Introduction
Neuroblastoma (NB) is the most common type of
extra-cranial malignant tumor in childhood and infancy, and
accounts for 8–10% of all childhood cancers and for ~15% of infant
cancer-related mortality (1–3). The
current treatment strategies include high-dose chemotherapy with
autologous stem cell transplantation, radiation and surgery. Even
with this aggressive treatment, <40% of children are likely to
achieve a long-term cure (4–6). As
a result, the patients usually experience tumor recurrence as well
as long-term complications following high-dose chemotherapy
(7,8). Therefore, identification of more
effective and less toxic therapies, and molecular target-directed
drugs is urgently required.
PIWI proteins are members of the Argonaute family,
and the human PIWI subfamily genes encode four PIWI proteins (also
known as PIWI-like proteins): PIWIL1 (HIWI, piwi homology), PIWIL2
(HILI, CT80, PIWIL1L, Miwi like, and Mili in mouse), PIWIL3
(HIWI3), and PIWIL4 (HIWI2, MIWI2) (9). Although the biological function of
PIWI proteins during tumorigenesis remains unclear, it has been
reported that PIWIs are critical in germline and hematopoietic stem
cell self-renewal, spermatogenesis, translational regulation and
chromatin remodeling (10–12). In addition, PIWIs were abnormally
expressed in a variety of cancers and may be important in the
maintenance and invasion of cancer cells (13,14).
Among them, the high expression of PIWIL2 contributed to the
resistance effect of cisplatin drugs in colon cancer cells
(15), and the knockout of the
PIWIL2 gene reduced the aggressive and malignant degree of colon
cancer cells (16). However, the
above functions of PIWIL2 in NB cells remain unclear.
As a vanadium compound, sodium orthovanadate (SOV;
molecular formula, NO3VO4) has a number of
biological activities, including the inhibition of nonselective
protein tyrosine phosphatases, activation of tyrosine kinases,
mitogenic, neuroprotective and antidiabetic effects (17,18).
In addition, SOV was also used as an inhibitor of the piRNA-PIWI
signal pathway in Drosophila (19). A recent study has also demonstrated
that it exhibited antineoplastic activity in certain types of human
cancer cells, including lung, kidney and prostate cancer (20), but the effects of SOV in human NB
cells have not yet been reported.
In this study, the anti-proliferative effect of SOV
on the human NB cell lines was investigated. The effect of SOV on
cell apoptosis was also observed and the role of PIWIL2 in this
effect was investigated.
Materials and methods
Cell culture and the PIWIL2
inhibitor
Human NB SH-SY5Y cells were obtained from the
American Type Culture Collection (Rockville, MA, USA), and cultured
in Dulbecco's modified Eagle's medium (DMEM; Hyclone, Logan, UT,
USA), with 10% fetal bovine serum (FBS; Gibco, Thermo Fisher
Scientific Inc., Waltham MA, USA) and 1% penicillin/streptomycin
(Sigma-Aldrich, St. Louis, MO, USA), and were grown in a 5%
CO2 incubator at 37°C. The PIWIL2 inhibitor SOV was
purchased from Sigma-Aldrich.
Cell viability assay
Cell viability was assessed by an MTT assay.
Briefly, SH-SY5Y cells were seeded in a 96-well culture plate, and
subsequently treated with different concentrations of SOV for 24,
48 or 72 h. Control cells were treated with phosphate-buffered
saline (PBS) in culture medium. Following treatment, the cells were
incubated with 20 µl MTT reagent (5 mg/ml; Sigma-Aldrich)
for 4 h. MTT was then removed and 150 µl dimethyl sulfoxide
was added, cells were then analyzed by colorimetric analysis using
a multi-label plate reader (Bio-Rad, Hercules, CA, USA) at 490 nm.
Experiments were conducted in triplicate, average activity rates
were relative to control and standard error was calculated.
Apoptosis assays
The Annexin V/fluorescein isothiocyanate (FITC)
Apoptosis Detection kit (KeyGEN Biotech, Nanjing, China) was used
for measuring apoptosis according to the manufacturer's protocol.
Firstly, equal numbers of SH-SY5Y cells treated with PBS or SOV for
24, 48 or 72 h, were incubated with Annexin V-FITC, followed by
staining of their DNA with propidium iodide (PI; Sigma-Aldrich) in
the dark. Then, each sample was analyzed by fluorescence-activated
cell sorting (FACSAria II; BD Biosciences, San Jose, CA, USA). The
percentages of cells stained positive for Annexin V were
calculated, and the means as well as the standard error were
plotted.
Count and viability testing
The quantitative analysis of cell count and
viability were determined using the Muse Count & Viability kit
(MCH100102; Merck KGaA, Darmstadt, Germany) on the Muse Cell
Analyzer (EMD Millipore, Billerica, MA, USA). The Muse Count &
Viability Reagent differentially stains viable and non-viable cells
based on their permeability to the two DNA binding dyes present in
the reagent. First, equal numbers of SH-SY5Y cells were treated
with PBS or SOV for 24, 48 or 72 h. Then a uniform cell suspension
was prepared with the cell concentrations adjusted in the range of
1×105–1×107 cells/ml. Stained cell samples
were then prepared by mixing cells with Muse Count & Viability
Reagent in a sample tube. Subsequently the cells were left to take
up the stain for a minimum of 5 min. The samples were then detected
on Muse Cell Analyzer according to the manufacturer's protocol. The
Muse Count & Viability Software Module (EMD Millipore) was then
used to perform calculations automatically and display data in two
dot plots.
Cell cycle analysis
Equal numbers of SH-SY5Y cells were plated in 10-cm
dishes and treated with PBS or SOV for 24, 48 or 72 h. In total,
106 cells were trypsinized (Sigma-Aldrich), fixed with
70% ethanol and incubated overnight at 4°C. Cells were then
incubated in 100 µl RNase (Merck Millipore, Darmstadt,
Germany) at 37°C for 30 min, followed by staining of the DNA with
400 µl PI for 30 min in the dark, and FACS analysis. The
average percentages of cells in G0/G1, S or G2/M phases of the cell
cycle were quantified and standard error was calculated for three
experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted using the RNeasy Mini kit
(Qiagen, Valencia, CA, USA) and quantified using NanoDrop 1000
(NanoDrop, Wilmington, DE, USA). Then the RNA was reverse
transcribed into cDNA using PrimeScript RT reagent kit with gDNA
Eraser (Takara Bio Inc., Otsu, Japan). RT-qPCR was performed using
SYBR Premix Ex Taq™ II (Takara Bio Inc.) on a ABI 7500 Real-Time
PCR system (Applied Biosystems, Thermo Fisher Scientific Inc.). A
total of 520 ng of cDNA was obtained. The following primer
sequences were used: Forward: 5′-CGGAATGACTGTGTGCTGGA-3 and
reverse: 5′-GGTGATAACAATATTGCCAACCAGA-3′ for human PIWIL2; and
forward: 5′-TGGCACCCAGCACAATGAA-3′ and reverse:
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ for human β-actin. Primers were
obtained from Takara Biotechnology Co., Ltd. (Dalian China) The PCR
amplification was conducted under the following conditions: 95°C
for 30 sec; 95°C for 5 sec, and 60°C for 34 sec for 40 cycles. The
relative changes in gene expression data were analyzed by the
2−ΔΔCt method. β-actin was used as an internal control.
Triplicates were run for each sample in three independent
experiments.
Western blot analysis
Equal numbers of SH-SY5Y cells were plated on 10-cm
plates and treated with PBS or SOV for 24, 48 or 72 h. Then the
cells were lysed with radioimmunoprecipitation assay buffer
(Beyotime Institute of Biotechnology, Shanghai, China) and protein
concentration was determined by the Bradford method using a
Bicinchoninic Acid Protein Assay kit (cat. no. P0012; Beyotime
Institute of Biotechnology) (21,22).
Equal amounts of protein (40 µg) were separated by sodium
dodecyl sufate polyacrylamide gel electrophoresis and then
transferred onto polyvinylidine difluoride membranes (EMD
Millipore). The blotted membranes were blocked with 5% non-fat dry
milk (w/v) in Tris-buffered saline with 0.1% Tween 20, and then
incubated at 4°C overnight with rabbit monoclonal anti-PIWIL2
(Bioss Inc., Woburn, MA, USA; cat. no. bs-3817R) and anti-Bcl-2
(Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA; cat. no.
sc-492) antibodies. Specific antibody binding was detected using
polyclonal horseradish peroxidase-conjugated goat anti-rabbit
antibodies (cat. no. A0208; Beyotime Institute of Biotechnloogy;
1:1,000) and visualized with an enhanced chemiluminescence reagent
(cat. no. sc-2048; Santa Cruz Biotechnology Inc.), according to the
manufacturer's protocol. Goat polyclonal IgG antibodies against
β-actin (cat. no. sc-1616; Santa Cruz Biotechnology, Inc.; 1:200)
was used to evaluate protein loading in each lane. Quantification
was performed using Image J software version 1.44 (National
Institutes of Health, Bethesda, MA, USA)
Statistical analysis
Each experiment was repeated three times. All
quantitative variables were analyzed using one way analysis of
variance followed by Tukey's post-hoc test using SPSS software,
version 16.0 (SPSS, Inc., Chicago, IL, USA). The results are
presented as the mean ± standard deviation, and the standard
deviation were presented as error bars in the figures. P<0.05
was considered to indicate a statistically significant
difference.
Results
SOV decreases cell growth and
proliferation in SH-SY5Y cells
SOV has been described as a potent small molecule
inhibitor of PIWIL2 and could induce cell apoptosis and inhibit
autophagy in human hepatocellular carcinoma (HCC) cells, in
vitro and in vivo (18). To elucidate the role of SOV in NB,
it was investigated how different concentrations of SOV affect cell
proliferation in SH-SY5Y cells with different concentrations.
SH-SY5Y cells showed reduction in cell proliferation after 24 h of
treatment with 5 µM SOV, with a maximum reduction at 72 h
(Fig. 1). This anti-proliferative
effect occurred in a dose- and time-dependent manner at 10 and 50
µM at 24, 48 and 72 h. These results indicate that SOV
attenuates NB cell proliferation. Thus 5 µM SOV was selected
for use in further assays.
SOV induces apoptosis in SH-SY5Y
cells
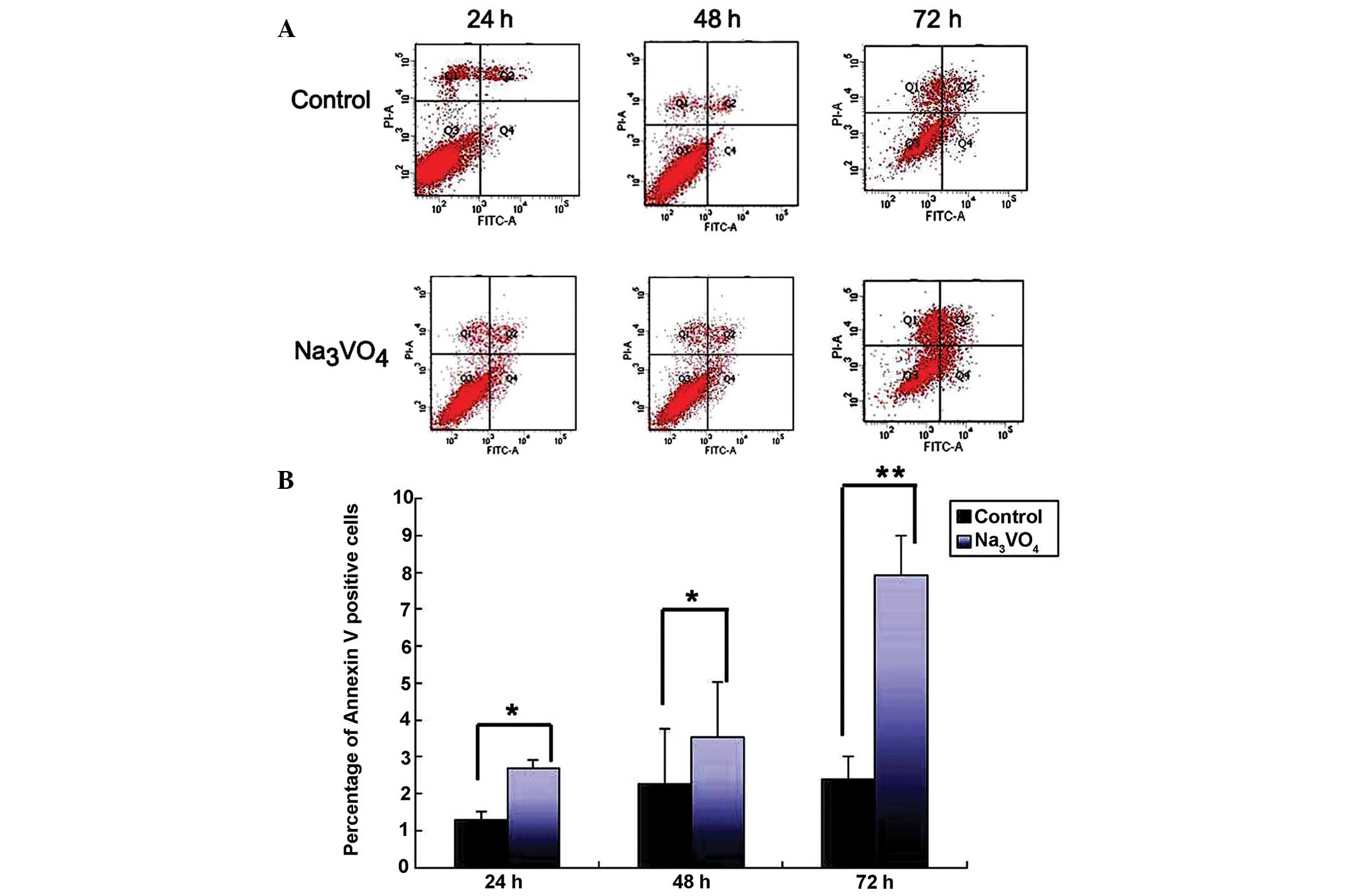
The early apoptotic cells were stained with Annexin
V, and are observed in the right lower quadrant (Fig. 2A). The results showed that the
apoptotic rates in the SOV group were 2.70±0.20, 3.53±1.50 and
7.93±1.07% respectively, at 24, 48 and 72 h, which were
significantly higher than those in control group (P<0.05,
Fig. 2B). In addition, the
apoptotic rates increased with time following SOV treatment, and
reached a climax at 72 h (Fig. 2B,
P<0.01). These results indicated that apoptosis was induced by
SOV in SH-SY5Y cells.
SOV reduces the percentage of viable
cells
Viable cells are located in the top left quadrant
and dead cells are located in the lower right quadrant (Fig. 3A). After SOV treatment, the average
percentages of viable cells were 57.59±8.72, 59.47±5.36 and
60.57±14.72% at 24, 48 and 72 h respectively, while those in
control group were 71.17±1.21, 70.9±0.91 and 80.98±2.30%,
respectively (Fig. 3B, P<0.05).
This suggested that SOV could reduce the percentage of viable
cells, particularly at 72 h where they were reduced by ~25.2%
(Fig. 3B, P<0.05).
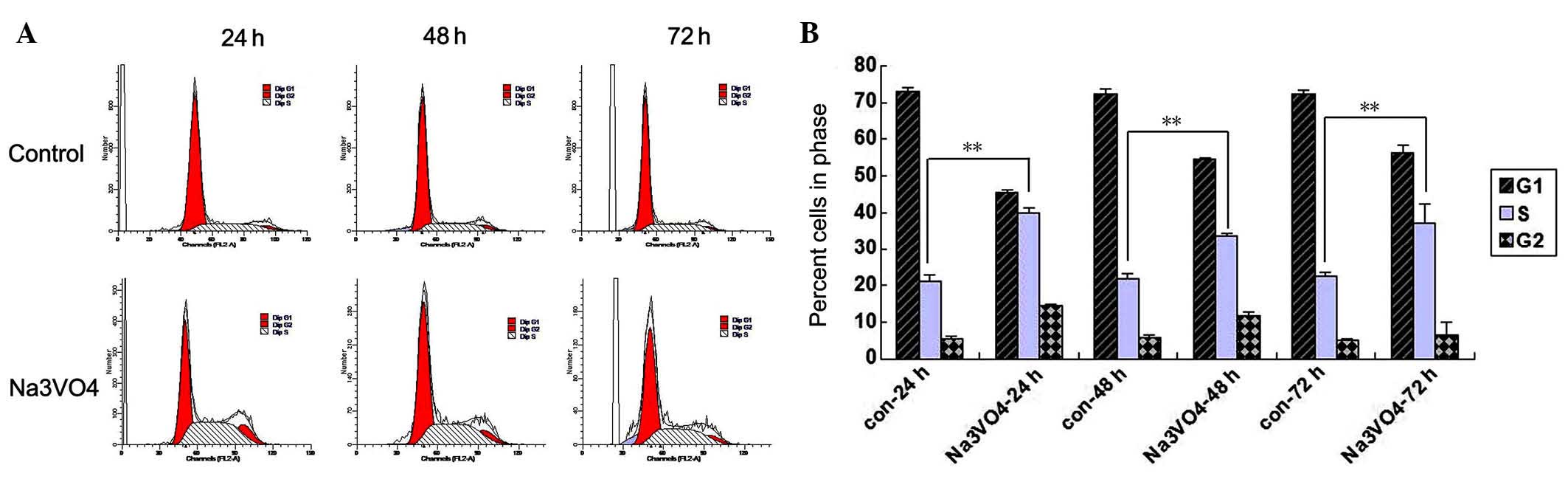
SOV induces the accumulation of SH-SY5Y
cells at the G2/M phase
As shown in Fig.
4A, the number of SH-SY5Y cells in the G0/G1 phase decreased,
while those in the S and G2/M phase increased following SOV
treatment. At 24 h after treatment, 45.48% of the SOV-treated
SH-SY5Y cells were in the G0/G1 phase, 40.10% at S and 14.41% at
G2/M (Fig. 4B, P<0.05). By
comparison, 73.15% of the PBS-treated SH-SY5Y cells were at G0/G1,
21.26% at S and 5.58% at G2/M (Fig.
4B, P<0.05). This trend was continued at later time points
(Fig. 4B). This suggested that
PIWIL2 is key in cell cycle regulation and that SOV induces
accumulation of SH-SY5Y cells at the G2/M and S phase of the cell
cycle.
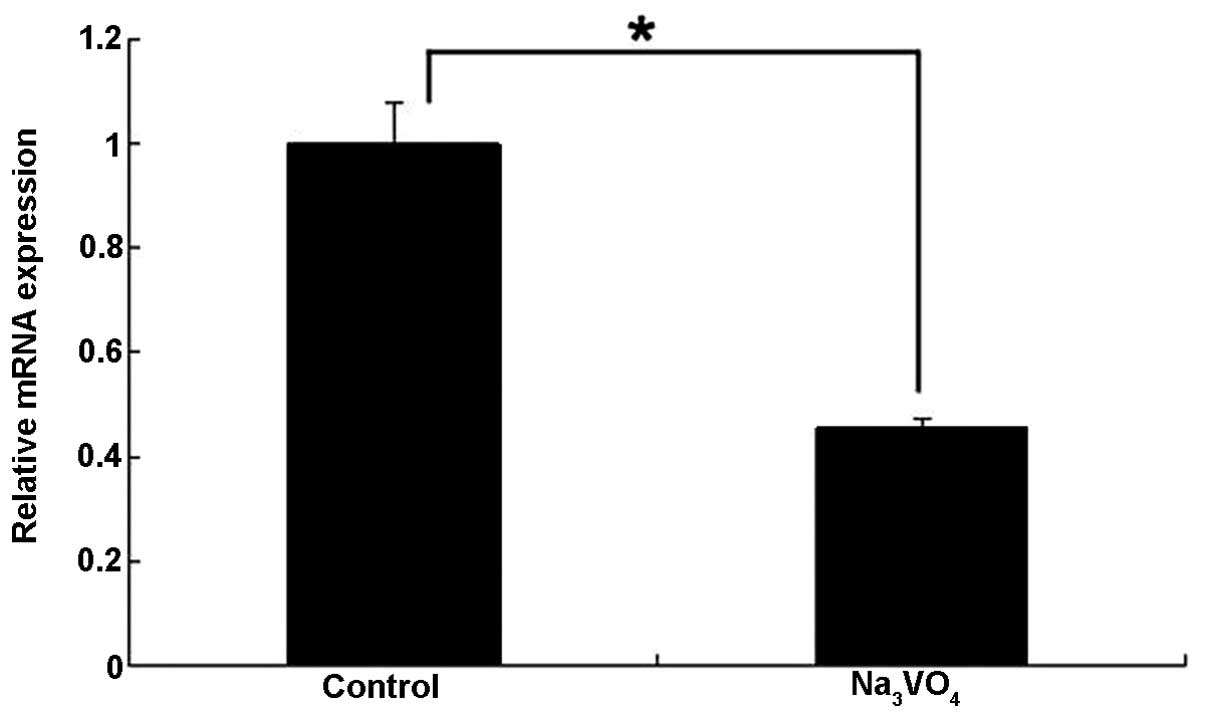
SOV treatment inhibits the expression of
PIWIL2 mRNA
RT-qPCR showed that the relative expression of
PIWIL2 mRNA was 0.45±0.02 following SOV treatment, which was lower
than that in the control group (Fig.
5, P<0.05). The results indicated that SOV treatment
suppressed the expression of PIWIL2 mRNA to a certain extent.
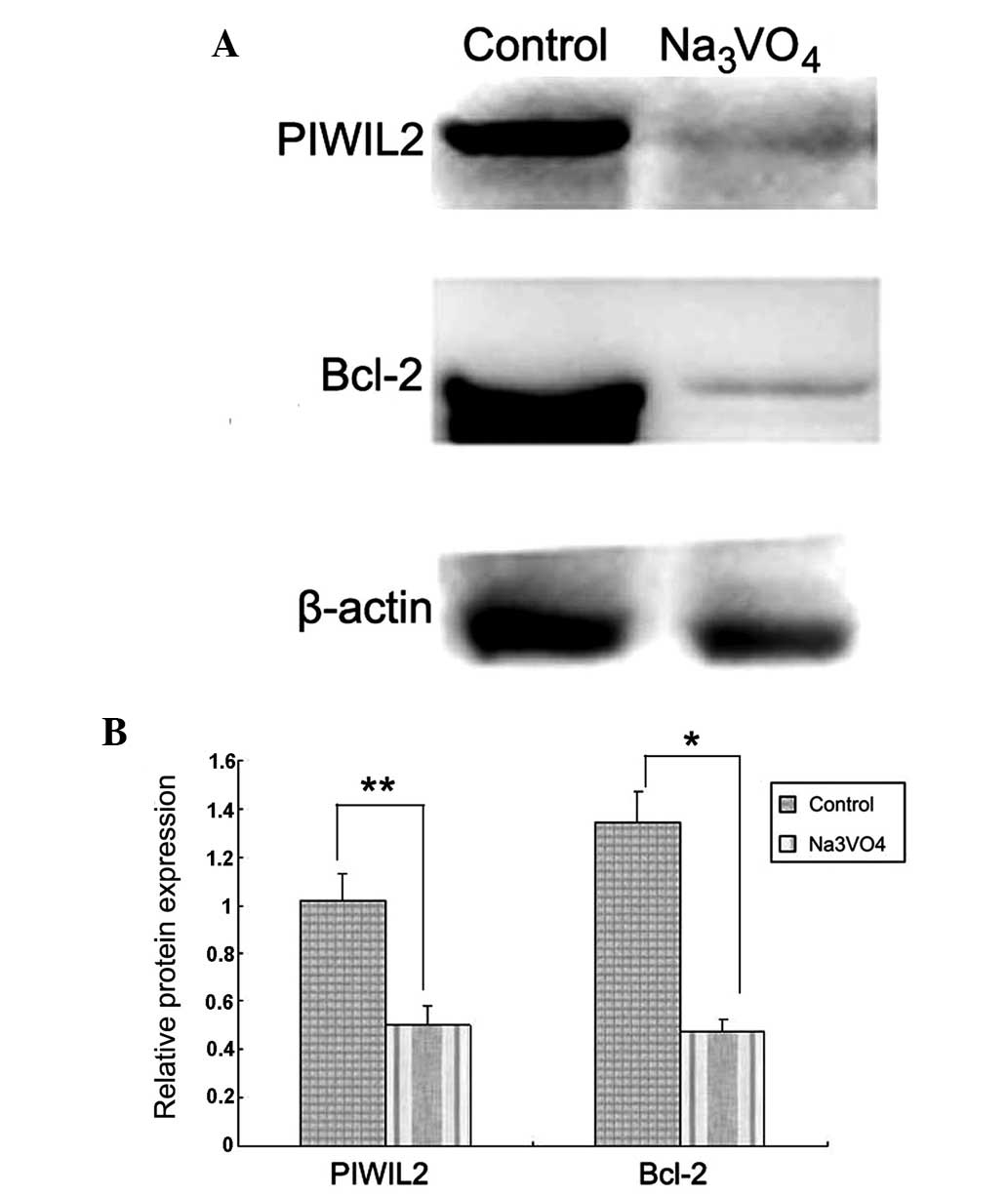
SOV reduces the expression of
anti-apoptotic protein Bcl-2
It has been reported that knocking out the PIWIL2
gene in SW620 and SW480 colon cancer cell lines inhibited cell
proliferation, invasion and metastasis in vitro, and
tumorigenicity in vivo (17). Western blot analysis was performed
to determine whether PIWIL2 inhibition induces apoptosis in SH-SY5Y
cells and whether Bcl-2 is involved. PIWIL2 expression in SH-SY5Y
cells was inhibited by 5 µM SOV. Following inhibition of
PIWIL2 in SH-SY5Y cells, protein levels of PIWIL2 and Bcl-2 were
reduced (Fig. 6, P<0.05). This
indicates that SOV may induce apoptosis in SH-SY5Y cells in part by
reducing the expression of the anti-apoptotic protein Bcl-2.
Discussion
NBs of higher-grade are aggressive and have low cure
rates even with combined modality treatments of radiation,
chemotherapy and surgery (23).
Understanding the molecular mechanism of this disease may aid in
the development of future therapeutic strategies. Recent evidence
has shown that PIWIL2 proteins are widely expressed in tumors and
can regulate genes involved in apoptosis and proliferation
(24), and SOV exhibited
anti-neoplastic activity in a variety of human cancer cells
(20). In addition, PIWIL2 has
also been shown to act as an oncogene by inhibiting apoptosis and
promoting proliferation via Stat3/Bcl-XL signaling (24). The overexpression of PIWIL2 in
certain cancers can cause cellular drug resistance, whereas
silencing the PIWIL2 gene can inhibit the expression of Stat3 and
Bcl-XL, and induce ovarian cancer cell apoptosis (25). These reports indicated that the
increase in the levels of PIWIL2 protein in cancer cells was one of
the reasons for the reduction in sensitivity to chemotherapy and
increasing chemo-resistance. For these reasons, this study aimed to
determine whether PIWIL2 was also involved in NB carcinogenesis and
whether SOV had an anti-proliferative effect in NB.
In the present study, the effect of different
concentrations of SOV on cell proliferation was detected using an
MTT assay, and the concentration of 5 µM was selected for
the follow-up tests, as cell activity decreased the most at this
concentration (~30%), compared with the other concentrations.
Higher concentrations resulted in a less marked decrease in
activity, suggesting potential toxicity (Fig. 1). The results showed that the
activity of SH-SY5Y cells reduced by ~48% after treatment with 5
µM SOV for 24 h. While in HCC cells, after 72 h treatment
with SOV, there was a significant difference in the cell viability
index between control and cells treated with 15 or 30 µM SOV
(P<0.05) (18). Moreover,
within the concentration range of 1-20 µM, SOV demonstrated
a time and dose-dependent inhibition of autocrine growth of the
A549 (lung), HTB44 (kidney) and DU145 (prostate) human carcinoma
cell lines, as compared with the appropriate controls (20). The difference may be associated
with varying cell type.
It was then demonstrated that SOV induced apoptosis
of SH-SY5Y cells using the Annexin V/FITC Apoptosis Detection kit
(Fig. 2), and reduced the
percentage of viable cells using count and viability testing
(Fig. 3). Similar results have
also been reported in HCC, A549, HTB44, DU145, SN56 (cholinergic
neuroblastoma) and H35-19 (rat hepatoma) cells (18,20,26,27).
In addition, Delwar et al (28) found that the combination of
menadione and SOV (17.5 µM:17.5 µM) eliminated and
inhibited migration of detached A549 and DBTRG-05MG human glioma
cells. The data contributed to the evaluation of SOV, its
implications in invasiveness and metastasis, and its sensitivity to
anticancer drugs.
Furthermore, the present study examined the
underlying mechanism and found that it may be associated with the
cell cycle and PIWIL2 inhibition. The present study demonstrated
that SOV induced accumulation of SH-SY5Y cells at the G2/M and S
phase of the cell cycle. Woo et al (29) showed that SOV caused G2/M phase
cell cycle arrest in Chinese hamster ovary cells, and Klein et
al (20) demonstrated that SOV
could induce G2/M phase cell cycle arrest in all three selected HCC
cell lines. The results of the present study were in accordance
with their findings. In addition, the expression of PIWIL2 was
investigated using RT-qPCR and western blot analysis in SH-SY5Y
cells, and it was found that SOV inhibited the expression of PIWIL2
mRNA and protein. This phenomenon was accompanied by the reduction
of Bcl-2. Therefore, it was speculated that SOV suppression of cell
proliferation may occur via the induction of mitochondria-dependent
apoptosis. Based on these findings, future studies in vivo
may define the mechanism of PIWIL2 in the downregulation of
Bcl-2.
In conclusion, to the best of our knowledge, the
present study demonstrated for the first time that SOV induced the
apoptosis of SH-SY5Y cells. Although the mechanism for inducing
Bcl-2 downregulation remains to be determined, the results suggest
that the underlying mechanisms may be, at least in part, due to SOV
suppression of proliferation, and induction of
mitochondria-dependent apoptosis and G2/M cell cycle arrest of
SH-SY5Y cells. These findings demonstrated activities of SOV and
supported its further evaluation as a treatment for human NB.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 30772215). The authors would
like to thank the Experimental Technology Center, Department of
Developmental Biology, Department of Pharmacology, and Department
of Pathophysiology in the China Medical University.
References
|
1
|
Maris JM and Matthay KK: Molecular biology
of neuroblastoma. J Clin Oncol. 17:2264–2279. 1999.PubMed/NCBI
|
|
2
|
Maris JM, Hogarty MD, Bagatell R and Cohn
SL: Neuroblastoma. Lancet. 369:2106–2120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharp SE, Gelfand MJ and Shulkin BL:
Pediatrics: Diagnosis of neuroblastoma. Semin Nucl Med. 41:345–353.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matthay KK, Villablanca JG, Seeger RC,
Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT,
Brodeur GM, et al: Treatment of high-risk neuroblastoma with
intensive chemotherapy, radiotherapy, autologous bone marrow
transplantation and 13-cisretinoic acid. Children's Cancer group. N
Engl J Med. 341:1165–1173. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pearson AD, Pinkerton CR, Lewis IJ, Imeson
J, Ellershaw C and Machin D; European Neuroblastoma Study Group,
Children's Cancer and Leukaemia Group (CCLG formerly United Kingdom
Children's Cancer Study Group): High-dose rapid and standard
induction chemotherapy for patients aged over 1 year with stage 4
neuroblastoma: A randomised trial. Lancet Oncol. 9:247–256. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zage PE, Kletzel M, Murray K, Marcus R,
Castleberry R, Zhang Y, London WB and Kretschmar C; Children's
Oncology Group: Outcomes of the POG 9340/9341/9342 trials for
children with high-risk neuroblastoma: A report from the Children's
Oncology Group. Pediatr Blood Cancer. 51:747–753. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lau L, Tai D, Weitzman S, Grant R,
Baruchel S and Malkin D: Factors influencing survival in children
with recurrent neuroblastoma. J Pediatr Hematol Oncol. 26:227–232.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Laverdière C, Cheung NK, Kushner BH,
Kramer K, Modak S, LaQuaglia MP, Wolden S, Ness KK, Gurney JG and
Sklar CA: Long-term complications in survivors of advanced stage
neuroblastoma. Pediatr Blood Cancer. 45:324–332. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen C, Liu J and Xu G: Overexpression of
PIWI proteins in human stage III epithelial ovarian cancer with
lymph node metastasis. Cancer Biomark. 13:315–321. 2013.
|
|
10
|
Sharma AK, Nelson MC, Brandt JE, Wessman
M, Mahmud N, Weller KP and Hoffman R: Human CD34 (+) stem cells
express the hiwi gene, a human homologue of the Drosophila gene
piwi. Blood. 97:426–434. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carmell MA, Girard A, van de Kant HJ,
Bourc'his D, Bestor TH, de Rooij DG and Hannon GJ: MIWI2 is
essential for spermatogenesis and repression of transposons in the
mouse male germline. Dev Cell. 12:503–514. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aravin A, Gaidatzis D, Pfeffer S,
Lagos-Quintana M, Landgraf P, Iovino N, Morris P, Brownstein MJ,
Kuramochi-Miyagawa S, Nakano T, et al: A novel class of small RNAs
bind to MILI protein in mouse testes. Nature. 442:203–207.
2006.PubMed/NCBI
|
|
13
|
Qiao D, Zeeman AM, Deng W, Looijenga LH
and Lin H: Molecular characterization of hiwi, a human member of
the piwi gene family whose overexpression is correlated to
seminomas. Oncogene. 21:3988–3999. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su C, Ren ZJ, Wang F, Liu M, Li X and Tang
H: PIWIL4 regulates cervical cancer cell line growth and is
involved in down-regulating the expression of p14ARF and p53. FEBS
Lett. 586:1356–1362. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu C, Qu L, Dong B, Xing X, Ren T, Zeng
Y, Jiang B, Meng L, Wu J and Shou C: Combined phenotype of 4
markers improves prognostic value of patients with colon cancer. Am
J Med Sci. 343:295–302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li D, Sun X, Yan D, Huang J, Luo Q, Tang H
and Peng Z: Piwil2 modulates the proliferation and metastasis of
colon cancer via regulation of matrix metallopeptidase 9
transcriptional activity. Exp Biol Med (Maywood). 237:1231–1240.
2012. View Article : Google Scholar
|
|
17
|
Korbecki J, Baranowska-Bosiacka I,
Gutowska I and Chlubek D: Biochemical and medical importance of
vanadium compounds. Acta Biochim Pol. 59:195–200. 2012.PubMed/NCBI
|
|
18
|
Wu Y, Ma Y, Xu Z, Wang D, Zhao B, Pan H,
Wang J, Xu D, Zhao X, Pan S, et al: Sodium orthovanadate inhibits
growth of human hepatocellular carcinoma cells in vitro and in an
orthotopic model in vivo. Cancer Lett. 351:108–116. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ipsaro JJ, Haase AD, Knott SR, Joshua-Tor
L and Hannon GJ: The structural biochemistry of Zucchini implicates
it as a nuclease in piRNA biogenesis. Nature. 491:279–283. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Klein A, Holko P, Ligeza J and Kordowiak
AM: Sodium orthovanadate affects growth of some human epithelial
cancer cells (A549, HTB44, DU145). Folia Biol (Krakow). 56:115–121.
2008. View Article : Google Scholar
|
|
21
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dryer RL and Lata GF: Experimental
biochemistry. New York: Oxford University Press; New York, NY: pp.
346–347. 1989
|
|
23
|
Maris JM: Recent advances in
neuroblastoma. N Engl J Med. 362:2202–2211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee JH, Schütte D, Wulf G, Füzesi L,
Radzun HJ, Schweyer S, Engel W and Nayernia K: Stem-cell protein
Piwil2 is widely expressed in tumors and inhibits apoptosis through
activation of Stat3/Bcl-XL pathway. Hum Mol Genet. 15:201–211.
2006. View Article : Google Scholar
|
|
25
|
Wang QE, Han C, Milum K and Wani AA: Stem
cell protein Piwil2 modulates chromatin modifications upon
cisplatin treatment. Mutat Res. 708:59–68. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suwalsky M, Fierro P, Villena F, Aguilar
LF, Sotomayor CP, Jemiola-Rzeminska M, Strzalka K, Gul-Hinc S,
Ronowska A and Szutowicz A: Human erythrocytes and neuroblastoma
cells are in vitro affected by sodium orthovanadate. Biochim
Biophys Acta. 1818:2260–2270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kordowiak AM, Klein A, Goc A and Dabroś W:
Comparison of the effect of VOSO4, Na3VO4 and NaVO3 on
proliferation, viability and morphology of H35-19 rat hepatoma cell
line. Pol J Pathol. 58:51–57. 2007.PubMed/NCBI
|
|
28
|
Delwar ZM, Siden A, Cruz MH and Yakisich
JS: Menadione: Sodium orthovanadate combination eliminates and
inhibits migration of detached cancer cells. ISRN Pharmacol.
2012:3071022012. View Article : Google Scholar
|
|
29
|
Woo ES, Rice RL and Lazo JS: Cell cycle
dependent subcellular distribution of Cdc25B subtypes. Oncogene.
18:2770–2776. 1999. View Article : Google Scholar : PubMed/NCBI
|