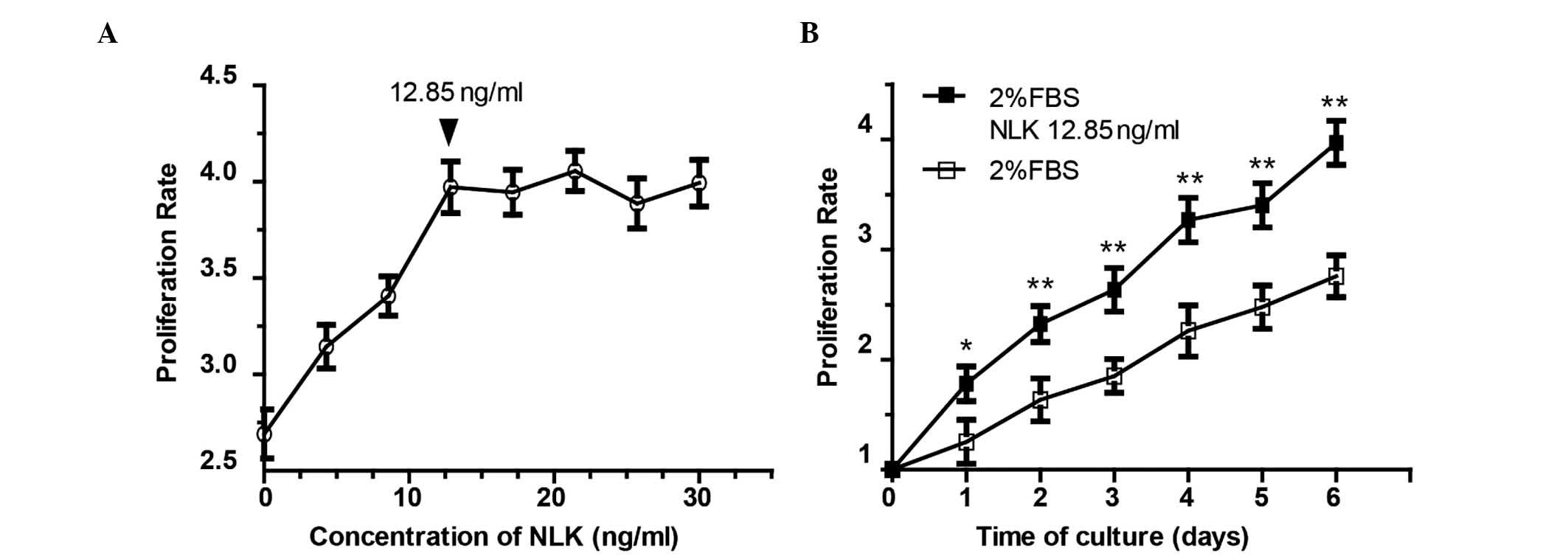
|
1
|
Wang Q, Huang C, Xue M and Zhang X:
Expression of endogenous BMP-2 in periosteal progenitor cells is
essential for bone healing. Bone. 48:524–532. 2011. View Article : Google Scholar :
|
|
2
|
Wilusz RE, Sanchez-Adams J and Guilak F:
The structure and function of the pericellular matrix of articular
cartilage. Matrix Biol. 39:25–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Demoor M, Ollitrault D, Gomez-Leduc T,
Bouyoucef M, Hervieu M, Fabre H, Lafont J, Denoix JM, Audigié F,
Mallein-Gerin F, et al: Cartilage tissue engineering: Molecular
control of chondrocyte differentiation for proper cartilage matrix
reconstruction. Biochim Biophys Acta. 1840:2414–2440. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang Y and Tuan RS: Origin and function
of cartilage stem/progenitor cells in osteoarthritis. Nat Rev
Rheumatol. 11:206–212. 2015. View Article : Google Scholar
|
|
5
|
Rodrigues MT, Gomes ME and Reis RL:
Current strategies for osteochondral regeneration: From stem cells
to pre-clinical approaches. Curr Opin Biotechnol. 22:726–733. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kock L, van Donkelaar CC and Ito K: Tissue
engineering of functional articular cartilage: The current status.
Cell Tissue Res. 347:613–627. 2012. View Article : Google Scholar :
|
|
7
|
van Osch GJ, Brittberg M, Dennis JE,
Bastiaansen-Jenniskens YM, Erben RG, Konttinen YT and Luyten FP:
Cartilage repair: Past and future–lessons for regenerative
medicine. J Cell Mol Med. 13:792–810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Edwards PK, Ackland T and Ebert JR:
Clinical rehabilitation guidelines for matrix-induced autologous
chondrocyte implantation on the tibiofemoral joint. J Orthop Sports
Phys Ther. 44:102–119. 2014. View Article : Google Scholar
|
|
9
|
Yu SM and Kim SJ: The thymoquinone-induced
production of reactive oxygen species promotes dedifferentiation
through the ERK pathway and inflammation through the p38 and PI3K
pathways in rabbit articular chondrocytes. Int J Mol Med.
35:325–332. 2015.
|
|
10
|
Goessler UR, Hörmann K and Riedel F:
Tissue engineering with chondrocytes and function of the
extracellular matrix (Review). Int J Mol Med. 13:505–513.
2004.PubMed/NCBI
|
|
11
|
Foldager CB: Advances in autologous
chondrocyte implantation and related techniques for cartilage
repair. Dan Med J. 60:B46002013.PubMed/NCBI
|
|
12
|
Yanagawa T, Funasaka T, Tsutsumi S,
Watanabe H and Raz A: Novel roles of the autocrine motility
factor/phosphoglucose isomerase in tumor malignancy. Endocr Relat
Cancer. 11:749–759. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Funasaka T and Raz A: The role of
autocrine motility factor in tumor and tumor microenvironment.
Cancer Metastasis Rev. 26:725–735. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsutsumi S, Hogan V, Nabi IR and Raz A:
Overexpression of the autocrine motility factor/phosphoglucose
isomerase induces transformation and survival of NIH-3T3
fibroblasts. Cancer Res. 63:242–249. 2003.PubMed/NCBI
|
|
15
|
Shih WL, Liao MH, Lin PY, Chang CI, Cheng
HL, Yu FL and Lee JW: PI 3-kinase/Akt and STAT3 are required for
the prevention of TGF-beta-induced Hep3B cell apoptosis by
autocrine motility factor/phosphoglucose isomerase. Cancer Lett.
290:223–237. 2010. View Article : Google Scholar
|
|
16
|
Kho DH, Nangia-Makker P, Balan V, Hogan V,
Tait L, Wang Y and Raz A: Autocrine motility factor promotes HER2
cleavage and signaling in breast cancer cells. Cancer Res.
73:1411–1419. 2013. View Article : Google Scholar :
|
|
17
|
Tsutsumi S, Yanagawa T, Shimura T,
Fukumori T, Hogan V, Kuwano H and Raz A: Regulation of cell
proliferation by autocrine motility factor/phosphoglucose isomerase
signaling. J Biol Chem. 278:32165–32172. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Silletti S and Raz A: Autocrine motility
factor is a growth factor. Biochem Biophys Res Commun. 194:446–457.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhi J, Sommerfeldt DW, Rubin CT and
Hadjiargyrou M: Differential expression of neuroleukin in osseous
tissues and its involvement in mineralization during osteoblast
differentiation. J Bone Miner Res. 16:1994–2004. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Qin J, Lin B and Zhang W: The
effects of insulin-like growth factor-1 and basic fibroblast growth
factor on the proliferation of chondrocytes embedded in the
collagen gel using an integrated microfluidic device. Tissue Eng
Part C Methods. 16:1267–1275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spahn G, Kahl E, Muckley T, Hofmann GO and
Klinger HM: Arthroscopic knee chondroplasty using a bipolar
radiofrequency-based device compared to mechanical shaver: Results
of a prospective, randomized, controlled study. Knee Surg Sports
Traumatol Arthrosc. 16:565–573. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gelse K, von der Mark K, Aigner T, Park J
and Schneider H: Articular cartilage repair by gene therapy using
growth factor-producing mesenchymal cells. Arthritis Rheum.
48:430–441. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haene R, Qamirani E, Story RA, Pinsker E
and Daniels TR: Intermediate outcomes of fresh talar osteochondral
allografts for treatment of large osteochondral lesions of the
talus. J Bone Joint Surg Am. 94:1105–1110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nukavarapu SP and Dorcemus DL:
Osteochondral tissue engineering: Current strategies and
challenges. Biotechnol Adv. 31:706–721. 2013. View Article : Google Scholar
|
|
25
|
Chan BP and Leong KW: Scaffolding in
tissue engineering: General approaches and tissue-specific
considerations. Eur Spine J. 17(Suppl 4): 467–479. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng CW, Solorio LD and Alsberg E:
Decellularized tissue and cell-derived extracellular matrices as
scaffolds for orthopaedic tissue engineering. Biotechnol Adv.
32:462–484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krejci P, Masri B, Fontaine V, Mekikian
PB, Weis M, Prats H and Wilcox WR: Interaction of fibroblast growth
factor and C-natriuretic peptide signaling in regulation of
chondrocyte proliferation and extracellular matrix homeostasis. J
Cell Sci. 118:5089–5100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Phornphutkul C, Wu KY, Yang X, Chen Q and
Gruppuso PA: Insulin-like growth factor-I signaling is modified
during chon-drocyte differentiation. J Endocrinol. 183:477–486.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bobick BE and Kulyk WM: The MEK-ERK
signaling pathway is a negative regulator of cartilage-specific
gene expression in embryonic limb mesenchyme. J Biol Chem.
279:4588–4595. 2004. View Article : Google Scholar
|
|
30
|
Gikas PD, Bayliss L, Bentley G and Briggs
TW: An overview of autologous chondrocyte implantation. J Bone
Joint Surg Br. 91:997–1006. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Araki K, Shimura T, Yajima T, Tsutsumi S,
Suzuki H, Okada K, Kobayashi T, Raz A and Kuwano H: Phosphoglucose
isomerase/autocrine motility factor promotes melanoma cell
migration through ERK activation dependent on autocrine production
of interleukin-8. J Biol Chem. 284:32305–32311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Niizeki H, Kobayashi M, Horiuchi I,
Akakura N, Chen J, Wang J, Hamada JI, Seth P, Katoh H, Watanabe H,
et al: Hypoxia enhances the expression of autocrine motility factor
and the motility of human pancreatic cancer cells. Br J Cancer.
86:1914–1919. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Timar J, Trikha M, Szekeres K, Bazaz R,
Tovari J, Silletti S, Raz A and Honn KV: Autocrine motility factor
signals integrin-mediated metastatic melanoma cell adhesion and
invasion. Cancer Res. 56:1902–1908. 1996.PubMed/NCBI
|
|
34
|
Niinaka Y, Paku S, Haga A, Watanabe H and
Raz A: Expression and secretion of neuroleukin/phosphohexose
isomerase/maturation factor as autocrine motility factor by tumor
cells. Cancer Res. 58:2667–2674. 1998.PubMed/NCBI
|
|
35
|
Dobashi Y, Watanabe H, Matsubara M,
Yanagawa T, Raz A, Shimamiya T and Ooi A: Autocrine motility
factor/glucose-6-phosphate isomerase is a possible predictor of
metastasis in bone and soft tissue tumours. J Pathol. 208:44–53.
2006. View Article : Google Scholar
|
|
36
|
Luo Y, Long JM, Lu C, Chan SL, Spangler
EL, Mascarucci P, Raz A, Longo DL, Mattson MP, Ingram DK and Weng
NP: A link between maze learning and hippocampal expression of
neuroleukin and its receptor gp78. J Neurochem. 80:354–361. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang Y, Cheng XR, Zhang GR, Zhou WX and
Zhang YX: Autocrine motility factor receptor is involved in the
process of learning and memory in the central nervous system. Behav
Brain Res. 229:412–418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Funasaka T, Haga A, Raz A and Nagase H:
Tumor autocrine motility factor is an angiogenic factor that
stimulates endothelial cell motility. Biochem Biophys Res Commun.
285:118–128. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bhise NS, Ribas J, Manoharan V, Zhang YS,
Polini A, Massa S, Dokmeci MR and Khademhosseini A: Organ-on-a-chip
platforms for studying drug delivery systems. J Control Release.
190:82–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Björnmalm M, Yan Y and Caruso F:
Engineering and evaluating drug delivery particles in microfluidic
devices. J Control Release. 190:139–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ye N, Qin J, Shi W, Liu X and Lin B:
Cell-based high content screening using an integrated microfluidic
device. Lab Chip. 7:1696–1704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kane BJ, Zinner MJ, Yarmush ML and Toner
M: Liver-specific functional studies in a microfluidic array of
primary mammalian hepatocytes. Anal Chem. 78:4291–4298. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tourovskaia A, Figueroa-Masot X and Folch
A: Differentiation-on-a-chip: A microfluidic platform for long-term
cell culture studies. Lab Chip. 5:14–19. 2005. View Article : Google Scholar
|
|
44
|
Zhong W, Tian K, Zheng X, Li L, Zhang W,
Wang S and Qin J: Mesenchymal stem cell and chondrocyte fates in a
multishear microdevice are regulated by Yes-associated protein.
Stem Cells Dev. 22:2083–2093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sanz-Ramos P, Duart J, Rodríguez-Goñi MV,
Vicente-Pascual M, Dotor J, Mora G and Izal-Azcárate I: Improved
Chondrogenic Capacity of Collagen Hydrogel-Expanded Chondrocytes:
In Vitro and in Vivo Analyses. J Bone Joint Surg Am. 96:1109–1117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bobick BE, Chen FH, Le AM and Tuan RS:
Regulation of the chondrogenic phenotype in culture. Birth Defects
Res C Embryo Today. 87:351–371. 2009. View Article : Google Scholar : PubMed/NCBI
|