Introduction
Withania somnifera has been applied for the
treatment of chronic diseases in Indian Ayuvedic medicine and its
therapeutic effects are attributed to steroidal lactones referred
to as withanolides. Among these, withaferin A (WA) is known to have
anti-inflammatory and anti-cancer properties (1–4). WA
inhibits the expression of inducible nitric oxide synthase as well
as nitric oxide (NO) production in lipopolysaccharide (LPS)-treated
macrophages by downregulating AKT and activating nuclear factor
(NF)-κB (5). It also exerts
inhibitory effects on high mobility group box 1-induced NF-κB
activation and production of interleukin (IL)-6 as well as tumor
necrosis factor (TNF)-α in human umbilical vein endothelial cells
(6). In addition, WA inhibits
constitutive or induced expression of inflammatory mediators,
including cytokines and intercellular or vascular adhesion
molecules in various types of cell, including epithelial cells
(1–4), suggesting that WA is able to exert
its anti-inflammatory effects in a wide range of host cells. In
addition, WA exhibits marked anti-tumor activity against multiple
types of tumor cell, including leukemia (7) as well as prostate (8) and lung (9) cancer cells. Induction of apoptosis
and inhibition of DNA synthesis have been suggested as the
underlying mechanisms of the anti-proliferative effects of WA on
multiple tumor types (10,11); however, the exact mechanisms remain
to be elucidated.
Gastric cancer is the fourth most common cancer type
worldwide and the third leading cause of mortality from cancer
(12,13). Among the various risk factors,
including gender, age or diet, Helicobacter (H.)
pylori infection is the best-known risk factor for gastric
adenocarcinoma and has been estimated to account for 60% of gastric
cancer cases worldwide (14,15).
The inflammatory response induced by H. pylori infection is
considered to be a major step in the initiation and development of
gastric cancer (16).
Reducing inflammation induced by H. pylori
infection may be an effective means of preventing and curing
gastric cancer. It is therefore of great global interest to
discover novel preventive and therapeutic agents from a pool of
natural products with activity against inflammatory diseases and
cancer (9,17–19).
Although WA possesses anti-inflammatory as well as anti-cancer
properties against a broad range of cell types, its efficacy
against gastric inflammation and cancer has not yet been evaluated,
to the best of our knowledge. Therefore, the present study assessed
the inhibitory effects of WA on H. pylori-induced production
of IL-8 and vascular endothelial growth factor (VEGF), which are
key inflammatory mediators associated with tumor progression
(20–24), as well as the mitogen-activated
protein kinase (MAPK) pathway. In addition, the inhibitory effect
of WA on the proliferation of gastric cancer cells was assessed and
the underlying molecular mechanisms were investigated.
Materials and methods
H. pylori strain and culture
conditions
The H. pylori strain 26695 (American Type
Culture Collection, Manassas, VA, USA) was grown on campylobacter
agar (BD Biosciences, Franklin Lakes, NJ, USA) or brucella broth
(BD Biosciences) containing 10% fetal bovine serum (FBS; Corning
Incorporated, Corning, NY, USA), 10 µg/ml vancomycin
(Sigma-Aldrich, St. Louis, MO, USA), 5 µg/ml trimethoprim
(Sigma-Aldrich), and 1 µg/ml nystatin (Sigma-Aldrich) at
37°C under microaerobic conditions. The bacteria were grown to an
optical density at 600 nm (OD600) of 0.6, measured using
an enzyme-linked immunosorbent assay (ELISA) reader (Epoch; Bio-Tek
Instruments, Inc., Winooski, VT, USA), which corresponds to
~109 colony-forming units (CFU)/ml, and diluted to the
desired concentrations (16).
Cell culture and treatment
The AGS human gastric epithelial cell line was
purchased from the Korean Cell Line Bank (Seoul, Korea) and
cultured with RPMI-1640 medium (Welgene, Inc., Daegu, Korea)
containing 10% FBS and 1X penicillin/streptomycin (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) in a humidified
atmosphere containing 5% CO2 at 37°C. To determine the
production of VEGF and IL-8, AGS cells (1×105 cells/well
in a 48-well plate) were infected with H. pylori 26695 at
the indicated multiplicity of infection (MOI; 1, 10, 50 or 100) in
the absence or presence of WA (10–500 nM; Sigma-Aldrich) for 24 h
at 37°C in an atmosphere containing 5% CO2. To evaluate
the levels of hypoxia-inducible factor (HIF)-1α, AGS cells were
infected with H. pylori 26695 at an MOI of 100 with or
without WA (500 nM) for 6 h.
Determination of IL-8 and VEGF
The concentration of IL-8 and VEGF in the culture
supernatants of H. pylori-infected AGS cells was determined
by commercial Duoset ELISA kits (cat no. DY208 for IL-8 and cat no.
DY293B for VEGF; R&D Systems, Minneapolis, MN, USA) according
to the manufacturer's protocol.
3-(4, 5-dimethythiazol-2-yl)-2,
5-diphenyltetrazolium bromide (MTT) assay
The MTT)-based assay was performed to determine the
cytotoxicity of WA on AGS cells. The cells were seeded at a density
of 5×105 cells/well in 48-well plates with growth
medium. After 24 h, the cells were exposed to different
concentrations of WA (0, 10, 25, 50, 100, 250, 500 and 1000 n M).
After 24 h, each well was incubated with MTT (4 mg/ml;
Sigma-Aldrich) in RPMI-1640 medium (Welgene, Inc.) for 4 h at 37°C.
After 4 h, the MTT solution was removed and replaced with 200
µl of dimethyl sulfoxide (Sigma-Aldrich). The plates were
shaken for 5 min to dissolve the MTT formazan crystals. The OD of
each well was determined using an ELISA reader (Epoch) at a
wavelength of 570 nm. Experiments were repeated in triplicate, and
6 parallel samples were measured each time.
Immunoblotting
AGS cells were infected with H. pylori 26695
(MOI, 100) with or without pre-treatment with WA (500 nM) for 6 h
and lysed at the indicated time-points (0, 15, 30 or 60 min) in a
buffer containing 1% Nonidet-P40 supplemented with protease
inhibitor (complete Mini EDTA-free; Roche, Mannheim, Germany),
phosphatase inhibitor (Phosphatase Inhibitor Cocktail 2;
Sigma-Aldrich) and 2 mM dithiothreitol (Sigma-Aldrich). The
extracted protein concentration was measured using a Protein Assay
kit (cat no. 500–0006; Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Samples of protein (30 µg) were cooled on ice
following an incubation at 95–100°C for 15 min, and the samples
were subse quently electrophoresed using 12% sodium dodecyl sulfate
polyacrylamide gel electrophoresis (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). For the electro phoresis, stacking gel and
separating gel were used at a constant voltage (100 V) for 90 min
and transferred onto nitrocellulose membranes (Bio-Rad
Laboratories, Inc.) by electroblotting. For the electrotransfer,
the apparatus was powered by a constant current (100 V) for 2 h.
The nitrocellulose membranes were blocked with blocking buffer of
5% skimmed milk (incubated at room temperature for 1 h), and subse
quently the blocking solution was discarded. The membranes were
immunoblotted with primary antibodies as follows: rabbit anti-human
polyclonal IκB-α (1:1000; cat. no. 9242; Cell Signaling Technology,
Inc., Danvers, MA, USA); rabbit anti-human polyclonal
phosphorylated (p)-c-Jun N-terminal kinase (JNK; 1:1000; cat. no.
9251; Cell Signaling Technology, Inc.); rabbit anti-human
polyclonal JNK antibody (1:1000; cat. no. 9252; Cell Signaling
Technology, Inc.); rabbit anti-human polyclonal p-p38 antibody
(1:1000; cat. no. sc-101759; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA); rabbit anti-human polyclonal p38 antibody
(1:1000; cat. no. sc-728; Santa Cruz Biotechnology, Inc.); mouse
anti-human monoclonal p-ERK antibody (1:1000; cat. no. sc-7383;
Santa Cruz Biotechnology, Inc.); rabbit anti-human polyclonal ERK
antibody (1:1000; cat. no. sc-94; Santa Cruz Biotechnology, Inc.);
mouse anti-human monoclonal HIF-1α antibody (1:1000; cat. no.
610958; BD Biosciences); and rabbit anti-human polyclonal
anti-β-actin antibody (1:1000; cat. no. sc-130656; Santa Cruz
Biotechnology, Inc.) were added, followed by an incubation with
agitation at 4°C overnight. The membrane was rinsed with
Tris-buffered saline with Tween® 20 (TBST) three times,
each time for 5 min. The membrane was incubated with secondary
horseradish peroxidase-conjugated goat anti-rabbit (1:4000; cat.
no. sc-2301; Santa Cruz Biotechnology, Inc.) or goat anti-mouse IgG
(1:2000; cat. no. sc-2031; Santa Cruz Biotechnology, Inc.)
antibodies for 2 h at room temperature, and again washed three time
with TBST for 10 min. Proteins were detected with SuperSignal™ West
Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Inc.).
Images of the blots were captured on CP-BU new film (Agfa
HealthCare, Mortsel, Belgium) by an Automatic X-ray Film processor
(JP-33; JPI, Seoul, Korea).
Bacterial growth rate
50 µl of the bacterial cultures
(1×109 CFU/ml) were diluted with 2 ml brucella broth
containing WA (10-1,000 nM) and incubated at 37°C under
microaerobic conditions for 12 and 24 h. Bacterial growth was
determined by measuring the OD600 of the culture
broth.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Differences between mean values among different groups
were tested and all statistical calculations were performed by one-
or two-way analysis of variance with Bonferroni's post-hoc test
using GraphPad Prism version 5.00 (GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference between values.
Results
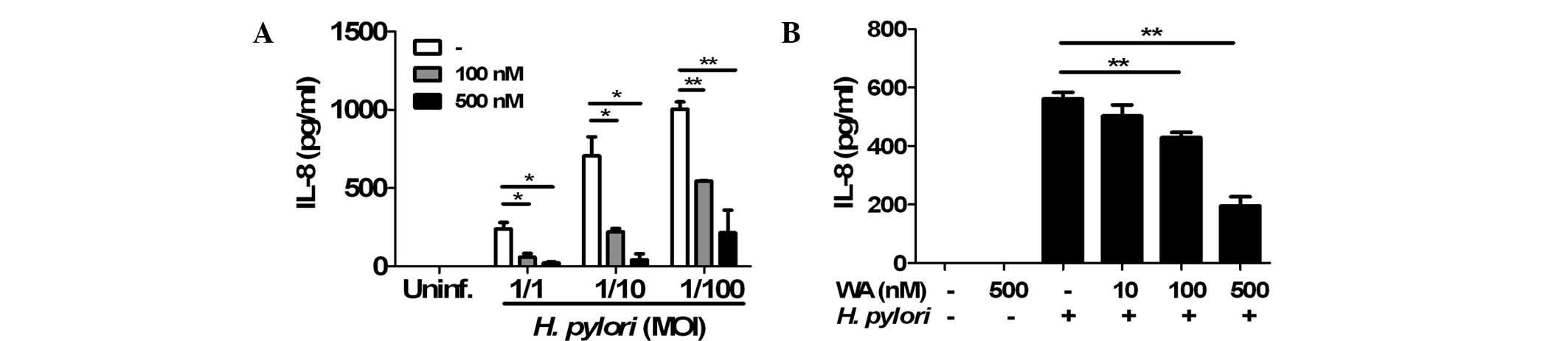
WA inhibits H. pylori-induced IL-8
production in gastric epithelial cells
To determine the effects of WA on H.
pylori-induced IL-8 production in gastric epithelial cells, AGS
cells were pre-treated with 100 or 500 nM WA for 6 h and
subsequently infected with H. pylori. An ELISA showed that
the two doses of WA significantly reduced IL-8 production induced
by H. pylori infection (Fig.
1A). In a further experiment, the cells were co-treated with
H. pylori (MOI, 1/100) and various doses of WA. Following
incubation for 24 h, H. pylori-induced production of IL-8
was decreased by WA in a dose-dependent manner (Fig. 1B). Furthermore, a preliminary MTT
assay revealed that WA was not cytotoxic at the concentrations used
(data not shown). These results indicated that pre-treatment and
co-treatment with WA effectively inhibited H. pylori-induced
IL-8 production in gastric epithelial cells.
WA inhibits the activation of NF-κB, but
not MAPKs, induced by H. pylori infection in gastric epithelial
cells
NF-κB and MAPKs are known to be involved in H.
pylori-induced IL-8 production in gastric epithelial cells
(25). Therefore, the present
study sought to determine whether WA affects the activation of
NF-κB and MAPKs in H. pylori-infected gastric epithelial
cells. Infection of AGS cells with H. pylori for 15 min led
to a marked degradation of IκB-α, which almost disappeared at 30
min (Fig. 2). Of note, this
reduction of IκB-α was restored by WA treatment (Fig. 2). By contrast, the MAPKs p38, ERK
and JNK were activated by H. pylori at 15 min of infection
and beyond, which was not affected by treatment with WA. These
results indicated that WA specifically inhibits the activation of
NF-κB induced by H. pylori in gastric epithelial cells,
while not affecting the activation of MAPKs,.
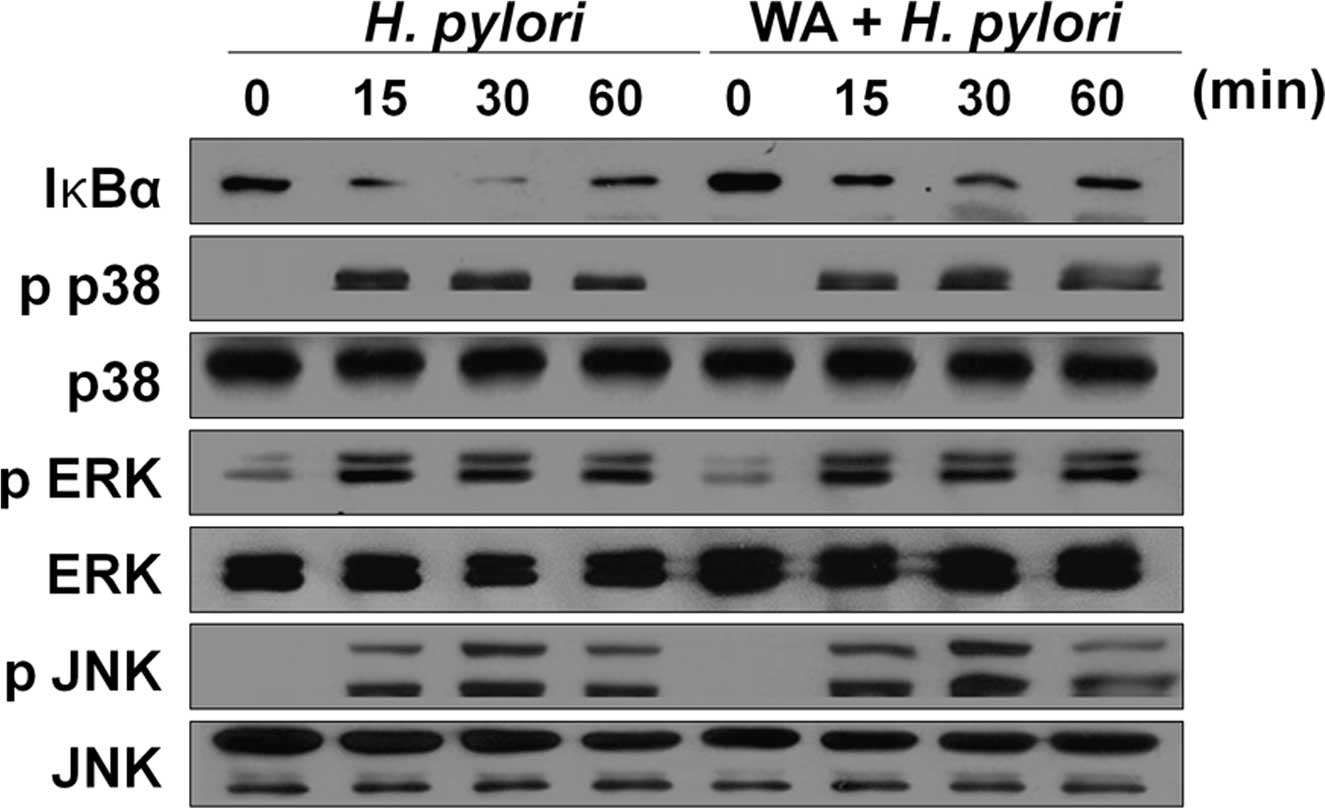 | Figure 2WA inhibits the activation of NF-κB,
but not MAPKs, induced by H. pylori infection in gastric
epithelial cells. AGS cells were pre-treated with or without 500 nM
of WA and subsequently infected with H. pylori (multiplicity
of infection, 1/100). The cellular proteins were harvested at the
indicated time-points and the amounts of phosphorylated or total
MAPKs (p38, ERK or JNK) and IκB-α were determined by western blot
analysis. MAPK, mitogen-activated protein kinase; p-ERK,
phosphorylated extracellular signal-regulated kinase; JNK, c-Jun
N-terminal kinase; NF-κB, nuclear factor kappa B; IκBα, inhibitor
of NF-κB; H. pylori, Helicobacter pylori; WA, withaferin
A. |
WA does not affect H. pylori-induced VEGF
production and HIF-1α stabilization in gastric epithelial
cells
To evaluate the effects of WA on VEGF production in
AGS cells induced by H. pylori infection, VEGF levels were
measured in the culture supernatants using an ELISA. The results
showed that pre-treatment as well as co-treatment with WA did not
inhibit basal or H. pylori-induced production of VEGF in the
cells (Fig. 3A and B). HIF-1 is a
transcriptional factor that regulates a number of genes, including
VEGF, involved in the hypoxic response (26). It is also known that H.
pylori can induce the stabilization of HIF-1α to promote VEGF
production (26–28). Therefore, the present study sought
to determine the effects of WA on H. pylori-mediated HIF-1α
stabilization using western blot analysis. As expected, H.
pylori led to HIF-1α stabilization in gastric epithelial cells,
which was not affected by pre-treatment of WA (Fig. 3C).
 | Figure 3WA does not affect H.
pylori-induced VEGF production and HIF-1α stabilization in
gastric epithelial cells. (A) ASG cells were pre-treated with or
without WA for 6 h, followed by infection with the indicated MOI of
H. pylori for 24 h. (B) In another experiment
(co-treatment), the cells were also infected with H. pylori
(MOI, 1/100) in the absence or presence of WA. At 24 h after
infection, the concentration of VEGF in culture supernatant was
determined by ELISA. Values are expressed as the mean ± standard
deviation of triplicate samples from one experiment representative
of three independent experiments. (C) The cells were pre-treated
with WA (500 nM) and infected with H. pylori at an MOI of
100 for 6 h. Cellular protein was extracted and the levels of
HIF-1α were analyzed by immunoblotting. β-actin was used as a
loading control. VEGF, vascular endothelial growth factor; HIF,
hypoxia-inducible factor; H. pylori, Helicobacter pylori;
MOI, multiplicity of infection; IL interleukin; WA, withaferin A;
Uninf., uninfected. |
WA does not exhibit any anti-bacterial
activity against H. pylori
To determine whether WA exerts any anti-bacterial
activity against H. pylori, bacterial growth was evaluated
by measuring their OD600 following incubation with WA.
The results showed that the growth of H. pylori was not
affected by WA, even at the high concentration of 1 µM.
These results suggested that the anti-inflammatory activity of WA
is not based on any bactericidal effect.
Discussion
IL-8 is a chemoattractant factor for neutrophil
recruitment and a critical immune mediator for the pathogenesis of
chronic gastritis caused by H. pylori infection. In
addition, various studies have reported that high expression of
IL-8 is correlated with poor prognosis of gastric cancers or
gastrointestinal tumorigenesis, including angiogenesis (29–31).
Therefore, IL-8 has been suggested as a therapeutic target in
gastric cancer. The present study revealed that in vitro
pre-treatment and co-treatment of WA effectively inhibits H.
pylori-induced production of IL-8 in gastric epithelial cells,
suggesting that WA may have preventive as well as therapeutic
effects on H. pylori-mediated inflammation.
Various host factors, including phosphoinositide-3
kinase, heat shock protein 90, toll-like receptor 4, nicotinamide
adenosine dinucleotide phosphate oxidase 1 and nucleotide-binding
oligomerization domain 1 have been suggested as mechanisms for
H. pylori-induced IL-8 production in gastric epithelial
cells (32). NF-κB and MAPKs are
known to be essential downstream molecules for the production of
IL-8 induced by H. pylori (32). Previous studies by our and another
group have also revealed that bacterial factors, including the type
IV secretion system, are required for H. pylori-induced IL-8
production and activation of NF-κB and MAPKs (25,32).
In the present study, pre-treatment with WA inhibited H.
pylori-induced activation of NF-κB, but not MAPKs. It is known
that WA inhibits NF-κB activation in a wide variety of cell types
exposed to several stimuli, including LPS and TNF-α (33). By contrast, WA was shown to induce
MAPK activation in breast cancer and leukemia cells (7,34,35),
suggesting that WA may differentially regulate the activation of
NF-κB and MAPKs in host cells. In the present study, although WA
pre-treatment did not affect H. pylori-induced activation of
MAPKs, the effect of WA on basal levels of MAPK activation in
gastric cancer cells should not be ignored. It has been reported
that WA induces apoptosis in various cancer types (33) and that MAPK-mediated signaling is
involved in cellular apoptosis. In most experiments performed to
evaluate the apoptotic effects of WA, a high concentration (>1
µM) of WA was used (7,34,35),
whereas low concentrations (<500 nM) of WA were used in the
present study. In fact, the MTT assay demonstrated that WA at
concentrations >1 µM exerted cytotoxic effects on AGS
cells (data not shown). Therefore, further experiments should be
performed to clarify the effects of WA on gastric
tumorigenesis.
VEGF is closely associated with poor prognosis of
gastric cancer due to its characteristics of tumor invasion and
lymph node metastasis (22,24).
In addition, Wu et al (36)
reported that NF-κB-mediated signaling is important for VEGF
production induced by H. pylori in gastric epithelial cells.
As the results indicated that WA inhibited H. pylori-induced
NF-κB activation in AGS cells, the present study further assessed
the effects of WA on VEGF production induced by H. pylori.
It was revealed that WA did not influence H. pylori-induced
VEGF production in gastric epithelial cells. In a recent study by
our group, an inhibitor assay revealed that NF-κB signaling is not
essential for H. pylori-induced production of VEGF (25). Such VEGF production was reduced by
digoxin (a HIF-1α inhibitor) and N-acetyl-L-cysteine, a
scavenger of reactive oxygen species (ROS) (25). In a study by Zhu et al
(37), the anti-oxidant compound
pyrrolidine dithiocarbamate was used as an NF-κB inhibitor. These
findings suggested that ROS and the HIF-1α axis are critical for
H. pylori-induced VEGF production in gastric epithelial
cells. In the present study, consistently with the results on VEGF
production, HIF-1α stabilization by H. pylori was not
affected by pre-treatment with WA. Therefore, it is expected that
WA does not influence H. pylori-induced ROS production and
any associated signaling.
Extracts from the leaf and root of Withania
somnifera are known to have anti-bacterial activity against
Escherichia coli, Staphylococcus aureus and Salmonella
typhimurium (38,39). Withanolides also inhibit the growth
of Proteus vulgaris (40).
These findings led to the speculation whether the inhibitory
effects of WA on H. pylori-induced IL-8 production and NF-κB
activation in gastric epithelial cells may be due to its
bactericidal effects. However, in the present study, the growth of
H. pylori was not affected by WA, suggesting that the
anti-inflammatory activity of WA is due to direct inhibition of
core signaling pathways, such as NF-κB, but not due to bactericidal
effects.
In conclusion, the results of the present study
showed that WA effectively inhibits H. pylori-induced IL-8
production and NF-κB activation in gastric epithelial cells. An
in vivo study using a murine infection model and a clinical
study are recommended to develop WA as a novel therapeutic agent or
functional additive for the prevention on H. pylori-mediated
inflammation or gastric diseases.
Acknowledgments
This work was supported by a program for Basic
Research in Science and Engineering (grant nos. 2009-0070845 and
2014R1A1A2055026) and by the World Class Institute program (grant
no. WCI 2009-002) of the National Research Foundation of Korea
funded by the Ministry of Science, Information and Communications
Technology and Future Planning.
References
|
1
|
Maitra R, Porter MA, Huang S and Gilmour
BP: Inhibition of NFkappaB by the natural product Withaferin A in
cellular models of cystic fibrosis inflammation. J Inflamm (Lond).
6:152009. View Article : Google Scholar
|
|
2
|
Mohan R, Hammers HJ, Bargagna-Mohan P,
Zhan XH, Herbstritt CJ, Ruiz A, Zhang L, Hanson AD, Conner BP,
Rougas J and Pribluda VS: Withaferin A is a potent inhibitor of
angiogenesis. Angiogenesis. 7:115–122. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vyas AR and Singh SV: Molecular targets
and mechanisms of cancer prevention and treatment by withaferin A,
a naturally occurring steroidal lactone. AAPS J. 16:1–10. 2014.
View Article : Google Scholar :
|
|
4
|
Hahm ER and Singh SV: Withaferin A-induced
apoptosis in human breast cancer cells is associated with
suppression of inhibitor of apoptosis family protein expression.
Cancer Lett. 334:101–108. 2013. View Article : Google Scholar
|
|
5
|
Oh JH, Lee TJ, Park JW and Kwon TK:
Withaferin A inhibits iNOS expression and nitric oxide production
by Akt inactivation and down-regulating LPS-induced activity of
NF-kappaB in RAW 264.7 cells. Eur J Pharmacol. 599:11–17. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee W, Kim TH, Ku SK, Min KJ, Lee HS, Kwon
TK and Bae JS: Barrier protective effects of withaferin A in
HMGB1-induced inflammatory responses in both cellular and animal
models. Toxicol Appl Pharmacol. 262:91–98. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oh JH, Lee TJ, Kim SH, Choi YH, Lee SH,
Lee JM, Kim YH, Park JW and Kwon TK: Induction of apoptosis by
withaferin A in human leukemia U937 cells through down-regulation
of Akt phosphorylation. Apoptosis. 13:1494–1504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roy RV, Suman S, Das TP, Luevano JE and
Damodaran C: Withaferin A, a steroidal lactone from Withania
somnifera, induces mitotic catastrophe and growth arrest in
prostate cancer cells. J Nat Prod. 76:1909–1915. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cai Y, Sheng ZY, Chen Y and Bai C: Effect
of Withaferin A on A549 cellular proliferation and apoptosis in
non-small cell lung cancer. Asian Pac J Cancer Prev. 15:1711–1714.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hahm ER, Lee J and Singh SV: Role of
mitogen-activated protein kinases and Mcl-1 in apoptosis induction
by withaferin A in human breast cancer cells. Mol Carcinog.
53:907–916. 2014. View
Article : Google Scholar
|
|
11
|
Lee DH, Lim IH, Sung EG, Kim JY, Song IH,
Park YK and Lee TJ: Withaferin A inhibits matrix
metalloproteinase-9 activity by suppressing the Akt signaling
pathway. Oncol Rep. 30:933–938. 2013.PubMed/NCBI
|
|
12
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
13
|
Fock KM: Review article: The epidemiology
and prevention of gastric cancer. Aliment Pharmacol Ther.
40:250–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Herrera V and Parsonnet J: Helicobacter
pylori and gastric adenocarcinoma. Clin Microbiol Infect.
15:971–976. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Forman D, de Martel C, Lacey CJ,
Soerjomataram I, Lortet-Tieulent J, Bruni L, Vignat J, Ferlay J,
Bray F, Plummer M and Franceschi S: Global burden of human
papillomavirus and related diseases. Vaccine. 30(Suppl 5): F12–F23.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kao JY, Zhang M, Miller MJ, Mills JC, Wang
B, Liu M, Eaton KA, Zou W, Berndt BE, Cole TS, et al: Helicobacter
pylori immune escape is mediated by dendritic cell-induced Treg
skewing and Th17 suppression in mice. Gastroenterology.
138:1046–1054. 2010. View Article : Google Scholar :
|
|
17
|
Aggarwal BB, Ichikawa H, Garodia P,
Weerasinghe P, Sethi G, Bhatt ID, Pandey MK, Shishodia S and Nair
MG: From traditional Ayurvedic medicine to modern medicine:
Identification of therapeutic targets for suppression of
inflammation and cancer. Expert Opin Ther Targets. 10:87–118. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Beelen Granlund A, Østvik AE, Brenna
Ø, Torp SH, Gustafsson BI and Sandvik AK: REG gene expression in
inflamed and healthy colon mucosa explored by in situ
hybridisation. Cell Tissue Res. 352:639–646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Xu Y, Lei B, Wang W, Ge X and Li J:
Rhein induces apoptosis of human gastric cancer SGC-7901 cells via
an intrinsic mitochondrial pathway. Braz J Med Biol Res.
45:1052–1059. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kitadai Y, Sasaki A, Ito M, Tanaka S, Oue
N, Yasui W, Aihara M, Imagawa K, Haruma K and Chayama K:
Helicobacter pylori infection influences expression of genes
related to angiogenesis and invasion in human gastric carcinoma
cells. Biochem Biophys Res Commun. 311:809–814. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan A, Chen JJ, Yao PL and Yang PC: The
role of interleukin-8 in cancer cells and microenvironment
interaction. Front Biosci. 10:853–865. 2005. View Article : Google Scholar
|
|
22
|
Maeda K, Kang SM, Onoda N, Ogawa M, Sawada
T, Nakata B, Kato Y, Chung YS and Sowa M: Expression of p53 and
vascular endothelial growth factor associated with tumor
angiogenesis and prognosis in gastric cancer. Oncology. 55:594–599.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song ZJ, Gong P and Wu YE: Relationship
between the expression of iNOS, VEGF, tumor angiogenesis and
gastric cancer. World J Gastroenterol. 8:591–595. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maeda K, Chung YS, Ogawa Y, Takatsuka S,
Kang SM, Ogawa M, Sawada T and Sowa M: Prognostic value of vascular
endothelial growth factor expression in gastric carcinoma. Cancer.
77:858–863. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kang MJ, Song EJ, Kim BY, Kim DJ and Park
JH: Helicobacter pylori induces vascular endothelial growth factor
production in gastric epithelial cells through hypoxia-inducible
factor-1 α-dependent pathway. Helicobacter. 19:476–483. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nizet V and Johnson RS: Interdependence of
hypoxic and innate immune responses. Nat Rev Immunol. 9:609–617.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bhattacharyya A, Chattopadhyay R, Hall EH,
Mebrahtu ST, Ernst PB and Crowe SE: Mechanism of hypoxia-inducible
factor 1 alpha-mediated Mcl1 regulation in Helicobacter
pylori-infected human gastric epithelium. Am J Physiol Gastrointest
Liver Physiol. 299:G1177–G1186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park JH, Kim TY, Jong HS, Kim TY, Chun YS,
Park JW, Lee CT, Jung HC, Kim NK and Bang YJ: Gastric epithelial
reactive oxygen species prevent normoxic degradation of
hypoxia-inducible factor-1alpha in gastric cancer cells. Clin
Cancer Res. 9:433–440. 2003.PubMed/NCBI
|
|
29
|
Lee KH, Bae SH, Lee JL, Hyun MS, Kim SH,
Song SK and Kim HS: Relationship between urokinase-type plasminogen
receptor, interleukin-8 gene expression and clinicopathological
features in gastric cancer. Oncology. 66:210–217. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Asfaha S, Dubeykovskiy AN, Tomita H, Yang
X, Stokes S, Shibata W, Friedman RA, Ariyama H, Dubeykovskaya ZA,
Muthupalani S, et al: Mice that express human interleukin-8 have
increased mobilization of immature myeloid cells, which exacerbates
inflammation and accelerates colon carcinogenesis.
Gastroenterology. 144:155–166. 2013. View Article : Google Scholar
|
|
31
|
Beales IL and Calam J: Stimulation of IL-8
production in human gastric epithelial cells by Helicobacter
pylori, IL-1beta and TNF-alpha requires tyrosine kinase activity,
but not protein kinase C. Cytokine. 9:514–520. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee KE, Khoi PN, Xia Y, Park JS, Joo YE,
Kim KK, Choi SY and Jung YD: Helicobacter pylori and interleukin-8
in gastric cancer. World J Gastroenterol. 19:8192–8202. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vanden Berghe W, Sabbe L, Kaileh M,
Haegeman G and Heyninck K: Molecular insight in the multifunctional
activities of Withaferin A. Biochem Pharmacol. 84:1282–1291. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang X, Mukerji R, Samadi AK and Cohen
MS: Down-regulation of estrogen receptor-alpha and rearranged
during transfection tyrosine kinase is associated with withaferin
a-induced apoptosis in MCF-7 breast cancer cells. BMC Complement
Altern Med. 11:842011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mandal C, Dutta A, Mallick A, Chandra S,
Misra L, Sangwan RS and Mandal C: Withaferin A induces apoptosis by
activating p38 mitogen-activated protein kinase signaling cascade
in leukemic cells of lymphoid and myeloid origin through
mitochondrial death cascade. Apoptosis. 13:1450–1464. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu CY, Wang CJ, Tseng CC, Chen HP, Wu MS,
Lin JT, Inoue H and Chen GH: Helicobacter pylori promote gastric
cancer cells invasion through a NF-κB and COX-2-mediated pathway.
World J Gastroenterol. 11:3197–3203. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhu BZ, Carr AC and Frei B: Pyrrolidine
dithiocarbamate is a potent antioxidant against hypochlorous
acid-induced protein damage. FEBS Lett. 532:80–84. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Owais M, Sharad KS, Shehbaz A and
Saleemuddin M: Antibacterial efficacy of Withania somnifera
(ashwagandha) an indigenous medicinal plant against experimental
murine salmonellosis. Phytomedicine. 12:229–235. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sundaram S, Dwivedi P and Purwar S: In
vitro evaluation of antibacterial activities of crude extracts of
Withania somnifera (Ashwagandha) to bacterial pathogens. Asian J
Biotechnol. 3:194–199. 2011. View Article : Google Scholar
|
|
40
|
Kharel P, Manandhar MD, Kalauni SK, Awale
S and Baral J: Isolation, identification and antimicrobial activity
of a Withanolide [WS-1] from the roots of Withania somnifera. Nepal
J Sci Technol. 12:179–186. 2011.
|