Introduction
Endothelial cells are monolayer cells located on the
inner side of vessels, and the integrity and function of
endothelial cells are considered important in the cardiovascular
system (1). Oxidized low-density
lipoprotein (oxLDL)-mediated endothelial cell apoptosis and
dysfunction are reported to be closely associated with
atherosclerosis through inflammatory processes and coagulation
activity, eventually resulting in lesion rupture and clinical
complications (2,3).
MicroRNAs (miRs) are small non-coding RNAs, which
regulate the expression of several proteins at the
post-transcriptional level (4). In
atherogenesis, several microRNAs, including miR-33, miR-133a and
the let-7 family have been demonstrated to be important in
atherogenesis (5–7). miR-590 is a microRNA located on
chromosome 7q11.23 (8). A previous
study reported the anti-apoptotic effects of miR-590 on
oxLDL-treated endothelial cells (9). The pilot studies also revealed a
marked decrease in the expression levels of miR-590 in endothelial
cells following oxLDL treatment. However, the mechanism underlying
the inhibition of oxLDL-induced endothelial cell apoptosis by
miR-590 remains to be elucidated.
Lectin-like low density lipoprotein receptor-1
(LOX-1) was first identified to be a receptor for oxLDL in
endothelial cells (10). The
upregulation in the expression of LOX-1 by oxLDL leads to the
overproduction of reactive oxygen species (ROS), the
phosphorylation of p38 mitogen-activated protein kinase (MAPK), the
activation of nuclear factor (NF)-κB and, eventually, endothelial
activation, dysfunction and apoptosis (11). The LOX-1/ROS/p38MAPK/NF-κB
signaling pathway is also involved in the protective effects of
traditional Chinese medicines (12,13).
As upregulation in the expression of LOX-1 is considered to be the
initial and crucial step in oxLDL-induced endothelial cell injury
(14), the present study
hypothesized that miR-590 may affect this signaling pathway,
regulate apoptosis-associated protein expression and, eventually,
prevent oxLDL-induced endothelial cell apoptosis. P53 is a tumor
suppressor which modulates the apoptotic process by regulating the
expression of B cell lymphoma 2 (Bcl-2) and Bcl-2 associated
protein X (Bax) directly (15,16).
Bcl-2 and Bax are located upstream of caspase-3, a key enzyme in
apoptosis signaling. The p53-Bcl-2/Bax-caspase-3 pathway serves an
important role in apoptosis (17).
Therefore, the aim of the present study was to
examine the anti-apoptotic effects of miR-590 on oxLDL-treated
endothelial cells, and to investigate the roles of the
LOX-1/ROS/p38MAPK/NF-κB and p53-B cell lymphoma 2
(Bcl-2)/Bcl-2-associated protein X (Bax)-caspase-3 apoptotic
signaling pathways in these effects.
Materials and methods
Materials
The human umbilical vascular endothelial cell
(HUVEC) line was obtained from the American Type Culture Collection
(Manassas, VA, USA); oxLDL was supplied by the the Institute of
Biochemistry, Peking Union Medical College (Beijing, China); a
SYBR® Premix DimerEraser™ (Perfect Real Time) assay kit
and a PrimeScript RT reagent kit with gDNA Eraser (Perfect Real
Time) kit were purchased from Takara (Biotechnology Co., Ltd.,
Dalian, China); miR-590 mimics (5′-GAG CUU AUU CAU AAA AGU GCA
G-3′) and primers were obtained from Guangzhou RiboBio Co., Ltd.
(Guangzhou, China); a lactate dehydrogenase (LDH) assay kit,
Annexin V-fluorescein isothiocyanate (FITC) kit and
dichloro-dihydro-fluorescein diacetate (DCFH-DA) were obtained from
Beyotime Institute of Biotechnology (Shanghai, China); Invitrogen
Lipofectamine® 2000 transfection reagent was purchased
from Thermo Fisher Scientific, Inc. (Waltham, MA, USA); rabbit
polyclonal anti-LOX-1 antibody was purchased from Abcam (Cambridge,
UK; cat. no. ab60178); mouse monoclonal anti-p38MAPK antibody (cat.
no. sc-81621), mouse monoclonal anti-phosphorylated (p)-p38MAPK
antibody (cat. no. sc-7973), mouse polyclonal anti-NF-κB (p65)
antibody (cat. no. sc-372), mouse monoclonal anti-p53 antibody
(cat. no. sc-126), rabbit polyclonal anti-Bax antibody (cat. no.
sc-526), rabbit polyclonal anti-Bcl-2 antibody (cat. no. sc-492),
rabbit polyclonal anti-caspase-3 antibody (cat. no. sc-7148) and
the goat anti-mouse (cat. no. sc-2005) and anti-rabbit (cat. no.
sc-2004) IgG-horseradish peroxidase secondary antibodies were
supplied by Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Cell culture and transfection
As previously described (13), the HUVECs (2×105) were
cultured in 6-well plates in complete medium [90% Dulbecco's
modified Eagle's medium supplemented with 10% fetal bovine serum
(GE Healthcare Life Sciences, Logan, UT, USA)], and maintained in a
humidified atmosphere containing 5% CO2 at 37°C.
For transfection, the endothelial cells were grown
to 80% confluence. The media were then replaced with the
transfection complexes and incubated for 6 h. Subsequently, the
medium was replaced with fresh normal growth medium, and incubated
for a further 24 h at 37°C.
The transfection complexes were obtained as follows:
Lipofectamine® 2000 (10 μl), miR-590 mimics (7.5
μl; 20 μM) or negative control (NC; 7.5 μl)
mimics were diluted in 1 ml Opti-MEM I Medium (Invitrogen; Thermo
Fisher Scientific, Inc.) and incubated for 5 min at room
temperature. Diluted Lipofectamine® 2000 (1 ml) was
mixed with diluted microRNA mimics (or NC mimics; 1 ml), and
incubated for a further 20 min at room temperature.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) detection of the expression
levels of miR-590
The HUVECs were seeded in 6-well plates at a density
of 2×105 cells/well. The cells were then transfected
with 25–75 nM miR-590 for 24 h at 37°C, as described above.
Separate groups of HUVECs were treated with oxLDL (150
μg/ml) for different durations (0, 6, 12, and 24 h) or at
different concentrations (50, 100 and 150 μg/ml) for 24 h at
37°C.
Following incubation at 37°C, total RNA was
extracted using invitrogen TRIzol reagent (Thermo Fisher
Scientific, Inc.), the concentration was measured using a
BioPhotometer Plus (Eppendorf, Hamburg, Germany) by optical density
at 260 nm, and the purity of the RNA was evaluated at 260/A280.
Reverse transcription reactions were performed using a PrimeScript
RT reagent kit with a gDNA Eraser (Perfect Real Time) kit and an
miR-590 stem loop RT primer. The PCR amplification reactions were
performed using 2 μl sample, 10 μl SYBR Premix
DimerEraser™ (Perfect Real Time) assay kit, 0.6 μl forward
primer (10 μM), 0.6 μl reverse primer (10 μM)
and 6.8 μl water in a Roche LC480 PCR system (Roche
Diagnostics, Basel, Switzerland). RT-qPCR was performed using the
following thermocycling conditions: Initial incubation at 95°C for
30 sec, followed by 40 cycles of 95°C 5 sec, and 60°C 30 sec. All
samples were run in triplicate, and U6 was used as an internal
reference. The results were quantified using the
2(−∆∆Cq) method (18).
Cell viability and detection of LDH
release
The HUVECs were transfected with 75 nM miR-590 for
24 h at 37°C, as described above. Following transfection, the cells
were treated with 150 μg/ml oxLDL for a further 24 h at
37°C. Cell viability was measured using the MTS method using the
CellTiter 96® AQueous One Solution Cell Proliferation
Assay (Promega Corporation, Madison, WI, USA), and LDH content in
the medium was detected using an LDH assay kit, as previously
described (12).
Cell apoptosis detection using flow
cytometry (FCM) and Hoechst staining
The HUVECs were cultured and transfected, as
described above. Following transfection, the cells were treated
with oxLDL (150 μg/ml) for a further 24 h at 37°C.
Endothelial cell apoptosis was evaluated using FCM using the FC 500
MCL/MPL flow cytometer (Beckman Coulter, Inc., Brea, CA, USA) and
Hoechst (Beyotime Institute of Biotechnology) staining, as
previously described (13). For
FCM, an Annexin V-FITC kit containing propidium iodide was used;
the cells were harvested by trypsinization (Beyotime Institute of
Biotechnology) and incubated with Annexin V-FITC and propidium
iodide for 15 min at room temperature in the dark. Cell apoptosis
rates were then analyzed and quantified using FCM. For the Hoechst
33342 staining, the cells were washed twice with phosphate-buffered
saline (PBS), and incubated in 50 μl Hoechst 33342 solution
(5 μg/ml in PBS) for 20 min in dark at room temperature
following 15 min fixing with 4% paraformaldehyde (Beyotime
Institute of Biotechnology). Morphological changes in the nuclear
chromatin of the apoptotic cells were examined and analyzed using
fluorescence microscopy (CX31; Olympus Corporation, Tokyo,
Japan).
FCM analysis of the production of
ROS
The HUVECs were cultured and transfected, as
described above. Following transfection, the cells were treated
with oxLDL (150 μg/ml) for a further 4 h at room
temperature. Intracellular ROS production was measured using
DCFH-DA, which was oxidized and transformed into high fluorescent
dichlorofluorescin by intracellular ROS. Following treatment, the
cells were incubated for 30 min at room temperature with DCFH-DA
(10 μM) at 37°C. FCM was used to detect the fluorescence
intensity.
Extraction of total, nuclear and cytosol
proteins
The HUVECs were cultured and transfected, as
described above. Following transfection, the cells were treated
with oxLDL (150 μg/ml) for a further 24 h. Following
treatment, the cells were collected and washed twice with PBS.
Total protein was extracted by adding 50 μl
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology), containing 1% phenylmethanesulfonyl fluoride (PMSF)
and 10 mM NaF, to the cells for 30 min.
For the cytosol and nuclear proteins, hypotonic or
hypertonic lysis buffer (Beyotime Institute of Biotechnology) was
used, respectively (12). The
cytosol proteins were extracted using 200 ml hypotonic buffer,
containing 10 mM Hepes (pH 7.9), 1.5 mM MgCl2, 10 mM
KCl, 5 mM dithiothreitol (DTT), 5 mM PMSF, 10 μg/ml
leupeptin, 1 μg/ml aprotinin and 1% NP-40, and incubated for
15 min at 4°C. The lysates were then centrifuged at 12,000 × g for
5 min at 4°C, and the supernatants containing cytosol proteins were
separated from the nuclei-containing pellet. The pellet was
re-suspended and incubated in 100 ml hypertonic buffer, containing
10 μM Hepes, 5 mM MgCl2, 12.5% glycerol, 0.2 M
NaCl, 0.5 mM DTT, 1 mM PMSF, 10 μg/ml leupeptin and 1
μg/ml aprotinin, for 30 min at 4°C. The lysates were
subsequently centrifuged at 12,000 × g for 10 min at 4°C, and the
supernatant containing the desired nuclear proteins were collected.
The protein concentrations were measured using a bicinchoninic acid
method (Beyotime Institute of Biotechnology).
Western blot analysis of the expression
levels of LOX-1, p53, Bcl-2, Bax and caspase-3, phosphorlyation of
p38 MAPK, nuclear translocation of NF-κB and degradation of
IκB
For the detection of LOX-1, p53, Bcl-2, Bax,
caspase-3, p38MAPK, p-p38MAPK and IκB, 40 μg total proteins
were separated by 12% SDS-PAGE and transferred onto polyvinylidene
fluoride (PVDF) membranes (EMD Millipore, Billerica, MA, USA). The
membranes were then incubated in blocking solution (4% non-fat
milk) for 1 h at room temperature, and subsequently incubated in
1:200 of the LOX-1, p53, Bcl-2, pro-caspase-3, caspase-3, p38MAPK,
p-p38MAPK or IκB primary antibodies overnight at 4°C. The membranes
were washed and incubated with a 1:10,000 dilution of secondary
antibody for 1 h at room temperature and were detected using an
enhanced chemiluminescence system (ChemiDoc XRS; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Relative intensities were
analyzed using Quantity One® software, version
4.6.2.
For quantification of the expression levels of NF-κB
the cytosol and nuclear extracts were separated using SDS-PAGE and
transferred onto PVDF membranes. The membranes were then incubated
in blocking solution (4% non-fat milk) for 1 h, followed by
incubation with 1:200 of NF-κB (p65) primary antibodies overnight
at 4°C, washing with Tris-buffered saline and incubated in a
1:10,000 dilution of secondary antibody for 1 h. Protein expression
was detected using an enhanced chemiluminescence system (P0018;
Beyotime Institute of Biotechnology), and the relative intensities
were analyzed using Quantity One® software. The relative
percentage of NF-κB (nuclear)/NF-κB (plasma) was determined to
evaluate the nuclear translocation of NF-κB.
Statistical analysis
All data were obtained from three independent
experiments and are expressed as the mean ± standard deviation. The
significance of differences were analyzed using one-way analysis of
variance or Student's t-test (unpaired). Multiple comparisons
between groups were performed using Tukey's method. SPSS software,
version 19.0 (SPSS, Inc., Chicago, IL, USA) was used to perform the
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression levels of miR-590 are altered
following treatment with oxLDL and miR-590 transfection
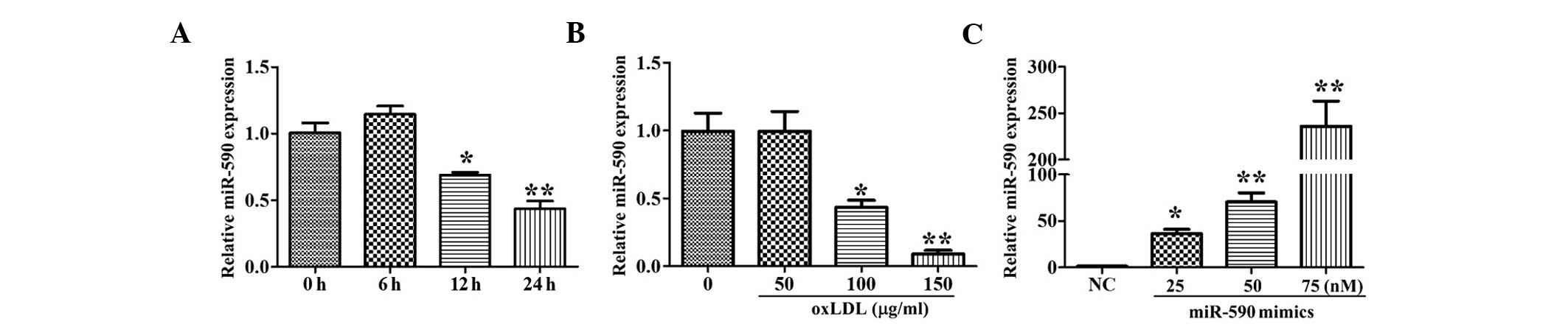
As shown in Fig. 1A and
B, treatment with oxLDL (150 μg/ml) for 12 and 24 h, or
treatment with 100–150 μg/ml oxLDL for 24 h significantly
inhibited the expression of miR-590, whereas treatment for a
relatively short duration (6 h) or low concentration (50
μg/ml) of oxLDL had no effect on the expression of miR-590.
These results suggested that miR-590 may function during the
process of oxLDL injury. Following transfection of the HUVECs with
various concentrations of miR-590 mimics, the expression levels of
miR-590 in the cells increased significantly, in a
concentration-dependent manner (Fig.
1C).
miR-590 has an anti-apoptotic effect on
oxLDL-treated HUVECs
To examine the effects of miR-590 on oxLDL-induced
HUVEC apoptosis, morphological changes in nuclear chromatin in the
apoptotic cells were evaluated using Hoechst staining, and the
apoptotic rates were measured using FCM. As shown in Fig. 2A, the cells in the NC group
exhibited uniform blue chromatin staining with an organized
structure, whereas oxLDL treatment resulted in apoptotic
morphological changes, featuring bright blue, fluorescent,
condensed nuclei and chromatin fragmentation under the fluorescence
microscope, with increased frequency, compared with the NC group.
The overexpression of miR-590 inhibited oxLDL-induced cell nuclear
chromatin morphological alterations. The results of the FCM
revealed that oxLDL treatment significantly increased the apoptotic
rates of the cells (3.55% for the NC group and 17.45% for the oxLDL
group), and these increases were significantly inhibited by
overexpression of miR-590 (Fig. 2B and
C). In addition, oxLDL treatment decreased cell viability and
significantly increased LDH release, and these effects were
attenuated by overexpression of miR-590 (Fig. 2D and E).
miR-590 reduces oxLDL-induced
overproduction of ROS
To investigate the mechanisms underlying the
anti-apoptotic effects of miR-590 on oxLDL-induced endothelial cell
injuries, the ROS levels in the HUVECs were measured. As shown in
Fig. 3, following 4 h treatment
with oxLDL, the ROS levels in the HUVECs increased ~2-fold,
compared with those in the NC group. This overproduction of ROS was
reversed by miR-590, suggesting that ROS may be involved in the
protective effects of miR-590.
Effects of miR-590 on the expression
levels of oxLDL-induced p53, Bcl-2, Bax, and caspase-3
To determine which apoptosis-associated proteins
were involved in the effects of miR-590, the expression levels of
p53, caspase-3, Bcl-2 and Bax were quantified in the HUVECs. oxLDL
treatment increased the expression levels of p53 and Bax and the
activation of caspase-3, but inhibited the expression of Bcl-2. The
ratio of Bcl-2/Bax was decreased to 32.7% of that in the control
group (Fig. 4A and B). The
overexpression of miR-590 significantly attenuated these
effects.
 | Figure 4Effects of miR-590 on oxLDL-induced
p53 induction, Bcl-2/Bax ratio reduction and caspase-3 activation.
(A) Protein expression levels, determined using western blotting.
(B) Relative band intensities, analyzed using Quantity
One® software. Data are presented as the mean ± standard
deviation of three independent experiments and are are relative to
the control, which was set as 1. ##P<0.01, vs.
control; *P<0.05 and **P<0.01, vs.
oxLDL. oxLDL, oxidized low-density lipoprotein; miR, microRNA;
Bcl-2, B cell lymphoma 2; Bax, Bcl-2-associated X protein; 1,
negative control; 2, oxLDL group; 3, miR-590 + oxLDL group. |
Effects of miR-590 on oxLDL-induced
upregulated expression of LOX-1, phosphorylation of p38MAPK,
activation of NF-κB and degradation of IκB
To determine the signals involved in the effects of
miR-590, the phosphorylation of p38MAPK, activation of NF-κB and
expression levels of LOX-1 were measured in the HUVECs. The
expression levels of LOX-1 were upregulated 2.05-fold, compared
with the NC cells following treatment with oxLDL, as previously
described (12,14). By contrast, the overexpression of
miR-590 reversed this upregulated expression of LOX-1 in the HUVECs
(Fig. 5A).
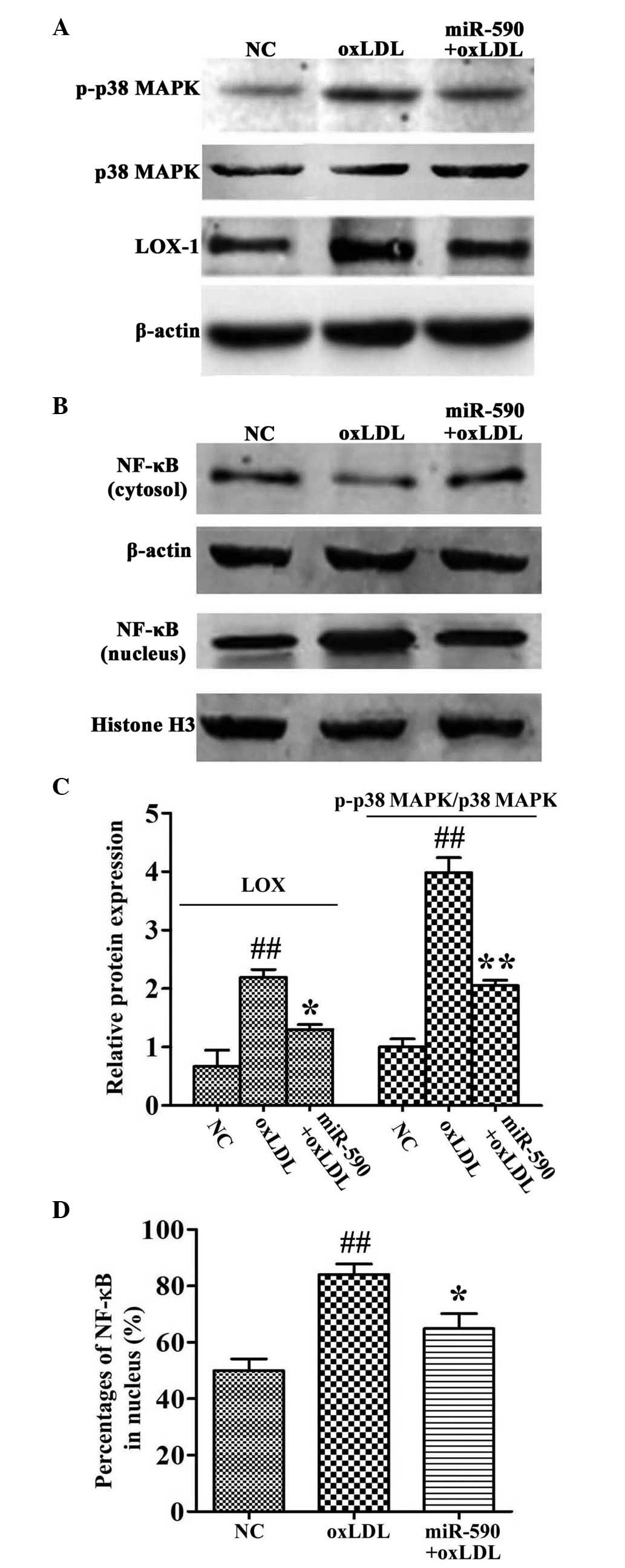 | Figure 5Effects of miR-590 on oxLDL-induced
phosphorylation of p38MAPK, upregulation of LOX-1 and nuclear
translocation of NF-κB. (A and B) Protein expression levels,
determined using western blot analysis. (C and D) Relative band
intensities, determined using Quantity One® software.
Data are presented as the mean ± standard deviation of three
independent experiments and are relative to the control, which was
set as 1. ##P<0.01, vs. control;
*P<0.05 and **P<0.01, vs. oxLDL. oxLDL,
oxidized low-density lipoprotein; miR, microRNA; p38MAPK, p38
mitogen-activated protein kinase; NF-κB, nuclear factor-κB; LOX-1,
lectin-like low-density lipoprotein receptor 1; NC, negative
control. |
OxLDL treatment also promoted the phosphorylation of
p38MAPK (3.98-fold, compared with the oxLDL group). The expression
levels of NF-κB in the nuclei also increased following oxLDL
treatment (49.8%, vs. NC group; 84.1%, vs. oxLDL group). The
phosphorylation of p38MAPK and nuclear translocation of NF-κB were
significantly inhibited by the overexpression of miR-590 (Fig. 5A–D).
Discussion
The present study demonstrated that oxLDL treatment
inhibited the expression levels of miR-590 in a time- and
concentration-dependent manner. The overexpression of miR-590
inhibited oxLDL-induced endothelial cell apoptosis. The mechanisms
underlying the anti-apoptotic effects of miR-590 partly involved
inhibiting the expression of LOX-1 and subsequent ROS generation,
p38MAPK phosphorylation and NF-κB activation. Apoptosis-associated
proteins, including p53, Bcl-2, Bax and caspase-3 were also
involved in the effects of miR-590.
Atherosclerosis is a severe vascular disorder, which
results in several complications, including coronary artery
disease, myocardial ischemia, cerebral ischemia and stroke
(19). During the pathogenesis of
atherosclerosis, oxLDL-induced endothelial cell apoptosis is key,
and may lead to a reduction in vascular integrity, the deposition
of lipids, invasion of vascular smooth muscle cells, migration of
monocytes and formation of atherosclerotic plaques (20). In the present study, an increase in
cell apoptosis following oxLDL treatment was observed, as
previously reported (13).
Notably, a time and concentration-dependent decrease in the
expression levels of miR-590 was observed following oxLDL
treatment. As the roles of several microRNAs in atherosclerosis
have been elucidated in previous years (21,22),
the present study hypothesized that miR-590 may be involved in
oxLDL-induced endothelial cell apoptosis. Therefore, the present
study induced the overexpression of miR-590 prior to treatment of
cells with oxLDL. As expected, the overexpression of miR-590
inhibited oxLDL-induced cell apoptosis. These results were
consistent with previous reports (9), and suggested that miR-590 was
involved in oxLDL-induced apoptosis. However, the mechanism
underlying the protective effects of miR-590 remain to be
elucidated, therefore, the present study investigated
apoptosis-associated proteins and signaling cascades in
oxLDL-treated endothelial cells.
p53 is a well-known tumor suppressor gene, which is
located on chromosome 17p13.1 (23). Previous studies have investigated
the roles of p53 in the apoptotic processes of several diseases,
including atherosclerosis, myocardial ischemia, infarction and
Alzheimer disease (24–27). In atherosclerosis, p53 has been
demonstrated to be involved in endothelial cell injuries, vascular
smooth muscle cell apoptosis and atherosclerotic plaque rupture
(27). The present study
investigated whether p53 was involved in the protective effects of
miR-590, and observed a significant upregulation in the expression
levels of p53 following oxLDL treatment in the endothelial cells,
and this upregulation was inhibited by the overexpression of
miR-590. In addition to the changes in the expression of p53, the
results also demonstrated a decrease in the Bcl-2/Bax ratio and an
increase in caspase-3 activation following oxLDL treatment, which
was also attenuated by the overexpression of miR-590. p53 is a
transcription factor, which regulates the expression levels of
Bcl-2 and Bax directly at the transcriptional level (15,16),
and the upregulation of Bax subsequently promotes caspase-3
activation. Therefore, the present study hypothesized that miR-590
may inhibit oxLDL-induced endothelial cell apoptosis via the
p53-Bcl-2/Bax-caspase-3 pathway.
LOX-1 is an important receptor involved in
oxLDL-induced cell apoptosis (14). Our previous study demonstrated the
LOX-1-ROS-p38MAPK-NF-κB signals in oxLDL-treated endothelial cells
(12). To examine whether these
signals were involved in the effects of miR-590, the overexpression
of miR-590 was induced in the present study, and the protein
expression levels of LOX-1, production of ROS, phosphorylation of
p38MAPK and nuclear translocation of NF-κB were quantified. The
results demonstrated that miR-590 significantly inhibited the
changes induced by oxLDL. As the interaction between oxLDL and
LOX-1 is considered to be the initial step in oxLDL-induced
endothelial cell injury, the inhibition of LOX-1 is regarded as
beneficial for the prevention of atherogenesis (14). In the present study, a significant
decrease in the expression of LOX-1 was observed following miR-590
overexpression, as previously reported (9). The present study also investigated
whether miR-590 inhibited the expression LOX-1 directly using dual
luciferase reporter assays. No effects of miR-590 on LOX-1 reporter
gene expression levels were observed (data not shown). A previous
study demonstrated that lipoprotein lipase (LPL) is a target for
miR-590 in human THP-1 macrophages (28). Whether LPL is responsible for the
downregulation in LOX-1 remains to be elucidated. Considering the
complicated signaling networks and interactions in cells, the
present study hypothesized that miR-590 downregulated the
expression of LOX-1 in an indirect manner. However, which molecule
is the target of miR-590, remains to be elucidated.
In the oxLDL-treated endothelial cells, the
upregulation of LOX-1 promoted the overproduction of ROS, with
subsequent p38MAPK phosphorylation and NF-κB activation. NF-κB is a
multifunctional transcriptional factor, which regulates the
expression of several apoptosis-associated genes, and several
studies have demonstrated that the activation of NF-κB is
associated with the activation of caspase-3, induction of Bax and
reduction of Bcl-2, either directly or indirectly (29–31).
The present study demonstrated that miR-590 led to marked
inhibition in the translocation of NF-κB, which subsequently
suppressed caspase-3 activation, Bax induction and the reduction of
Bcl-2, and was responsible for the anti-apoptotic effects of
miR-590.
p53 and NF-κB are transcription factors, which
regulate the expression of certain apoptosis-associated genes,
including Bcl-2 and Bax. However, the association between p53 and
NF-κB remains controversial (32,33).
In cancer cells, NF-κB and p53 antagonize the activity of one
another (32), whereas, in
oxLDL-treated endothelial cells, the expression levels of p53 and
NF-κB are upregulated (33). The
results of the present study demonstrated an increase in NF-κB and
p53 in the oxLDL-treated endothelial cells, and these effects were
inhibited by the overexpression of miR-590. These results indicated
that p53 and NF-κB may co-regulate the apoptosis of endothelial
cells, and may be involved in the anti-apoptotic effects of
miR-590.
In conclusion, the present study demonstrated that
miR-590 exerted anti-apoptotic effects on oxLDL-treated endothelial
cells. These anti-apoptotic effects of miR-590 were caused, in
part, by the p53-Bcl-2/Bax-caspase-3/apoptotic pathway and the
LOX-1-ROS-p38MAPK-NF-κB signaling cascade. The results of the
present study may provide novel insights into the protective
properties of miR-590 in the prevention of endothelial dysfunction
associated with cardiovascular disease, including
atherosclerosis.
Acknowledgments
The present study was supported by the Construct
Program of the Key Discipline in Hunan Province, National Sciences
Foundation of China (grant no. 81300231), and the Hunan Provincial
Natural Sciences Foundation of China (grant no. 13JJ4112).
References
|
1
|
Pohl U, Holtz J, Busse R and Bassenge E:
Crucial role of endothelium in the vasodilator response to
increased flow in vivo. Hypertension. 8:37–44. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang F, Gibson AP and Dusting GJ:
Endothelial dysfunction induced by oxidized low-density
lipoproteins in isolated mouse aorta: A comparison with
apolipoprotein-E deficient mice. Eur J Pharmacol. 424:141–149.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li R, Mittelstein D, Fang K, Beebe T,
Quigley K, Berliner J and Hsiai TK: Angiopoeitin-2 modulates
survivin expression in OxLDL-induced endothelial cell apoptosis.
Biochem Biophys Res Commun. 417:619–622. 2012. View Article : Google Scholar :
|
|
4
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen WJ, Zhang M, Zhao GJ, Fu Y, Zhang DW,
Zhu HB and Tang CK: MicroRNA-33 in atherosclerosis etiology and
pathophysiology. Atherosclerosis. 227:201–208. 2013. View Article : Google Scholar
|
|
6
|
Gao S, Wassler M, Zhang L, Li Y, Wang J,
Zhang Y, Shelat H, Williams J and Geng YJ: MicroRNA-133a regulates
insulin-like growth factor-1 receptor expression and vascular
smooth muscle cell proliferation in murine atherosclerosis.
Atherosclerosis. 232:171–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bao MH, Zhang YW, Lou XY, Cheng Y and Zhou
HH: Protective Effects of let-7a and let-7b on oxidized low-density
lipoprotein induced endothelial cell injuries. PLoS One.
9:e1065402014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu XM, Han T, Sargent IL, Yin GW and Yao
YQ: Differential expression profile of microRNAs in human placentas
from preeclamptic pregnancies vs normal pregnancies. Am J Obstet
Gynecol. 200:661.e1–7. 2009. View Article : Google Scholar
|
|
9
|
Qin B, Xiao B, Jiang T and Yang H: Effects
of miR-590-5p on ox-LDL-induced endothelial cells apoptosis and
LOX-1 expression. Journal of Central South University. Medical
sciences. 37:675–681. 2012.In Chinese.
|
|
10
|
Sawamura T, Kume N, Aoyama T, Moriwaki H,
Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T and Masaki
T: An endothelial receptor for oxidized low-density lipoprotein.
Nature. 386:73–77. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li D and Mehta JL: Antisense to LOX-1
inhibits oxidized LDL-mediated upregulation of monocyte
chemoattractant protein-1 and monocyte adhesion to human coronary
artery endothelial cells. Circulation. 101:2889–2895. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bao MH, Zhang YW and Zhou HH: Paeonol
suppresses oxidized low-density lipoprotein induced endothelial
cell apoptosis via activation of LOX-1/p38MAPK/NF-κB pathway. J
Ethnopharmacol. 146:543–551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bao MH, Zhang YW, Lou XY, Xiao Y, Cheng Y
and Zhou HH: Puerarin protects endothelial cells from oxidized low
density lipoprotein induced injuries via the suppression of LOX-1
and induction of eNOS. Can J Physiol Pharmacol. 92:299–306. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen M, Masaki T and Sawamura T: LOX-1,
the receptor for oxidized low-density lipoprotein identified from
endothelial cells: Implications in endothelial dysfunction and
atherosclerosis. Pharmacol Ther. 95:89–100. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamaguchi H, Chen J, Bhalla K and Wang HG:
Regulation of bax activation and apoptotic response to
microtubule-damaging agents by p53 transcription-dependent and
-independent pathways. J Biol Chem. 279:39431–39437. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Y, Mehew JW, Heckman CA, Arcinas M and
Boxer LM: Negative regulation of bcl-2 expression by p53 in
hematopoietic cells. Oncogene. 20:240–251. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Borghetti G, Yamaguchi AA, Aikawa J,
Yamazaki RK, de Brito GA and Fernandes LC: Fish oil administration
mediates apoptosis of Walker 256 tumor cells by modulation of p53,
Bcl-2, caspase-7 and caspase-3 protein expression. Lipids Health
Dis. 14:942015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–8. 2001.
View Article : Google Scholar
|
|
19
|
Beckman JA, Creager MA and Libby P:
Diabetes and atherosclerosis: Epidemiology, pathophysiology, and
management. JAMA. 287:2570–2581. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Choy JC, Granville DJ, Hunt DW and Mcmanus
BM: Endothelial cell apoptosis: Biochemical characteristics and
potential implications for atherosclerosis. J Mol Cell Cardiol.
33:1673–1690. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang T, Tian F, Wang J, Jing J, Zhou SS
and Chen YD: Endothelial cell autophagy in atherosclerosis is
regulated by miR-30-mediated translational control of ATG6. Cell
Physiol Biochem. 37:1369–1378. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bazan HA, Hatfield SA, O'Malley CB, Brooks
AJ, Lightell D Jr and Woods TC: Acute loss of miR-221 and miR-222
in the atherosclerotic plaque shoulder accompanies plaque rupture.
Stroke. 46:3285–3287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Levne AJ: p53, the cellular gatekeeper for
growth and division. Cell. 88:323–331. 1997. View Article : Google Scholar
|
|
24
|
Bennett MR, Littlewood TD, Schwartz SM and
Weissberg PL: Increased sensitivity of human vascular smooth muscle
cells from atherosclerotic plaques to p53-mediated apoptosis. Circ
Res. 81:591–599. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bialik S, Geenen DL, Sasson IE, Cheng R,
Horner JW, Evans SM, Lord EM, Koch CJ and Kitsis RN: Myocyte
apoptosis during acute myocardial infarction in the mouse localizes
to hypoxic regions but occurs independently of p53. J Clin Invest.
100:1363–1372. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alves da Costa C, Sunyach C,
Pardossi-piquard R, Sévalle J, Vincent B, Boyer N, Kawarai T,
Girardot N, St George-Hyslop P and Checler F: Presenilin-dependent
gamma-secretase-mediated control of p53-associated cell death in
alzheimer's disease. J Neurosci. 26:6377–6385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mercer J and Bennett M: The role of p53 in
atherosclerosis. Cell Cycle. 5:1907–1909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Khan MS, Halagowder D and Devaraj SN:
Methylated chrysin induces co-ordinated attenuation of the
canonical Wnt and NF-kB signaling pathway and upregulates apoptotic
gene expression in the early hepatocarcinogenesis rat model. Chem
Biol Interact. 193:12–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kiang JG, Agravante NG, Smith JT and
Bowman PD: 17-DMAG diminishes hemorrhage-induced small intestine
injury by elevating Bcl-2 protein and inhibiting iNOS pathway,
TNF-α increase and caspase-3 activation. Cell Biosci. 1:212011.
View Article : Google Scholar
|
|
30
|
Nakai M, Qin Z, Wang Y and Chase TN: NMDA
and non-NMDA receptor-stimulated IkappaB-alpha degradation:
Differential effects of the caspase-3 inhibitor DEVD.CHO, ethanol
and free radical scavenger OPC-14117. Brain Res. 859:207–216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pal S, Bhattacharjee A, Ali A, Mandal NC,
Mandal SC and Pal M: Chronic inflammation and cancer: Potential
chemoprevention through nuclear factor kappa B and p53 mutual
antagonism. J Inflamm (Lond). 11:232014. View Article : Google Scholar
|
|
32
|
Aoki M, Nata T, Morishita R, Matsushita H,
Nakagami H, Yamamoto K, Yamazaki K, Nakabayashi M, Ogihara T and
Kaneda Y: Endothelial apoptosis induced by oxidative stress through
activation of NF-kappaB: Antiapoptotic effect of antioxidant agents
on endothelial cells. Hypertension. 38:48–55. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang L, Zhou G, Song W, Tan X, Guo Y,
Zhou B, Jing H, Zhao S and Chen L: Pterostilbene protects vascular
endothelial cells against oxidized low-density lipoprotein-induced
apoptosis in vitro and in vivo. Apoptosis. 17:25–36. 2012.
View Article : Google Scholar
|