Introduction
Vascular inflammation is crucial in a diverse group
of diseases, including sepsis, atherosclerosis, diabetes and
rheumatoid arthritis (1,2). Once an inflammatory response is
activated, circulating leukocytes (primarily monocytes and T
lymphocytes) migrate across the vascular wall (1). The vascular endothelium responds with
increased endothelial cell permeability, which enables the passage
of plasma proteins and leukocytes from the capillary lumen to the
subendothelial tissues, triggering further tissue damage (3). Vascular permeability is essential for
the homeostasis of normal tissues, and hyperpermeability of
vascular tissue is an important characteristic of inflammation.
The permeability properties of the endothelium are
dynamically regulated by a vascular barrier, which is primarily
formed by adherens junctions and tight junctions between
endothelial cells (4). Adherens
junctions are composed of membrane spanning vascular endothelial
(VE)-cadherins, which interact with VE-cadherins expressed on
neighboring cells via a homotropic mechanism in order to restrict
paracellular permeability (5). The
cytoplasmic tail of VE-cadherin binds to β-catenin linked to the
cytoskeleton. Tight junctions are comprised of a branching network
of sealing strands consisting of integral membrane-spanning
proteins, such as occludin, which is directly linked to the actin
cytoskeleton and mediates a series of cellular processes (6). During inflammation, proinflammantory
cytokines, such as tumor necrosis factor (TNF)-α and interferon
(IFN)-γ, induce the downregulation or re-distribution of the
junctional proteins, leading to the breakdown of the vascular
barrier (4).
Catalpol is an iridoid glucoside isolated from the
root of Rehmannia glutinosa (7). A recent study has demonstrated the
cardioprotective and anti-inflammatory properties of catalpol
(8). In mouse models, catalpol
protects against lipopolysaccharide-induced acute lung injury via
the suppression of TNF-α, interleukin (IL)-1β, IL-4 and IL-6
(9). Catalpol also protects
against cerebral ischemia/reperfusion injury by reducing free
radicals and lipid peroxidation (10). In addition, catalpol suppresses
inflammation-induced toxicity in dopaminergic neurons (11). However, the underlying mechanisms
of its effects on inflammation have not been fully elucidated.
The present study examined the effects of catalpol
on vascular permeability. Using real-time intercellular resistance
analysis, it was demonstrated that catalpol induces an increase in
the permeability of monolayers of human umbilical vein endothelial
cells (HUVECs). In addition, the effects of catalpol on the
expression of VE-cadherin, which is regulated by the ETS
transcriptional factor ERG, was examined. This study identified a
novel effect of catalpol on vascular permeability and provides a
basis for catalpol-related drug development.
Materials and methods
Cell culture
HUVECs (American Type Culture Collection, Manassas,
VA, USA) were cultured in EBM2 basal endothelial cell medium
supplemented with the EGM-2-MV bullet kit (Lonza, Basel,
Switzerland) and antibiotics (100 IU/ml penicillin and 100
µg/ml streptomycin; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). The cells were cultured in humidified air at 37°C with 5%
CO2. Catalpol (1 M) was purchased from Sigma-Aldrich
(St. Louis, MO, USA) and dissolved in dimethyl sulfoxide (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China).
Fluorescein isothiocyanate (FITC)-Dextran
Transwell assay
HUVEC monolayers were plated on the Transwell insert
(Corning Incorporated, New York, NY, USA) and cultured until 100%
confluent. Following pre-treatment with catalpol (0. 0.01, 0.1, 1
mM) at different concentrations, FITC-Dextran (Invitrogen; Thermo
Fisher Scientific, Inc.) was added to the top chamber. Samples were
removed from the bottom chamber after 24 h and read in a
fluorometer (SpectraMax M3; Molecular Devices, Sunnyvale, CA, USA)
at an excitation of 485 nm and emission of 520 nm. The data
represent the mean of four experiments.
Electric cell-substrate impedance sensing
(ECIS) analysis
The transendothelial electrical resistance (TEER)
across a monolayer of HUVECs was measured using the ECIS technique
(ECIS Zθ; Applied BioPhysics, Troy, NY, USA) and were analyzed
using the integrated ECIS software (12). Briefly, HUVECs were plated in ECIS
8W10E+ arrays and allowed to grow to 100% confluence. Following
catalpol treatment, the resistance across the EC layer was
determined every 8 sec by measuring the alternating current through
the cells using electrodes. Data plots are representative of
triplicate experiments, with each graph showing impedance readings
from a separate well, at 40 distinct electrodes per well.
Western blotting
HUVECs treated with catalpol were lysed in
radioimmunoprecipitation assay buffer (containing 20 mM Tris, pH
7.5; 150 mM NaCl, 50 mM NaF, 1% NP40, 0.1% DOC, 0.1% SD and 1 mM
EDTA; EMD Millipore, Billerica, MA, USA) supplemented with 1
µg/ml aprotonin (Roche Diagnostics GmbH, Mannheim, Germany),
10 µg/ml leupeptin (Roche Diagnostics GmbH) and 1 mM PMSF
protease inhibitors (Beyotime Institute of Biotechnology, Shanghai,
China). An equal quantity of protein for each sample was
electrophoresed through a 10% SDS-PAGE gel (Beyotime Institute of
Biotechnology) and then transferred to a nitrocellulose membrane
(EMD Millipore). The membrane was blocked with 2.5% non-fat milk in
Tris-buffered saline with Tween-20 (TBST; Beyotime Institute of
Biotechnology) at 37°C for 1.5 h prior to being incubated with the
following primary antibodies overnight at 4°C. After washing with
TBST three times, the membrane was incubated with secondary
antibody at 37°C for 1 h. The primary antibodies were used as
follows: Rabbit polyclonal anti-VE-cadherin (1:500; ab33168),
rabbit polyclonal anti-occludin (1:500; ab31721), rabbit polyclonal
anti-ERG (1:1,000; ab28662) (Abcam, Cambridge, MA, USA), and rabbit
monoclonal anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH;
1:2,500; #2118) (14C10) mAb (Cell Signaling Technology, Inc.,
Danvers, MA, USA). The secondary antibody was horseradish
peroxidase-linked anti-rabbit IgG (1:200; #7074; Cell Signaling
Technology, Inc.). The blots were developed with enhanced
chemiluminescence reagents (EMD Millipore).
Immunofluorescence
HUVECs were allowed to grow to 100% confluence on
fibronectin-coated glass chamber slides (Sigma-Aldrich) and were
then treated with 1 mmol catalpol. After 24 h, the medium was
aspirated and the monolayers were washed with phosphate-buffered
saline (PBS), fixed with 4% paraformaldehyde for 10 min, and washed
three times with PBS for 10 min. Immunofluorescence was performed
by staining with a primary antibody against human VE-cadherin
(ab33168) or occludin (ab31721) (Abcam) at a dilution of 1:500
overnight at 4°C and a rhodamine-labeled secondary antibody (1:200;
SA00007-2; Proteintech Group, Inc., Chicago, IL, USA) for 30 min.
The slides were photographed using an Olympus LCX100 Imaging system
(Olympus Corporation, Tokyo, Japan) with an excitation wavelength
of 546 nm.
ERG knockdown with small interfering RNA
(siRNA)
siRNA against human ERG (NM_004440) and control
non-targeting siRNA were purchased from GE Dharmacon (Lafayette,
CO, USA). Transfections were performed according to the
manufacturer's instructions. HUVECs were seeded into 6-well plates
and cultured for 24 h. Subsequently, 200 nM siRNA in combination
with 3 µl DharmaFECT4 Transfection Reagent (GE Dharmacon).
were added to each well. After 24 h, the cells were harvested for
ECIS analysis. The knockdown of ERG was analyzed by RT-qPCR and
immunoblot analysis.
Reverse transcription-quantitative
polymerase chain reaction
Confluent HUVECs on 6-well plates were treated with
1 mmol catalpol and collected at 24 h. Total RNA was extracted with
the RNeasy Mini kit (Qiagen, Hilden, Germany) according to the
manufacturer's instructions. Reverse transcription was performed
using the RevertAid First Strand cDNA synthesis kit (Thermo Fisher
Scientific, Inc.). qPCR was conducted using SYBR Premix Ex TaqII
(Takara Bio, Inc., Shita, Japan) on the ViiA 7 DX Real-Time PCR
System (Thermo Fisher Scientific, Inc.). The sequences of primers
for ERG, CDH5 (encoding VE-cadherin) and OLDN
(encoding occludin) genes are shown in Table I. The reaction conditions were as
follows: 94°C for 5 min, 30 cycles of 94°C for 30 sec, 57°C for 30
sec and 72°C for 2 min, then a final extension at 72°C for 5 min.
All PCR reactions were repeated in triplicate. Gene expression was
assessed by comparing the relative expression level of each gene
with the internal reference GAPDH using the ΔΔCt
method.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Sequence | Size (bp) | Tm (°C) |
|---|
| CDH5 | | | |
| Sense |
GCGACTACCAGGACGCTTTCA | 150 | 60.5 |
| Antisense |
CATGTATCGGAGGTCGATGGTG | | |
| OCLN | | | |
| Sense |
GAAGCCAAACCTCTGTGAGC | 227 | 59.0 |
| Antisense |
GAAGACATCGTCTGGGGTGT | | |
| ERG | | | |
| Sense |
TCTTGGACCAACAAGTAGCC | 151 | 57.5 |
| Antisense |
GTCGGGATCCGTCATCTTG | | |
| GAPDH | | | |
| Sense |
TGATGACATCAAGAAGGTGGTGAAG | 240 | 57.9 |
| Antisense |
TCCTTGGAGGCCATGTGGGCCAT | | |
Statistical analysis
Statistical significance was assessed using
paired-sample t-tests. P<0.05 was considered to indicate a
statistically significant difference. Statistical analyses were
performed using SPSS 18.0 software (SPSS, Inc, Chicago, IL, USA).
All experiments were repeated three times unless otherwise
stated.
Results
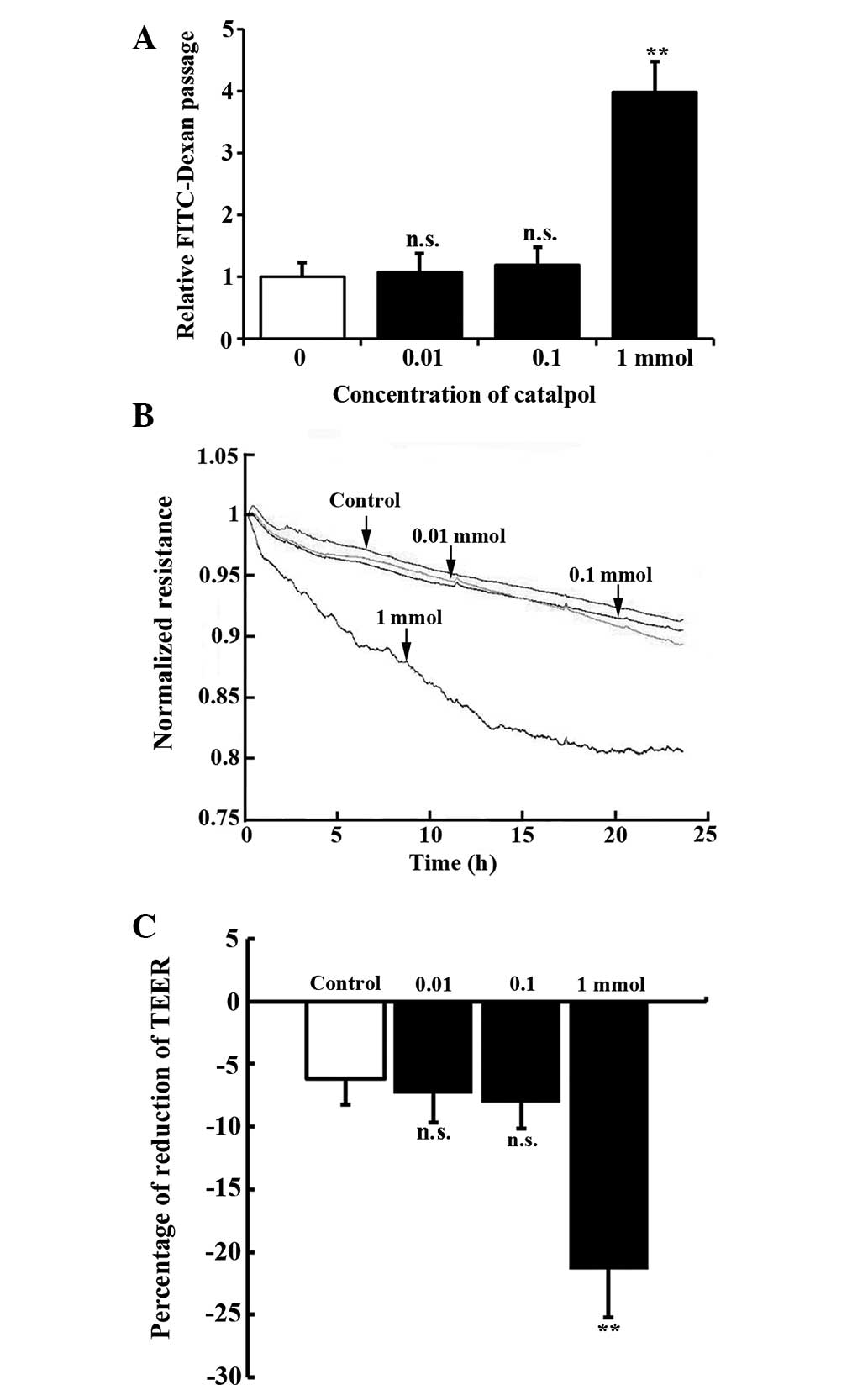
Catalpol induces vascular permeability in
a dose-dependent manner
In order to examine the effects of catalpol on the
permeability of endothelial cells, a FITC-Dextran Transwell assay
was performed for HUVEC monolayers treated with different
concentrations of catalpol. It was demonstrated that the
FITC-Dextran passage remained unchanged following treatment with
0.01 (1.13±0.08 fold, P=0.33) and 0.1 mmol catalpol (1.22±0.15
fold, P=0.18), but significantly increased with 1 mmol catalpol
treatment (3.98±0.49 fold, P<0.01) (Fig. 1A). In addition, catalpol-induced
permeability was measured using an ECIS system, which allows for
real-time measurements of TEER. In the ECIS circuit, current flows
across confluent endothelial cells while the intercellular barrier
functions as a resistor. As shown in Fig. 1B and C, 1 mmol catalpol treatment
induced a significant decrease in TEER in HUVECs during the 24 h
measurement while the TEER of the control, and 0.01 mmol and 0.1
mmol catalpol treatment groups remained unchanged.
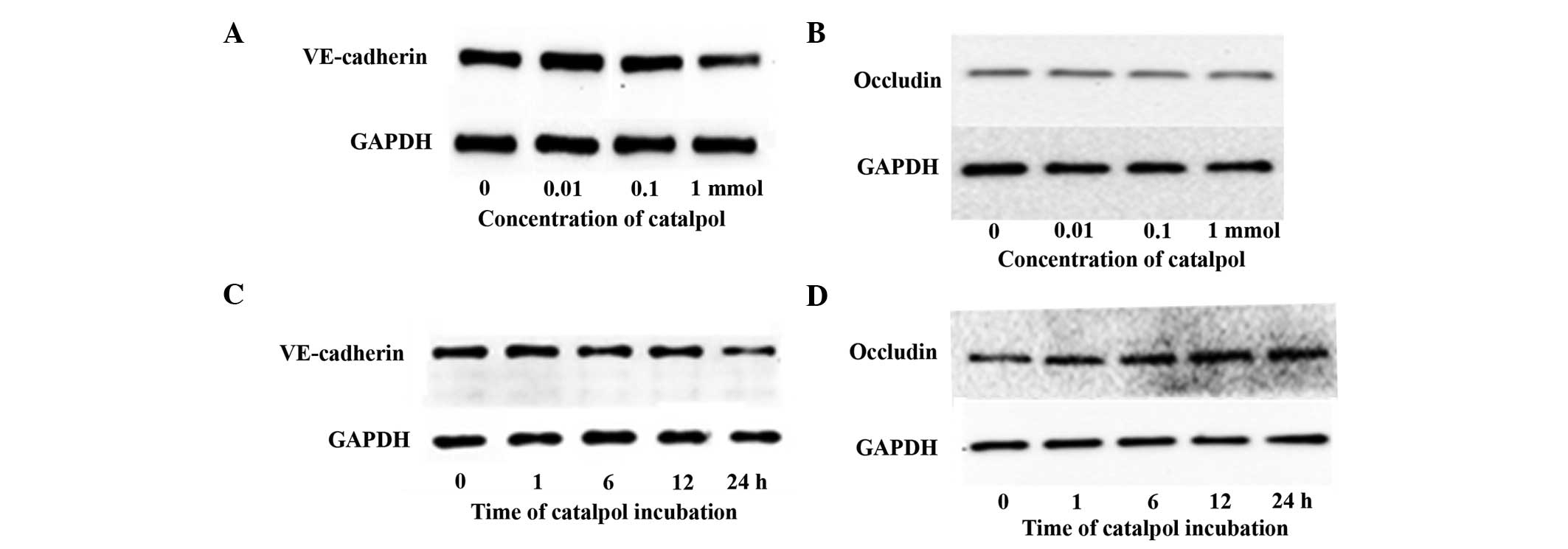
Catalpol reduces the expression of
VE-cadherin but not occluding
Adherens junctions and tight junctions are key in
regulating vascular permeability (13). VE-cadherin and occludin are the key
components of adherens junctions and tight junctions, respectively
(13). The effects of catalpol on
VE-cadherin and occludin expression were examined. In HUVECs
treated with 0.01 or 0.1 mmol catalpol, the levels of VE-cadherin
were similar to that of non-treated cells (Fig. 2A). At a concentration of 1 mmol,
catalpol significantly decreased the quantity of VE-cadherin
(Fig. 2A). However, the protein
levels of occludin did not show a significant change in HUVECs
treated with catalpol at all concentrations (Fig. 2B). In a time course study with 1
mmol catalpol, the level of VE-cadherin protein decreased over time
while occludin remained unchanged (Fig. 2C and D). The expression of
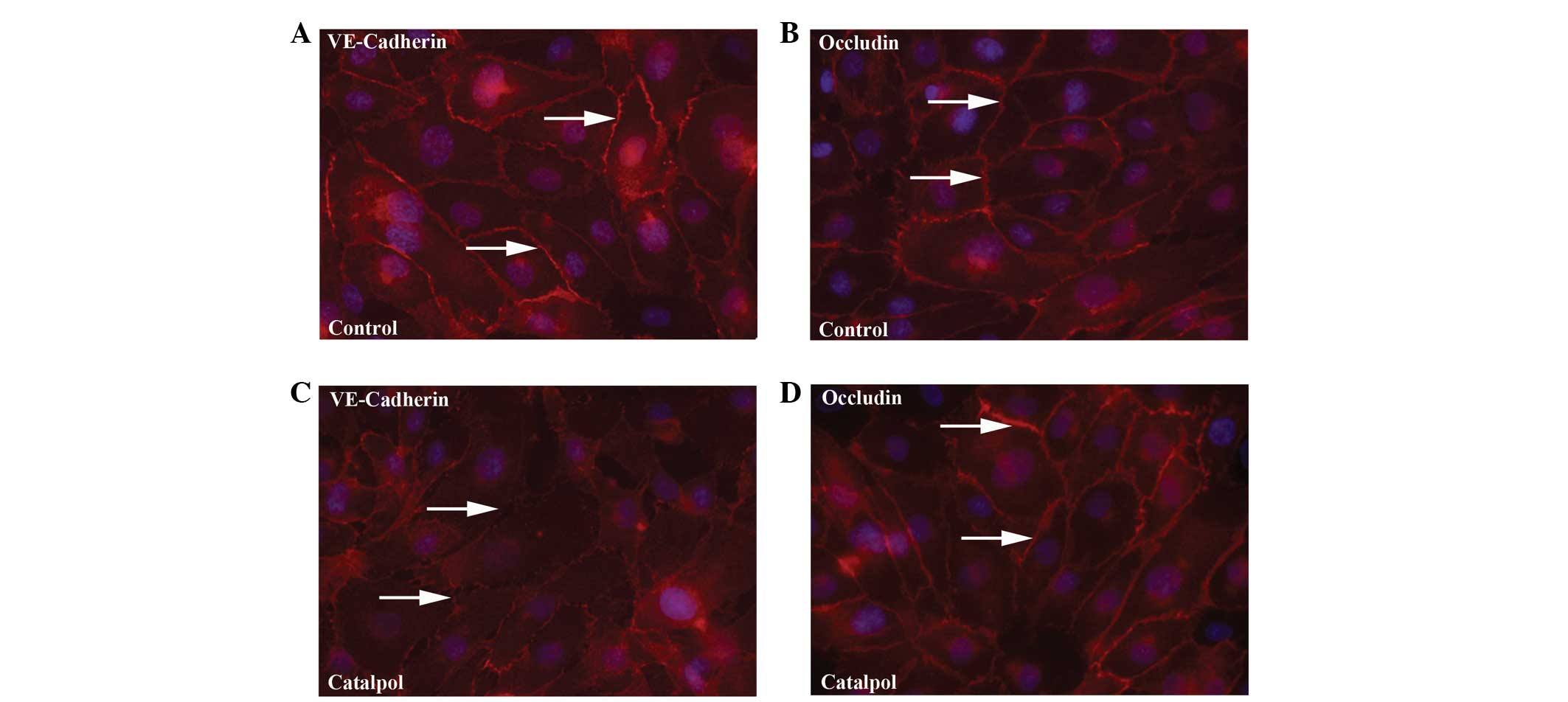
VE-cadherin and occludin was also examined in HUVEC monolayers by
immunofluoresence. As expected, VE-cadherin and occludin were
highly localized to the cell-cell contacts throughout the monolayer
of non-treated HUVECs (Fig. 3A and
B). Upon treatment with 1 mM catalpol, lower levels of
VE-cadherin were found to be associated with junctions while the
expression of occludin was similar to that of the controls
(Fig. 3C and D). VE-cadherin and
occludin remained localized on the membrane of the endothelial
cells, suggesting the distribution of these proteins was not
affected by catalpol treatment. These results indicate that
catalpol specifically reduced protein expression of
VE-cadherin.
Catalpol inhibits the expression of the
ERG transcription factor
The ERG (ETS-related gene) transcription factor
regulates VE-cadherin expression (14). To investigate the mechanisms
underlying the function of catalpol, the transcription of
ERG, CDH5 (encoding VE-cadherin) and OLDN
(encoding occludin) genes were examined in HUVECs treated with 1 mM
catalpol for 24 h. The results of the RT-qPCR demonstrated a
significant decrease in mRNA levels of ERG (P<0.01) and
VE-cadherin (P<0.01), but not occludin (P=0.27) (Fig. 4A). The protein level of ERG was
also examined in HUVECs treated with catalpol by western blot
analysis. The ERG protein level was decreased by treatment with
catalpol in a dose- and time-dependent manner (Fig. 4B and C). To verify the role of ERG
in catalpol-induced vascular permeability, the expression of ERG
was knocked down in HUVECs using siRNA, and these cells were
subsequently plated as monolayers for examination of permeability
using the ECIS system. In the ERG-deficient cells, no significant
change in TEER was observed in the absence or presence of 1 mM
catalpol at any time point during a 24-h incubation (Fig. 4D). Thus, this demonstrated that
catalpol increases vascular permeability via downregulation of the
ERG transcriptional factor.
 | Figure 4Catalpol inhibits the expression of
the ERG transcription factor. (A) Relative VE-cadherin, ERG and
occludin mRNA expression measured by reverse
transcription-quantitative polymerase chain reaction in HUVECs
treated with 1 mM catalpol for 24 h. Error bars represent the
standard error of the mean (n=4). n.s., non-significant;
**P<0.01 compared with control. Representative blots
of ERG and GAPDH from protein samples of HUVECs treated with (B)
catalpol at 0, 0.01, 0.1 and 1 mM for 24 h and (C) at a
concentration of 1 mM for 0, 1, 6, 12, 24 h. (D) Real-time TEER
measurement of ERG-knockdown HUVEC monolayer in the absence or
presence of 1 mM catalpol. VE, vascular endothelial; HUVECs, human
umbilical vein endo-thelial cells; GAPDH, glyceraldehyde
3-phosphate dehydrogenase; TEER, transendothelial electrical
resistance. |
Discussion
Catalpol has been widely used in traditional Chinese
medicine (15). Similar to other
drugs, catalpol enters the circulation and interacts with the
vascular endothelial cells that line the blood vessels. In the
present study, the effects of catalpol on endothelial cells were
analyzed, and it was demonstrated that catalpol induces vascular
permeability in a dose-dependent manner. In addition, catalpol
significantly inhibits the expression of VE-cadherin but not
occludin. The ERG transcription factor, a positive regulator of
VE-caherin expression, was downregulated by catalpol. Knockdown of
ERG expression compromised catalpol-induced hyperpermeability in
HUVECs. From these results, it was concluded that catalpol induces
the downregulation of the ERG transcription factor, which decreases
VE-cadherin expression and increases vascular permeability.
Increased vascular permeability is a key
pathophysiologic event associated with inflammation (1). Previous studies reported the
anti-inflammatory effects of catalpol (8,9,16);
however, its effects on the vascular system have not yet been
investigated. The present study demonstrated an increase in
permeability of HUVEC monolayers in response to 1 mM catalpol using
a Transwell permeability assay and ECIS measurement. This effective
dose is consistent with previous dose-response studies of catalpol,
wherein concentrations of 0.5–1 mM catalpol affected cellular
function (16,17). Catalpol may reduce the inflammatory
response by inhibiting the expression of pro-inflammatory cytokines
and proteins, such as inducible nitric oxide synthase,
cyclooxygenase-2 and Toll-like receptor 4 (16). However, the catalpol-induced
hyper-permeability may exaggerate the inflammatory responses of the
endothelium and increase the passage of plasma proteins. This study
determined the previously unknown effects of catalpol on vascular
endothelium and suggests a pro-inflammatory role.
VE-cadherin is specifically expressed in endothelial
cells and is the major component of adherens junctions (18). In this study, it was demonstrated
that catalpol decreases the mRNA and protein level of VE-cadherin
but not occludin, suggesting that catalpol specifically disrupts
adherens junctions to increase vascular permeability. The
expression and distribution of VE-cadherin is tightly regulated by
the micro-environment of the endothelium (19,20).
Extracellular stimuli, such as TNF-α, thrombin and cadmium,
increase vascular permeability by disruption of the homophilic
interaction of VE-cadherin (18,21,22).
Other factors, such as basic fibroblast growth factor, may enhance
VE-cadherin expression (23). In
this study, it was identified that catalpol significantly inhibits
the mRNA and protein level of VE-cadherin. In a rat model of
stroke, catalpol increases infarcted-brain angiogenesis by
upregulating vascular endothelial growth factor (VEGF) expression
(24). VEGF, also known as a
vascular permeability factor, is a potent enhancer of microvascular
permeability (25). In addition,
VEGF directly inhibits VE-cadherin expression, and induces its
phosphorylation and internalization in endothelial cells (26–28).
Thus, increased VEGF may contribute to catalpol-induced inhibition
of VE-cadherin.
The ETS family member ERG is specifically and
constitutively expressed in endothelial cells (29). The ERG transcription factor drives
the expression of genes involved in endothelial homeostasis and
angiogenesis (30). ERG binds to
the VE-cadherin promoter and enhances its activity (14). Inhibition of ERG expression by
siRNA in HUVECs also decreased the expression of VE-cadherin. In
this study, it was demonstrated that catalpol inhibits the mRNA and
protein expression of ERG, suggesting that catalpol is an inhibitor
of ERG. During inflammation, TNF-α downregulates ERG expression in
endothelial cells, which subsequently modifies the expression of
genes mediating inflammatory responses, including IL-8,
intracellular adhesion molecule-2, von Willibrand factor and
VE-cadherin (14,30–32).
In this context, catalpol may have a similar pro-inflammatory
effect on endothelial cells. Moreover, catalpol failed to induce
additional reduction of TEER in HUVECs following knockdown of ERG.
This confirmed that ERG transcription factor mediates
catalpol-induced vascular permeability.
In conclusion, it was demonstrated that catalpol
increases vascular permeability in HUVEC monolayers. Catalpol
inhibits the expression of the junctional molecule VE-cadherin, but
not occludin. In addition, catalpol downregulates the expression of
the ERG transcription factor, which mediates catalpol-induced
hyperpermeability. These results are important for the further
exploration of the clinical potential of catalpol.
Acknowledgments
This study was supported by the grants from Science
and Technology Development Plan of Shandong Province (grant no.
2013GSF11805) and the Shandong Taishan Scholarship (awarded to
Professor Ju Liu).
References
|
1
|
Phillipson M and Kubes P: The neutrophil
in vascular inflammation. Nat Med. 17:1381–1390. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Popović M, Smiljanić K, Dobutović B,
Syrovets T, Simmet T and Isenović ER: Thrombin and vascular
inflammation. Mol Cell Biochem. 359:301–313. 2012. View Article : Google Scholar
|
|
3
|
Lindbom L: Regulation of vascular
permeability by neutrophils in acute inflammation. Chem Immunol
Allergy. 83:146–166. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aghajanian A, Wittchen ES, Allingham MJ,
Garrett TA and Burridge K: Endothelial cell junctions and the
regulation of vascular permeability and leukocyte transmigration. J
Thromb Haemost. 6:1453–1460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vestweber D: VE-cadherin: The major
endothelial adhesion molecule controlling cellular junctions and
blood vessel formation. Arterioscler Thromb Vasc Biol. 28:223–232.
2008. View Article : Google Scholar
|
|
6
|
Van Itallie CM and Anderson JM:
Architecture of tight junctions and principles of molecular
composition. Semin Cell Dev Biol. 36:157–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Z, An LJ, Duan YL, Li YC and Jiang B:
Catalpol protects rat pheochromocytoma cells against oxygen and
glucose deprivation-induced injury. Neurol Res. 30:106–112. 2008.
View Article : Google Scholar
|
|
8
|
Zhang X, Jin C, Li Y, Guan S, Han F and
Zhang S: Catalpol improves cholinergic function and reduces
inflammatory cytokines in the senescent mice induced by
D-galactose. Food Chem Toxicol. 58:50–55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fu K, Piao T, Wang M, Zhang J, Jiang J,
Wang X and Liu H: Protective effect of catalpol on
lipopolysaccharide-induced acute lung injury in mice. Int
Immunopharmacol. 23:400–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu YR, Li PW, Suo JJ, Sun Y, Zhang BA, Lu
H, Zhu HC and Zhang GB: Catalpol provides protective effects
against cerebral ischaemia/reperfusion injury in gerbils. J Pharm
Pharmacol. 66:1265–1270. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tian YY, An LJ, Jiang L, Duan YL, Chen J
and Jiang B: Catalpol protects dopaminergic neurons from
LPS-induced neurotoxicity in mesencephalic neuron-glia cultures.
Life Sci. 80:193–199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hong J, Kandasamy K, Marimuthu M, Choi CS
and Kim S: Electrical cell-substrate impedance sensing as a
non-invasive tool for cancer cell study. Analyst. 136:237–245.
2011. View Article : Google Scholar
|
|
13
|
Wallez Y and Huber P: Endothelial adherens
and tight junctions in vascular homeostasis, inflammation and
angiogenesis. Biochim Biophys Acta. 1778:794–809. 2008. View Article : Google Scholar
|
|
14
|
Yuan L, Le Bras A, Sacharidou A, Itagaki
K, Zhan Y, Kondo M, Carman CV, Davis GE, Aird WC and Oettgen P:
ETS-related gene (ERG) controls endothelial cell permeability via
transcriptional regulation of the claudin 5 (CLDN5) gene. J Biol
Chem. 287:6582–6591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li DQ, Bao YM, Li Y, Wang CF, Liu Y and An
LJ: Catalpol modulates the expressions of Bcl-2 and Bax and
attenuates apoptosis in gerbils after ischemic injury. Brain Res.
1115:179–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bi J, Jiang B, Zorn A, Zhao RG, Liu P and
An LJ: Catalpol inhibits LPS plus IFN-gamma-induced inflammatory
response in astrocytes primary cultures. Toxicol In Vitro.
27:543–550. 2013. View Article : Google Scholar
|
|
17
|
Bi J, Jiang B, Liu JH, Lei C, Zhang XL and
An LJ: Protective effects of catalpol against H2O2-induced
oxidative stress in astrocytes primary cultures. Neurosci Lett.
442:224–227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hordijk PL, Anthony E, Mul FP, Rientsma R,
Oomen LC and Roos D: Vascular-endothelial-cadherin modulates
endothelial monolayer permeability. J Cell Sci. 112:1915–1923.
1999.PubMed/NCBI
|
|
19
|
Dejana E and Giampietro C: Vascular
endothelial-cadherin and vascular stability. Curr Opin Hematol.
19:218–223. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gavard J: Breaking the VE-cadherin bonds.
FEBS lett. 583:1–6. 2009. View Article : Google Scholar
|
|
21
|
Yuan SY: Protein kinase signaling in the
modulation of micro-vascular permeability. Vascul Pharmacol.
39:213–223. 2002. View Article : Google Scholar
|
|
22
|
Dong F, Guo F, Li L, Guo L, Hou Y, Hao E,
Yan S, Allen TD and Liu J: Cadmium induces vascular permeability
via activation of the p38 MAPK pathway. Biochem Biophys Res Commun.
450:447–452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Prandini MH, Dreher I, Bouillot S,
Benkerri S, Moll T and Huber P: The human VE-cadherin promoter is
subjected to organ-specific regulation and is activated in tumour
angiogenesis. Oncogene. 24:2992–3001. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu HF, Wan D, Luo Y, Zhou JL, Chen L and
Xu XY: Catalpol increases brain angiogenesis and up-regulates VEGF
and EPO in the rat after permanent middle cerebral artery
occlusion. Int J Biol Sci. 6:443–453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dvorak HF: VPF/VEGF and the angiogenic
response. Semin Perinatol. 24:75–78. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hebda JK, Leclair HM, Azzi S, Roussel C,
Scott MG, Bidère N and Gavard J: The C-terminus region of
β-arrestin1 modulates VE-cadherin expression and endothelial cell
permeability. Cell Commun Signal. 11:372013. View Article : Google Scholar
|
|
27
|
Gavard J and Gutkind JS: VEGF controls
endothelial-cell permeability by promoting the
beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol.
8:1223–1234. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Esser S, Lampugnani MG, Corada M, Dejana E
and Risau W: Vascular endothelial growth factor induces VE-cadherin
tyrosine phosphorylation in endothelial cells. J Cell Sci.
111:1853–1865. 1998.PubMed/NCBI
|
|
29
|
Oettgen P: Regulation of vascular
inflammation and remodeling by ETS factors. Circ Res. 99:1159–1166.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sperone A, Dryden NH, Birdsey GM, Madden
L, Johns M, Evans PC, Mason JC, Haskard DO, Boyle JJ, Paleolog EM
and Randi AM: The transcription factor Erg inhibits vascular
inflammation by repressing NF-kappaB activation and proinflammatory
gene expression in endothelial cells. Arterioscler Thromb Vasc
Biol. 31:142–150. 2011. View Article : Google Scholar
|
|
31
|
Yuan L, Nikolova-Krstevski V, Zhan Y,
Kondo M, Bhasin M, Varghese L, Yano K, Carman CV, Aird WC and
Oettgen P: Antiinflammatory effects of the ETS factor ERG in
endothelial cells are mediated through transcriptional repression
of the interleukin-8 gene. Circ Res. 104:1049–1057. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu J, Yuan L, Molema G, Regan E, Janes L,
Beeler D, Spokes KC, Okada Y, Minami T, Oettgen P and Aird WC:
Vascular bed-specific regulation of the von Willebrand factor
promoter in the heart and skeletal muscle. Blood. 117:342–351.
2011. View Article : Google Scholar :
|