocarcinomas
of the lung. Cancer Res. 62:3659–3662. 2002.PubMed/NCBI
|
|
29
|
Bourguignon LY, Singleton PA, Zhu H and
Diedrich F: Hyaluronan-mediated CD44 interaction with RhoGEF and
Rho kinase promotes Grb2-associated binder-1 phosphorylation and
phosphatidylinositol 3-kinase signaling leading to cytokine
(macrophage-colony stimulating factor) production and breast tumor
progression. J Biol Chem. 278:29420–29434. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Babina IS, McSherry EA, Donatello S, Hill
AD and Hopkins AM: A novel mechanism of regulating breast cancer
cell migration via palmitoylation-dependent alterations in the
lipid raft affiliation of CD44. Breast Cancer Res. 16:R192014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bourguignon LY, Zhu H, Shao L and Chen YW:
CD44 interaction with c-Src kinase promotes cortactin-mediated
cytoskeleton function and hyaluronic acid-dependent ovarian tumor
cell migration. J Biol Chem. 276:7327–7336. 2001. View Article : Google Scholar
|
|
32
|
Wang SJ, Wong G, de Heer AM, Xia WL and
Bourguignon LYW: CD44 variant isoforms in head and neck squamous
cell carcinoma progression. Laryngoscope. 119:1518–1530. 2009.
View Article : Google Scholar : PubMed/NCBI
|