Introduction
Respiratory syncytial virus (RSV) has become
increasingly recognized as an important pathogen in pediatric viral
bronchiolitis and pneumonia, and also causes severe respiratory
infection in immunocompromised adults and the elderly (1). A possible link between RSV infection
and asthma has been suggested in early childhood, and in subsequent
manifestations of atopy and persistent asthma (2,3).
However, the mechanisms by which RSV may be involved in the
development of post-bronchiolitis asthma and allergy remain to be
fully elucidated.
The airway epithelium is central in initiating
pulmonary inflammation, particularly in the case of RSV, as this
virus productively replicates only in the respiratory mucosa
(4,5). Enzymes involved in degradation of the
extracellular matrix, which have a number of important
physiological effects, including remodeling of the extracellular
matrix, facilitating cell migration, cleaving cytokines and
activating defensins, may be important in initiating pulmonary
diseases (6,7). Reports from clinical investigations
and animal models have shown that abnormal metalloproteinase causes
matrix breakdown in patients with asthma (8,9),
suggesting that RSV infection may result in abnormality of the
activities of certain metalloproteinases and trigger lung
remodeling.
Whether the role of RSV in the pathogenesis of
airway hyperresponsiveness is associated with abnormalities in the
expression levels of metalloproteinase remains to be elucidated. In
the present study, the diverse expression of metalloproteinases in
RSV-infected 16-HBE human bronchial epithelial cells were screened
using a cDNA microarray. Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis and ELISA were also
used to further identify abnormalities in the expression of MMP-19.
The correlation between the expression of MMP-19, the proliferation
of epithelial cells and fibroblasts, and epithelial-mesenchymal
transition (EMT) were also examined.
Materials and methods
Preparation of the RSV
The A2 strain of human RSV was propagated in a HeLa
cell monolayer (1×106 cells at 90% confluence), both
from the Research Institute of Virology (Wuhan, China) at 37° C in
5% CO2 with 2% heat-inactivated fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). At
maximum cytopathic effect (large quantity of syncytia and residue
of scattered islands of cells), the cells were repeatedly frozen
and thawed three tomes to facilitate rupture of the cells.
Subsequently, the supernatants were harvested and cellular debris
was removed by centrifugation. The resulting RSV viral suspension
was purified by centrifugation at 1,000 × g for 15 min at 4° C,
filtered through a 0.22 µm filter, aliquoted and stored at
−80° C until use. The viral titre was determined using a plaque
assay.
Cell culture and RSV infection
The 16HBE human bronchial epithelial cells were
cultured in Dulbecco's modified Eagle's medium combined with F12
(1:1; Cyclone, Logan, UT, USA) at 37° C in 5% CO2 with
10% FBS. Following 2 days in culture, the cells at 90% confluence
were infected with RSV at a multiplicity of infection (MOI) of
0.01. The infected cells were collected after 3 days, when the
cells exhibited a healthy cell monolayer morphology (10), and after 7 days, when a number of
small syncytia began to form. In addition, a separate group of
16HBE cells were treated using the same procedure, but with
uninfected HeLa cell lysate, and were used as a mock control group.
RSV persistence was verified and monitored using an RSV Real-time
PCR kit (Huayin Medicine Biotechnology Co., Ltd., Huayin, China).
According to the manufacturer's protocol, the samples were
considered negative for RSV when the quantification cycle
(Ct) value was >32.0. Samples with a Ct
value ≤28.9 were considered positive for RSV.
Examination of the expression spectrum of
metalloproteinase
The gene expression array was established by
selecting all 84 known metalloproteinases, negative control
(PUC18DNA and blank) and the housekeeping genes (β-actin, GAPDH,
cylcophilllin A and ribose body protein L13a) from a region of the
whole chip. Shanghai Kangcheng Biological Technology Co., Ltd
(Guangzhou, China) assisted with the establishment of the cDNA
assay and the subsequent examination. cDNA were obtained from the
cells by reverse transcription, labeling was performed with
fluorescence at the 3′ end, and the biotintylated cDNAs were
hybridized to the designed metalloproteinase chip. The results were
scanned using a GenePix 4000B chip scanner (Molecular Devices,
Sunnyvale, CA, USA) and transformed into fluorescence signal
intensity. The primary data were initially subtracted from the
background value, and were subsequently adjusted by the
housekeeping genes.
RT-qPCR
Total RNA was extracted from the 16HBE cells using
TRIzol reagent, and reverse transcription was performed using a
QuantiTect Reverse Transcription kit (cat. no. 205311; Takara
Biotechnology, Co., Ltd., Dalian, China). qPCR was performed using
an ABI Prism 7000 Sequence Detection system and software (Applied
Biosystems; Thermo Fisher Scientific, Inc.) in a final volume of 50
µl containing 2 µl of cDNA synthesized from the RT
reaction, 5 pmol of each primer, 25 µl of SYBR Green Master
Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.), and 23
µl of water. The amplification parameters included an
initial 95° C for 5 min, followed by 20 cycles of 95° C for 30 sec
and 60° C for 30 sec The primers (Takara Biotechnology Co., Ltd,
Dalian, China) used were as follows: MMP-19, forward
5′-GTTGGGCTCTTATTGACGG-3′ and reverse 5′- GAGA AG G CA AG G CTG GA
A-3′ (295 bp); E-cadherin, forward 5′-TCATAACCCACAGA TCCATT-3′ and
reverse 5′-CCAGGCGTAGACCAAGAA-3, (37 bp); N-cadherin, forward
5′-ATCCTACTGGACGGTTCG-3′ and reverse 5′-TTGGCTAATGGCACTTGA-3′ (139
bp); and GAPDH forward 5′-CCACTCCTCCACCTTTGAC-3′ and reverse
5′-ACCCTGTTGCTGTAGCCA-3′. Normalization of the RNA expression data
was achieved by comparing the gray values of the target RNA with
that of human GAPDH for each run. The PCR amplification products
were sequenced following T-A cloning with a TOPO® TA
cloning kit (Invitrogen, Thermo Fisher Scientific, Inc.) to direcly
ligate the PCR products, to verify the specificity. Quantitative
analysis of target gene expression data was based on the
2−△Ct method (11).
Determination of the secretion of MMP-19
using ELISA
The supernatants of the mock-infected and
RSV-infected cultures were collected on day 3 and day 7 following
infection. Subsequently, ELISA was performed using an MMP-19 ELISA
kit [cat no. YY(bio)-elisa-014490; R&D Systems, Inc.,
Minneapolis, MN, USA], according to the manufacturer's protocol.
Briefly, the cellular supernatants were centrifuged for 5 min at
500 × g. The total supernatants or control samples (100 µl)
were added to a 96-well plate and incubated for 2 h at 37° C.
Following aspiration, the samples were incubated with 100 µl
Detection Reagent A for 1 h at 37° C. Following washing three times
with washing buffer, the Detection Reagent B was added and
incubated for 30 min at 37° C. Then, the samples were washed 5
times and 90 µl Substrate Solution was added and incubated
for 20 min at 37° C. Subsequently 50 µl Stop Solution was
added to terminate the reaction. The 450 nm absorbance was
determined using a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Each sample was repeated three times. The
minimum detectable dose of MMP-19 was 0.01 ng/ml.
Construction of recombinant vectors
Fragments encoding the full coding sequence of
MMP-19, containing a flag insert following ATG, were synthesized by
GenScript Co., Ltd. (Nanjing, China) and cloned into the
BamHI and XhoI sites of the pcDNA3.1(+) plasmid to
construct pcDNA/MMP-19. The constructed plasmids were verified by
restriction enzyme mapping, involving the double digestion with
BamHI and XhoI (Invitrogen) to visualize the desired
bands at 5.1 and 1.8 kb, and direct DNA sequencing using T7 and sp6
primers. To generate MMP-19 small interfering (si)RNA expression
constructs, three siRNA sequences were cloned into the site of a
pGCU6/Neo/RFP vector to construct pGCU6/MMP-19siRNA. The most
effective silenced plasmid (siRNA, ag CUCGUACUGUUCCAAUACUuu, was
selected for use in the subsequent investigations.
Transfection and selection of recombinant
plasmids
The 16HBE cells were seeded into six-well plates at
a density of 5×105 cells per well. The recombinant
plasmid DNA (4 µg) and 8 µl X-treme GENE HP DNA
Transfection Reagent (Roche Diagnostics GmbH, Mannheim, Germany)
were mixed with 200 µl medium without antibiotics or FBS,
and incubated at room temperature for 10 min. Without removing the
growth medium, this mixture was added to the 16HBE cells. pcDNA
3.1(+) and pGCU6/Neo/RFP were used for vector controls of the
overexpressed and silenced plasmid, respectively. After 24 hr, the
plasmids were selected with G418 (Ceresco, USA) at 1,000 mg
ml−1 and subsequently cultured with G418 at 200 mg
ml−1.
Measurement of cell cycle of using flow
cytometry
Following treatment, the cells were fixed in cold
70% ethanol and stored at −20° C overnight. The fixed cells were
washed twice with PBS, stained with propidium iodide
(Sigma-Aldrich, St. Louis, MO, USA) solution (50 µg/ml) for
1 h and treated with a ribonuclease A solution (20 µg/ml;
Sigma-Aldrich) for 30 min. Flow cytometry (BD Accuri C6; BD Accuri
Cytometers, Ann Arbor, MI, USA) was then performed to examine the
cell cycle.
Western blot analysis
The mock- and RSV-infected 16HBE cells were lysed in
protease inhibitor cocktail solution (Roche Diagnostics). The cell
lysates were quantified using spectrophotometery (BioSpectometer;
Eppendorf, Hamburg, Germany) and 60 µg were separated by
SDS-PAGE (10%; Bio-Rad Laboratories, Inc.) and transferred onto a
nitrocellulose membrane (EMD Millipore, Billerica, MA, USA). The
membrane was blocked with 3% bovine serum albumin in PBS for 2 h,
followed by incubation with 1:250 dilutions of polyclonal
rabbit-anti-human N-cadherin and E-cadherin antibodies (Abcam; cat.
nos. 15148 and 12221) and polyclonal goat-anti human MMP-19
antibody [cat no. AF6790; R&D Systems, Inc., Minneapolis, MN,
USA] at 4° C overnight. The membrane was then incubated with
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (1:5,000; EMD Millipore) for 2 h at room temperature.
Detection was performed using an enhanced bioluminescence system
(Gene Co., Ltd., Hong Kong, China). The bands were analyzed using
ImageJ software (National Institutes of Health, Bethesda, MA,
USA)
Co-culture of 16HBE cells with human lung
fibroblasts (HLFs)
In the co-culture experiments, the 16HBE cells were
seeded at the bottom of a 24-well plate at a density of
105 cells (1 ml/well) with normal growth media, and were
grown to ~50% confluence. The HLFs were seeded into Transwell
chambers (Corning Inc., Corning, NY, USA) at a density of
2×104 with normal growth media for 12 h at 37° C,
following which the medium was replaced with 1 ml medium containing
1% serum for another 12 h. Subsequently, the Transwell chambers
were placed in the wells with the 16HBE cells for co-culture.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Statistical significances were assessed using either the
variance among multiple samples or q-test between groups.
P<0.05 was considered to indicate a statistically significant
difference. Analysis was performed using SPSS 11.0 for windows
(SPSS, Inc., Chicago, IL, USA).
Results
Expression of MMP-19 gradually decreases
in RSV-infected 16HBE cells
The chip results were scanned and analyzed using
software packages. The genes, in which expression levels were
increased more than twice were regarded as upregulated genes, and
those in which expression levels decreased by >0.5 times were
regarded as downregulated genes. The results showed that, compared
with the mock-infected control cells, there were five upregulated
genes, including MMP2, MMP-15, a disintegrin and metalloprotease
domain (ADAM)9, ADAM33 and ADAMTS2, and nine downregulated genes,
including MMP-7, MMP-17, MMP-19, uPA, TIMP-1, TIMP2, ADAMTS1,
ADAMTS10 and ADAM10. Among these, MMP-19 was downregulated 0.65 and
1.33 times at day 3 and day 7, respectively. The present study
subsequently examined the expression and function of MMP-19 in the
cultured 16HBE cells.
mRNA expression and secretion of MMP-19
in 16HBE cells
To verify the effects of RSV on the expression of
MMP-19, mRNA from obtained from the mock- and RSV-infected 16HBE
cells and analyzed using RT-qPCR 3 and 7 days following infection.
The results showed that the mRNA expression of MMP-19 decreased
significantly on days 3 and 7 following infection (Fig. 1). Enzyme immunoassay analyses of
the culture supernatants also demonstrated that the expression of
MMP-19 decreased on days 3 and 7, whereas the expression of MMP-19
in the control cells remained unchanged, as shown in Fig. 1.
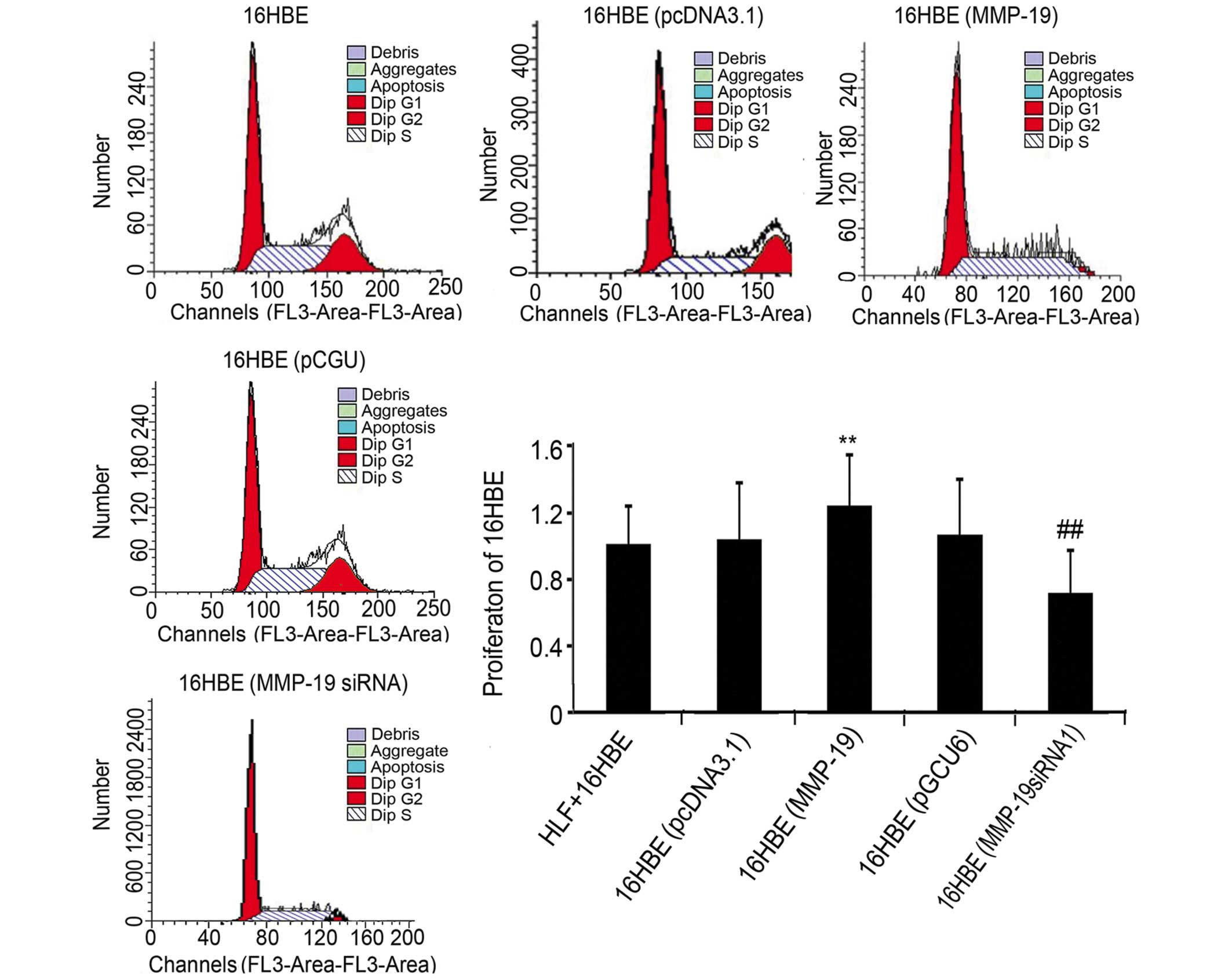
Downregulation of MMP-19 inhibits cell
cycle in 16HBE cells
To further examine the role of MMP-19 on the
proliferation of 16HBE cells, the present study examined the cell
cycle of normal, pcDNA3.1-transfected, pcDNA/MMP-19-transfected,
pGCU6/Neo/RFP-transfected and pGCU6/MMP-19siRNA-transfected 16HBE
cells using flow cytometry. The results revealed that, compared
with the corresponding empty vector-transfected groups, the
percentage of cells in the (G2+S)/G1 phase increased by 9.48% in
the MMP-19-overexpressing group, and decreased by 13.27% in the
MMP-19-silenced group (Fig.
2).
Downregulation of MMP-19 promotes
cadherin switching in 16HBE cells
Proteins were collected from the cultured cells in
the five treatment groups and evaluated using immunoblotting to
determine the expression levels of E-cadherin and N-cadherin. The
results showed that the expression of E-cadherin increased in the
MMP-19-overexpressed cells, compared with the
pcDNA3.1(+)-transfected cells. Cadherin switching, indicative of
EMT, was observed in the MMP-19-silenced cells, compared with the
pGCU6/Neo/RFP-transfected group (Fig.
3).
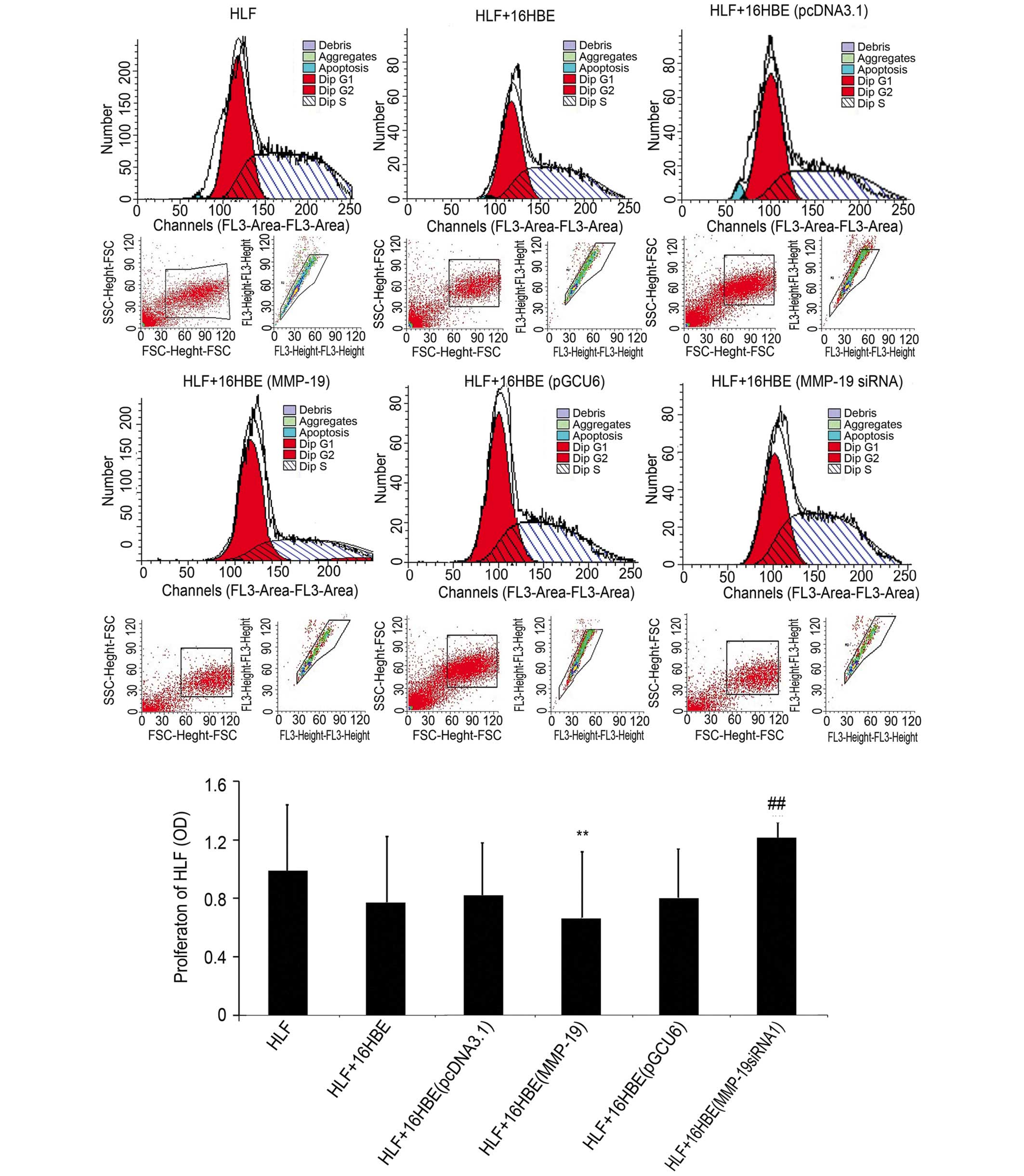
Downregulation of MMP-19 promotes the
proliferation of HLFs
In order to examine the effect of the downregulation
of MMP-19 in 16HBE cells on the proliferation of lung fibroblasts
under co-culture conditions, the proliferative activity of lung
fibroblasts were determined using flow cytometry. The results
(Fig. 4) demonstrated that the
proliferation of the HLFs co-cultured with the
MMP-19-overexpressing 16HBE cells were lower than that of the
pcDNA3.1(+)-transfected group (P<0.01). However, the
proliferation of the HLFs co-cultured with MMP-19-silenced 16HBE
increased significantly, compared with that of the
pGCU6/Neo/RFP-transfected cells (P<0.01).
Discussion
The formation of airway hyper-responsiveness is a
type of response to airway epithelial injury (12). A number of clinical and basic
investigations have confirmed that RSV in early childhood is an
important risk factor for subsequent airway hyperresponsiveness;
however, the underlying mechanism remains to be fully elucidated.
Persistent RSV infections have been established in several human
and animal epithelial cell lines (10); however, whether human epithelial
cells of bronchial origin can permit viral persistent infections
in vitro is an area of debate. The present study aimed to
determine whether RSV is able to infect the 16HBE human bronchial
epithelial cell line over multiple generations. The results showed
that, when RSV at an MOI of 0.01 was used to infect the 16HBE
cells, RSV survived to four generations. The establishment of an
in vitro model of infected human bronchial epithelial cells,
which persists for four generations prior to death, provides a
novel system for characterizing persistent RSV mechanisms.
MMP-19 was fist cloned from a human liver cDNA
library in 1997 (13), which has
been shown to degrade a variety of substrates of the extracellular
matrix and the basement membrane, including collagen type 4, large
tenascin-C isoform, fibronectin, type 1 gelatin, laminin-5,
nidogen-1, aggrecan and cartilage oligomeric matrix protein
(14–16). Brauer et al (17) reported that MMP-19 digests
plasminogen to produce fragments with bioactivities of angiostatin,
which inhibit proliferation and capillary-growth of endothelial
cells and implicate MMP-19 in vascular remodeling and angiogenesis.
Gueders et al (18) showed
that MMP-19 deficiency promotes the accumulation of tenascin-C and
allergen-induced airway inflammation. The present study, which
investigated the cellular levels of MMP-19, revealed that human RSV
infection in cultured 16HBE cells resulted in downregulated
expression levels of MMP-19.
EMT is a mechanism, which may account for the
accumulation of subepithelial mesenchymal cells, thereby
contributing to increased contractile cell mass and airway
hyperresponsiveness. EMT is predominantly characterized by the loss
of epithelial markers, including E-cadherin, and the acquisition of
mesenchymal markers including vimentin and N-cadherin (19). A previous study involving a mouse
model of chronic house dust mite-driven allergic airway
inflammation demonstrated the capacity of airway epithelial cells
to acquire mesenchymal characteristics under these conditions
(20). The results of the present
study demonstrated that downregulation in the expression levels of
MMP-19 induced loss of the characteristic airway epithelial cell
marker.
Under normal conditions, reciprocal inhibition in
proliferation exists between bronchial epithelial cells and lung
fibroblasts, which is essential for maintenance of homeostasis in
the airway architecture. In this state, bronchial epithelial cells
inhibit the proliferation of lung fibroblasts. However,
downregulation of MMP-19 in the 16HBE bronchial epithelial cells
promoted the proliferation of lung fibroblasts, indicating the
activation of lung fibroblasts following RSV infection.
In conclusion, the mechanism underlying the
pathogenesis of RSV in airway hyperresponsiveness may include
abnormal expression levels of certain metalloproteinases to inhibit
the function of epithelial cells and assist in the proliferation
and migration of lung fibroblasts. The present study is the first,
to the best of our knowledge, to report that the expression of
MMP-19 decreased in cultured 16HBE cells following RSV infection,
which provides an experimental basis for further elucidation of the
mechanism of RSV-induced airway hyper-responsiveness.
Acknowledgments
This study was supported by a grant from the
Scientific and Technological Research Project of Shandong Province
(grant. no. 2007GG3002008).
References
|
1
|
Backman K, Piippo-Savolainen E, Ollikainen
H, Koskela H and Korppi M: Adults face increased asthma risk after
infant RSV bronchiolitis and reduced respiratory health-related
quality of life after RSV pneumonia. Acta Paediatr. 103:850–855.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Simões EA, Carbonell-Estrany X, Rieger CH,
Mitchell I, Fredrick L and Groothuis JR; Palivizumab Long-Term
Respiratory Outcomes Study Group: The effect of respiratory
syncytial virus on subsequent recurrent wheezing in atopic and
nonatopic children. J Allergy Clin Immunol. 126:256–262. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Silver E, Yin-DeClue H, Schechtman KB,
Grayson MH, Bacharier LB and Castro M: Lower levels of plasmacytoid
dendritic cells in peripheral blood are associated with a diagnosis
of asthma 6 yr after severe respiratory syncytial virus
bronchiolitis. Pediatr Allergy Immunol. 20:471–476. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tan Y, Yang T, Liu S, Liu H, Xiang Y, Qu
F, Li H and Qin X: Infection with respiratory syncytial virus
alters peptidergic innervation in the lower airways of guinea-pigs.
Exp Physiol. 93:1284–1291. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu X, Qin X, Xiang Y, Liu H, Gao G, Qin
L, Liu C and Qu X: Progressive changes in inflammatory and matrix
adherence of bronchial epithelial cells with persistent respiratory
syncytial virus (RSV) infection (progressive changes in RSV
infection). Int J Mol Sci. 14:18024–18040. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zariffard MR, Anastos K, French AL,
Munyazesa E, Cohen M, Landay AL and Spear GT: Cleavage/alteration
of interleukin-8 by matrix metalloproteinase-9 in the female lower
genital tract. PLoS One. 10:e01169112015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilson CL, Schmidt AP, Pirilä E, Valore
EV, Ferri N, Sorsa T, Ganz T and Parks WC: Differential Processing
of {alpha}- and {beta}-Defensin Precursors by Matrix
Metalloproteinase-7 (MMP-7). J Biol Chem. 284:8301–8311. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barbaro MP, Spanevello A, Palladino GP,
Salerno FG, Lacedonia D and Carpagnano GE: Exhaled matrix
metallopro-teinase-9 (MMP-9) in different biological phenotypes of
asthma. Eur J Intern Med. 25:92–96. 2014. View Article : Google Scholar
|
|
9
|
Weitoft M, Andersson C, Andersson-Sjöland
A, Tufvesson E, Bjermer L, Erjefält J and Westergren-Thorsson G:
Controlled and uncontrolled asthma display distinct alveolar tissue
matrix compositions. Respir Res. 15:672014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu X, Qin X, Xiang Y, Liu H, Gao G, Qin
L, Liu C and Qu X: Progressive changes in inflammatory and matrix
adherence of bronchial epithelial cells with persistent respiratory
syncytial virus (RSV) infection (progressive changes in RSV
infection). Int J Mol Sci. 14:18024–18040. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dwivedi S, Goel A, Mandhani A, Khattri S,
Sharma P, Misra S and Pant KK: Functional genetic variability at
promoters of pro-(IL-18) and anti-(IL-10) inflammatory affects
their mRNA expression and survival in prostate carcinoma patients:
Five year follow-up study. Prostate. 75:1737–1746. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan YR, Yang T, Liu SP, Xiang Y, Qu F, Liu
HJ and Qin XQ: Pulmonary peptidergic innervation remodeling and
development of airway hyperresponsiveness induced by RSV persistent
infection. Peptides. 29:47–56. 2008. View Article : Google Scholar
|
|
13
|
Pendás AM, Knäuper V, Puente XS, Llano E,
Mattei MG, Apte S, Murphy G and López-Otín C: Identification and
characterization of a novel human matrix metalloproteinase with
unique structural characteristics, chromosomal location and tissue
distribution. J Biol Chem. 272:4281–4286. 1997. View Article : Google Scholar
|
|
14
|
Stracke JO, Fosang AJ, Last K, Mercuri FA,
Pendás AM, Llano E, Perris R, Di Cesare PE, Murphy G and Knäuper V:
Matrix metalloproteinases 19 and 20 cleave aggrecan and cartilage
oligomeric matrix protein (COMP). FEBS Lett. 478:52–56. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sadowski T, Dietrich S, Koschinsky F,
Ludwig A, Proksch E, Titz B and Sedlacek R: Matrix
metalloproteinase 19 processes the laminin 5 gamma 2 chain and
induces epithelial cell migration. Cell Mol Life Sci. 62:870–880.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Titz B, Dietrich S, Sadowski T, Beck C,
Petersen A and Sedlacek R: Activity of MMP-19 inhibits
capillary-like formation due to processing of nidogen-1. Cell Mol
Life Sci. 61:1826–1833. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brauer R, Beck IM, Roderfeld M, Roeb E and
Sedlacek R: Matrix metalloproteinase-19 inhibits growth of
endothelial cells by generating angiostatin-like fragments from
plasminogen. BMC Biochem. 12:382011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gueders MM, Hirst SJ, Quesada-Calvo F,
Paulissen G, Hacha J, Gilles C, Gosset P, Louis R, Foidart JM,
Lopez-Otin C, et al: Matrix metalloproteinase-19 deficiency
promotes tenascin-C accumulation and allergen-induced airway
inflammation. Am J Respir Cell Mol Biol. 43:286–295. 2010.
View Article : Google Scholar
|
|
19
|
Johnson JR, Nishioka M, Chakir J, Risse
PA, Almaghlouth I, Bazarbashi AN, Plante S, Martin JG, Eidelman D
and Hamid Q: IL-22 contributes to TGF-β1-mediated
epithelial-mesenchymal transition in asthmatic bronchial epithelial
cells. Respir Res. 14:1182013. View Article : Google Scholar
|
|
20
|
Johnson JR, Roos A, Berg T, Nord M and
Fuxe J: Chronic respiratory aeroallergen exposure in mice induces
epithelial-mesenchymal transition in the large airways. PLoS One.
6:e161752011. View Article : Google Scholar : PubMed/NCBI
|