Introduction
Excessive neuronal cell loss through synapse loss
and neurite damage is a common characteristic of numerous brain
diseases, particularly of neurodegenerative diseases, including
Alzheimer's, Parkinson's and Huntington's disease (1). Studies have shown that several
endogenous neurotrophic factors (NTFs), including brain-derived
neurotrophic factor (BDNF), nerve growth factor (NGF) as well as
neurotrophin-3, -4 and -5 have critical roles in neuronal survival,
process outgrowth, synaptic connectivity and nervous system
plasticity (2,3). Thus, NTFs are likely to have a
potential role in ameliorating neurodegeneration. However, due to
their peptidyl properties and their resulting inability to cross
the blood-brain barrier as well as their fast metabolic breakdown,
the clinical use of NTFs is impossible (4). Identification of small molecules with
similar functions to those of NTFs may provide novel therapeutics
for neurodegenerative diseases. Of note, numerous compounds,
including 4-O-methylhonokiol, magnolol and macranthol, have
been identified to promote neurite process outgrowth and neuronal
survival due to their similar functions to those of NTFs in
vitro (5–8).
Senegenin is a compound extracted from the Chinese
herb Polygala tenuifolia Willd and has been shown to exert a
range of biological activities, including neuroprotective and
antioxidant effects (9,10). A pharmacological study showed that
Polygala tenuifolia Willd extracts improve cognitive
function and have neuroprotective properties (11). Previous studies by our group
revealed that senegenin promoted neurite outgrowth and upregulated
the mRNA expression of microtubule-associated protein 2 (MAP2) and
brain-derived neurotrophic factor in cultured cortical neurons
(12,13). The present study further
investigated the neurotrophic effects of senegenin as well as the
underlying mechanisms.
Materials and methods
Materials
Senegenin was purchased from Guangdong Institute for
Drug Control (Guangzhou, China). Dulbecco's modified Eagle's medium
(DMEM)/F12 and B27 supplement were from Gibco (Thermo Fisher
Scientific, Waltham, MA, USA). K252a, GÖ6976, PD98059, LY294002 and
MAP2 antibody were purchased from Sigma-Aldrich (St. Louis, MO,
USA). ZM241385 was purchased from Tocris Bioscience Co. Ltd.
(Bristol, UK). Paraformaldehyde was purchased from Guangzhou
Chemical Reagent Factory (Guangzhou, China). Trypsin was purchased
from Amresco, LLC (Solon, OH, USA). The cell strainer was obtained
from Dingguo Biotechnology Co., Ltd. (Beijing, China). Gibco goat
serum was purchased from Thermo Fisher Scientific, Inc. The Hoechst
stain, lysis buffer components, and electrochemiluminescence
reagent (ECL; cat no. P0018) were purchased from Beyotime Institute
of Biotechnology (Shanghai, China). Polyvinylidene fluoride (PVDF)
membranes and bicinchoninic acid (BCA) assay were purchased from
Sigma-Aldrich. The anti-MAP2 mouse monoclonal antibody (cat no.
M9942) was purchased from Sigma-Aldrich. Anti-Akt (pan) rabbit
monoclonal antibody (cat no. C67E7) and anti-phospho-Akt monoclonal
antibody (Ser473) rabbit monoclonal antibody (cat no. 4060) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Cy3 Affini Pure goat anti-mouse polyclonal IgG (H+L; (cat no.
115-095-003) was purchased from Jackson ImmunoResearch
Laboratories, Inc. (West Grove, PA, USA). Rabbit GAPDH polyclonal
antibody (cat no. 10494-1-AP) was purchased from Proteintech Group,
Inc. (Chicago, IL, USA). Horseradish peroxidase (HRP)-conjugated
goat anti-rabbit IgG secondary antibody (H+L; cat no. A0208) was
purchased from Beyotime Institute of Biotechnology, Inc.
Primary culture of cortical neurons
Neonatal Sprague-Dawley (SD) rats (<24 h after
birth) obtained from the Medical Laboratory Animal Center of
Guangdong province [license no. CXK (Yue) 2008-0002] were used for
the preparation of the primary culture of cortical neurons as
described previously (14).
Briefly, after neonatal SD rats were decapitated under anesthesia
(diethyl ether; Beyotime Institute of Biotechnology), the brains
were placed into petri dishes containing ice-cold D-Hank's balanced
salt solution. Cortices were separated and minced into small
pieces. The cells were dissociated by digestion with 0.125% (w/v)
trypsin for 10 min at 37°C followed by passing through a
74-µm cell strainer and centrifugation for 5 min at 1,000 ×
g. The cell pellet was re-suspended to the desired concentration
with DMEM/F12 supplemented with 0.4% (v/v) B27. Freshly isolated
cells were cultured in poly-l-lysine-coated 24-/96-well
plates. These cells were cultured at 37°C in a humidified
atmosphere containing 5% CO2. After three days of
culture, cells were collected for subsequent experiments. The study
was approved by the ethics committee of the Medical School of Jinan
University (Guangzhou, China).
Immunocytochemistry assay
Cortical neurons were identified by immunostaining
for MAP2, which is a specific neuronal marker. After 3 days of
culture, cells were fixed with 4% (w/v) paraformaldehyde at room
temperature for 20 min and subsequently incubated with 0.1% (v/v)
Triton X-100 in phosphate-buffered saline (PBS) containing 10%
(v/v) normal goat serum for 30 min. Cells were then incubated with
mouse anti-MAP2 monoclonal antibody (1:200 dilution) overnight at
4°C followed by staining with Cy3 AffiniPure goat anti-mouse IgG
(1:500 dilution) for 1 h and Hoechst 33258 for 5 min. After each
incubation step, the cells were washed three times with PBS for 5
min each. Images of randomly selected fields of view of stained
neurons were captured using an Olympus IX71 microscope (Olympus
Corp., Tokyo, Japan).
Morphological analysis
Cortical neurons were cultured in 24-well plates at
a density of 2.0×104 cells/cm2 and treated
for three days with or without senegenin (2 µM), K252a (50
nM), ZM241385 (10 nM), GÖ6976 (100 nM), PD98059 (10 µM) or
LY294002 (10 µM) as described previously (15–18).
Neurons were fixed and stained with MAP2 antibody to observe the
morphology of cortical neurons. Images of randomly selected fields
of view of stained neurons were captured using an Olympus IX71
microscope (Olympus Corp.) at ×400 magnification and the average
length of neurite outgrowth in each group was measured using Image
Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA) by
evaluating 200 neurons in a randomly selected field of view.
Western blot analysis
Cortical neurons were cultured in a six-well plate
at a density of 2.5×105 cells/cm2 and treated
with or without senegenin and 10 µM LY294002 for three days.
Following washing with 1 ml ice-cold PBS, cells were lysed in 100
µl lysis buffer [115 mM Tris-HCl (pH 6.8), 4% (w/v) SDS, 10%
(v/v) glycerol, 10 mM dithiothreitol and 2.5 mg/ml bromophenol
blue]. Protein was quantified using a BCA Protein Quantification
kit. Equivalent amounts of protein (10 µg) were subjected to
12% SDS-PAGE and transferred onto a PVDF membrane, followed by
incubation of the membrane with antibodies against phospho (p)-Akt
(1:2,000 dilution), Akt (1:1,000 dilution) and GAPDH (1:1,000
dilution). Subsequently, membranes were incubated with the
HRP-conjugated secondary antibody (1:3,000 dilution). The
non-phosphorylated form of Akt from the same cell lysates was
quantified by immunoblotting to ensure that phosphorylated Akt was
detected in an identical amount of protein. The blots were
visualized using ECL reagent. The autoradiograms were scanned and
the protein level was quantified by densitometry using an image
analysis system. using a Versa Doc™ Imaging system (Model 4000;
Bio-Rad Laboratories, Inc., Hercules, CA, USA) and the intensity of
the protein bands were analyzed using Quantity One 1-D Analysis
Software v.4.6.6 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Statistical significance among groups was determined by
one-way analysis of variance with Bonferroni's post hoc test.
Statistical analyses were conducted using SPSS 16.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference between values.
Results
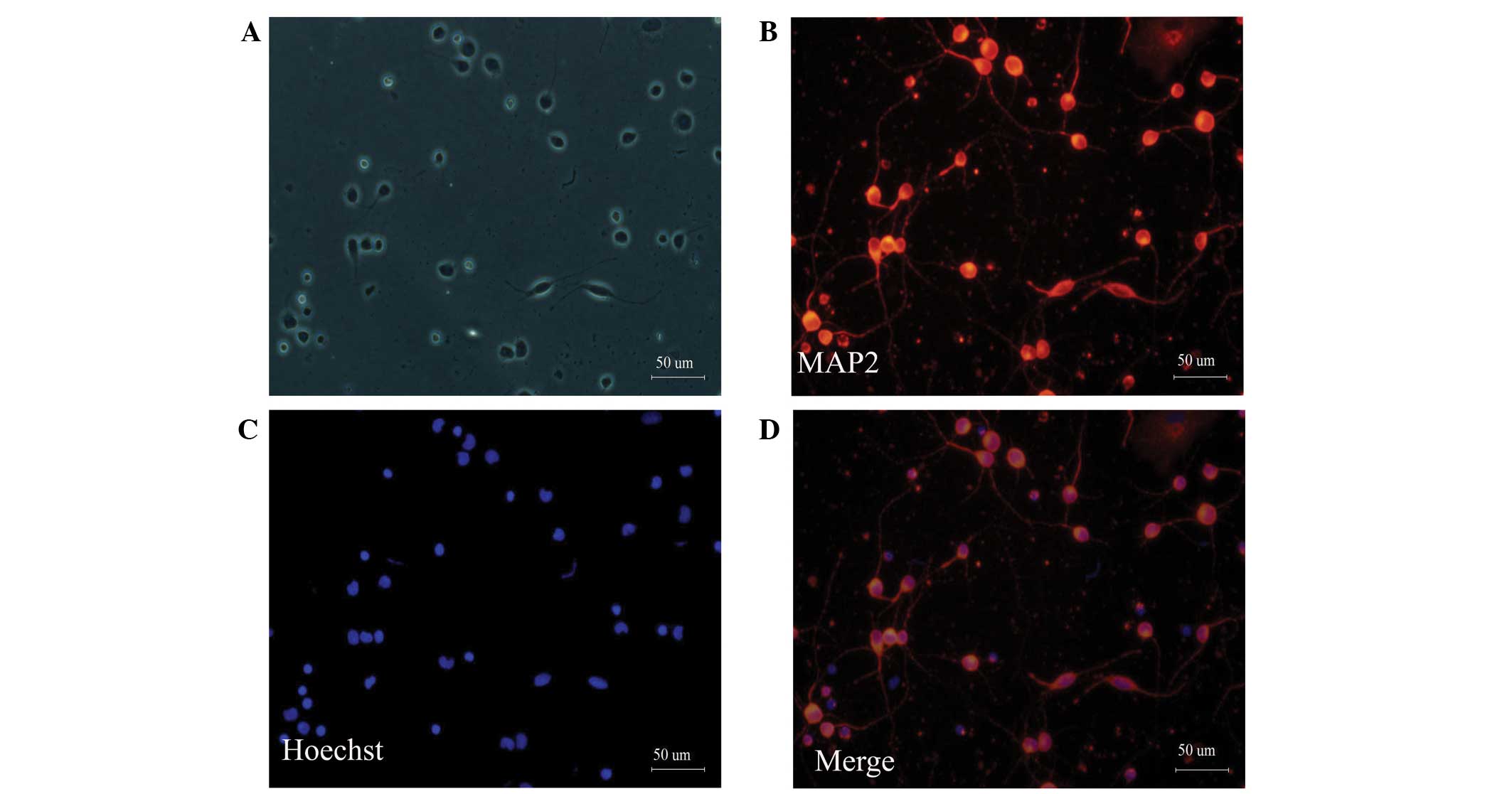
Morphology and phenotype of cultured
cortical neurons
Neonatal rat cortical neurons were cultured in
serum-free DMEM/F12 supplemented with 0.4% (v/v) B27 to prevent the
proliferation of gliocytes. MAP2 is a neuron-specific cytoskeletal
protein that is enriched in the cell body and dendrites (19). MAP2 staining can distinguish
neurons from other cells. After cortical neurons were cultured for
three days, MAP2 immunofluorescence staining was performed. MAP2
immunostaining revealed that ~95% of the cultured cells were
neurons (Fig. 1).
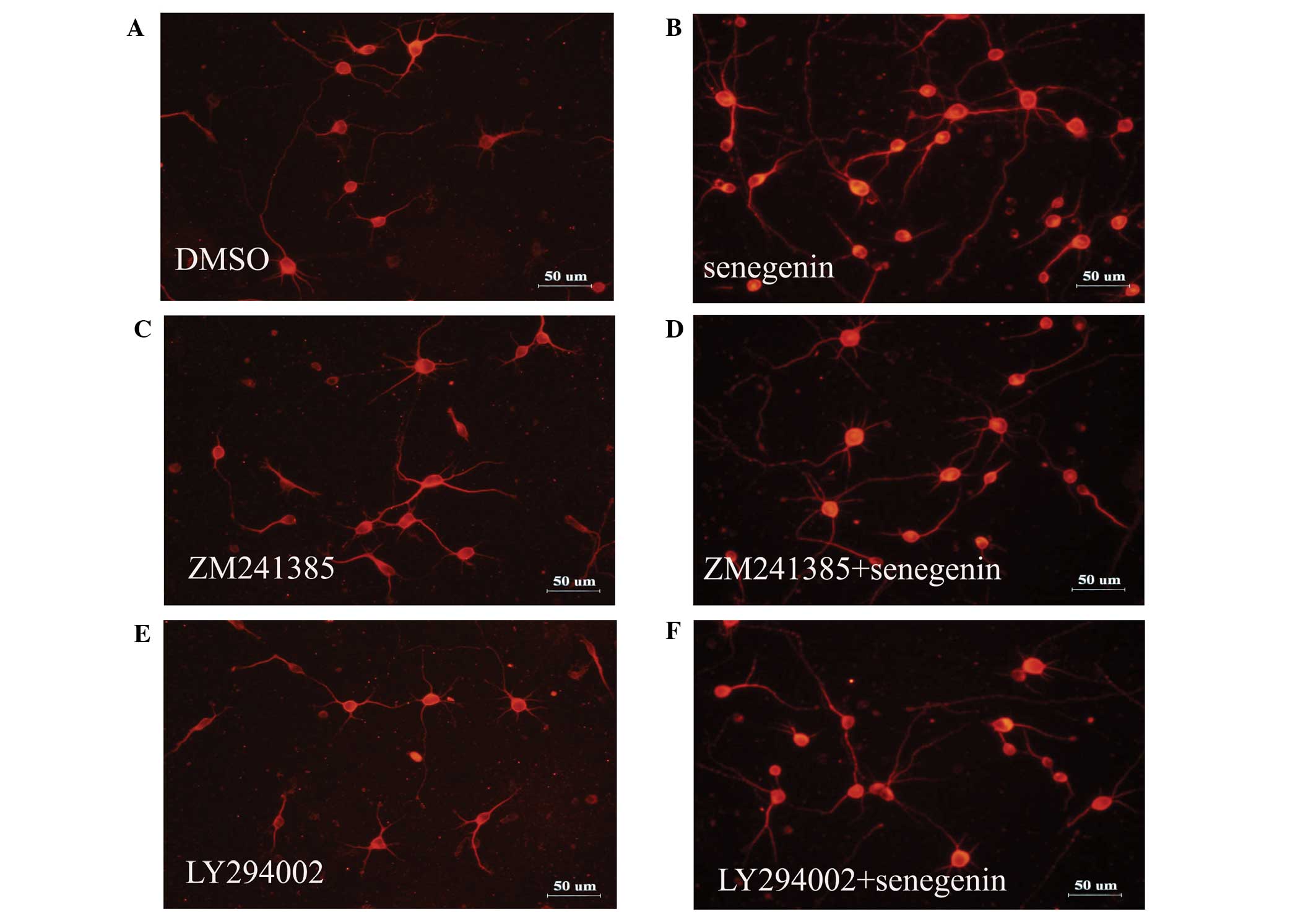
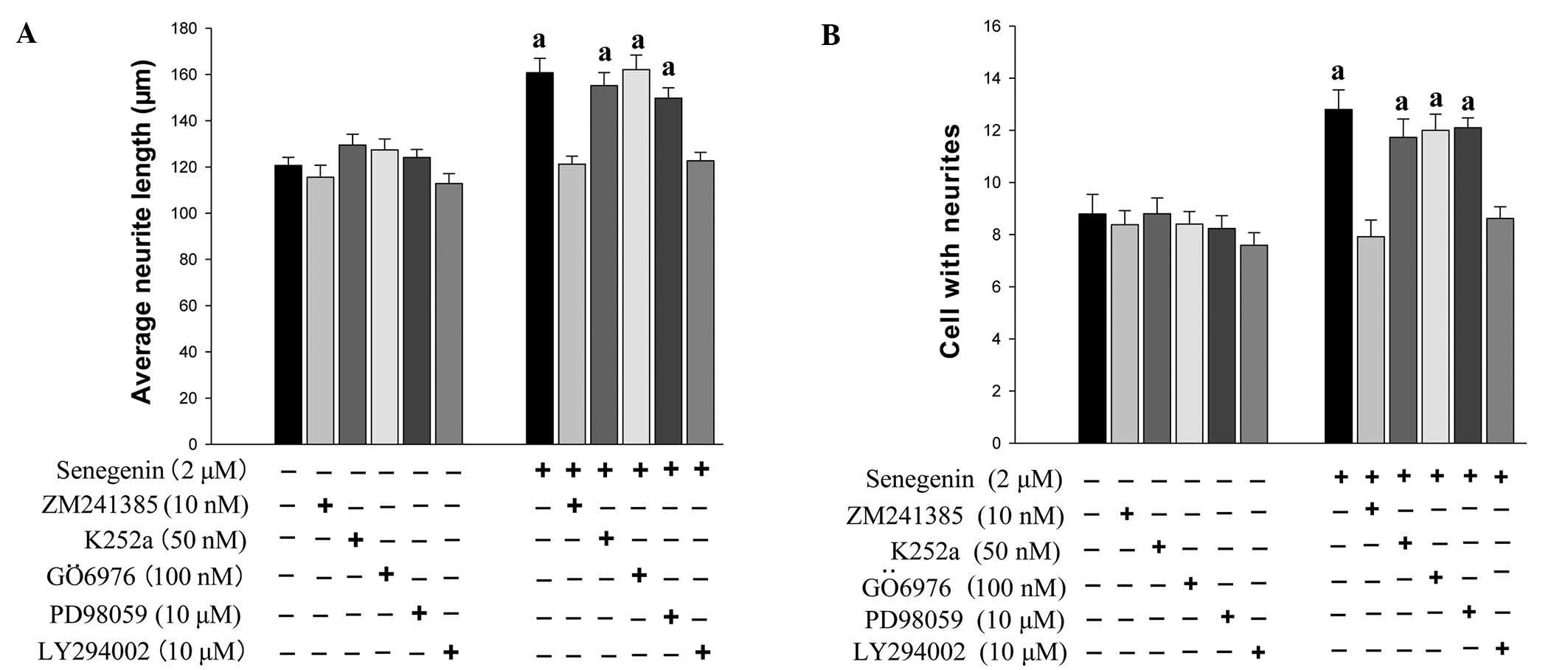
Senegenin promotes neurite outgrowth and
neuronal survival, which is inhibited by ZM241385 and LY294002
As the phosphoinositide-3 kinase (PI3K) and the
mitogen-activated protein kinase (MAPK) signaling pathways have
been implicated in neurite outgrowth and neuronal survival, which
is mediated via tropomyosin receptor kinase (Trk) receptors
(20), the present study assessed
the effect of TrkA inhibitor K252a, A2A receptor
antagonist ZM241385, PI3K inhibitor LY294002, protein kinase C
inhibitor GÖ6976 and MAPK kinase inhibitor PD98059 on
senegenin-induced neurotrophic activity. The effects of senegenin
on neurite outgrowth were assessed using immunostaining for MAP2.
As shown in Figs. 2 and 3, the neurite outgrowth and neuronal
survival induced by senegenin was prevented by treatment with
ZM241385 and LY294002, but not by K252a, GÖ6976 and PD98059. These
results indicated that senegenin-induced neurotrophic activity
required a signaling pathway involving PI3K.
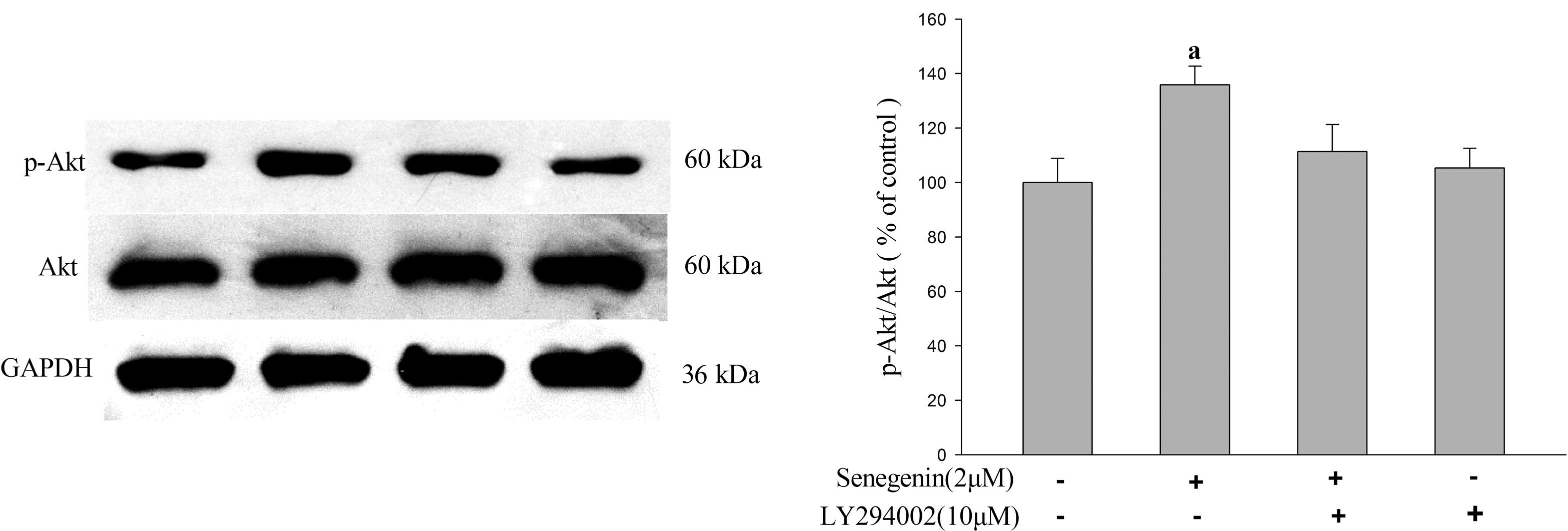
Senegenin promotes Akt
phosphorylation
To further confirm the involvement of the PI3K/Akt
signaling pathway in the neurotrophic effects of senegenin, the
phosphorylation of Akt was investigated using western blot analysis
(Fig. 4). The results demonstrated
that senegenin significantly enhanced the phosphorylation of Akt as
compared with that in the vehicle-treated control group. However,
the enhancing effect of senegenin on Akt phosphorylation was
blocked by LY294002, a specific inhibitor of PI3K, suggesting that
the neurotrophic activity of senegenin may be mediated via the
PI3K/Akt signaling pathway.
Discussion
NTFs and numerous neurotrophic small molecules are
regulators of neuronal development, growth, survival, plasticity,
and differentiation in the nervous system by activating Trk
receptor tyrosine kinases (19–22).
The results of the present study suggested that the neurotrophic
effects of senegenin were counteracted with the antagonist
ZM241385, an inhibitor of the A2A receptor, but not
K252a, an inhibitor of Trk receptors. This indicated that the
A2A receptor may be involved in the neurotrophic effects
of senegenin, whereas previous studies (19–22)
demonstrated that neurotrophic effects were mediated by receptor
tyrosine kinases. A2A receptors are a type of G
protein-coupled receptors, which are expressed in the neuropil of
the striatum, the cortex, hippocampus, cerebellum, and olfactory
striatum (23). Furthermore, the
A2A receptor is involved in the induction of the
MAPK/Erk or PI3K/Akt signaling pathway (17), which regulate neuronal
differentiation, neurite outgrowth, and neuronal survival (24–26),
as well as neurotrophic effects (27). In addition, Liot et al
(28) demonstrated that NT-3
displayed an anti-apoptotic effect on cultured cortical neurons via
activation of the PI3K/Akt signaling pathway. To date, numerous
previous studies have demonstrated that the PI3K/Akt signaling
pathway has an important role in mediating the survival and/or
neurite outgrowth processes. Ashcroft et al (29) demonstrated that the selective and
inducible activation of endogenous PI3K in PC12 cells results in
efficient NGF-mediated survival and neurite outgrowth. In addition,
López-Carballo et al (30)
showed that the PI3K/Akt signaling pathway is important in the
regulation of RA-induced neuronal survival in SH-SY5Y human
neuroblastoma cells. Zhang et al (31) showed that the PI3K/Akt signaling
pathway contributed to neuronal survival and neurite outgrowth
induced by methyl 3,4-dihydroxybenzoate. The results of the present
study demonstrated that the PI3K inhibtor LY294002, but not the MEK
inhibitor PD98059, prevented senegenin-induced neurotrophic
activity. Akt phosphorylation was enhanced in cortical neurons
treated with senegenin but phosphorylation levels were attenuated
by LY294002. Therefore, these results indicated that PI3K
activation is essential for senegenin-induced neurite outgrowth and
neuronal survival in cortical neurons, and also suggested that Akt
phosphorylation was enhanced in cortical neurons treated with
senegenin, but could be attenuated by LY294002. The data also
suggested that Akt phosphorylation has a role in neurite outgrowth
and survival induced by senegenin. Senegenin may enhance Akt
activity by activating A2A receptors in order to exert
its neurotrophic activity. The neurotrophic effects may be
important in the protection of neurons against damage. Honokio,
magnolol and methyl 3,4-dihydroxybenzoate exhibit neurotrophic
effects, and these compounds are also known to protect neurons from
various types of damage (32–34).
Brain amyloid β (Aβ) aggregates into clumps called oligomers that
can accumulate and form deposits called amyloid plaques, which are
thought to be a pathological mechanism underlying Alzheimer's
disease (35,36). Previous studies demonstrated that
Aβ may cause neuronal death (34,37).
Future studies will clarify the neuroprotective effects of
senegenin against Aβ-induced apoptosis in rat primary cortical
neurons.
In conclusion, the results of the present study
demonstrated that the A2A/PI3K/Akt signaling cascade is
involved in the neurotrophic effects of senegenin. These results
provide novel insights for treating Alzheimer's disease and
neurodegenerative diseases. Further studies are currently taking
place to clarify which downstream proteins are activated by Akt,
and which molecules in neurons are associated with
senegenin-induced neurotrophic activities.
Acknowledgments
This present study was supported by grants from the
National Natural Science Foundation Committee of China (grant nos.
81173037, 30672450 and 81202519) and the Guangdong Provincial
Department of Science and Technology (grant nos. 2012B050300018 and
2010B030700018).
Abbreviations:
|
DMEM/F12
|
Dulbecco's modified Eagle's medium:
nutrient mixture F12
|
|
MAP2
|
microtubule-asscociated protein 2
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
NTF
|
neurotrophic factor
|
References
|
1
|
Christensen DZ, Bayer TA and Wirths O:
Intracellular Aβ triggers neuron loss in the cholinergic system of
the APP/PS1KI mouse model of Alzheimer's disease. Neurobiol Aging.
1153–1163. 2008.
|
|
2
|
Mullen LM, Pak KK, Chavez E, Kondo K,
Brand Y and Ryan AF: Ras/p38 and PI3K/Akt but not Mek/Erk signaling
mediate BDNF-induced neurite formation on neonatal cochlear spiral
ganglion explants. Brain Res. 1430:25–34. 2012. View Article : Google Scholar
|
|
3
|
Zhai Hf, Nakatsukasa M, Mitsumoto Y and
Fukuyama Y: Neurotrophic effects of talaumidin, a neolignan from
Aristolochia arcuata, in primary cultured rat cortical neurons.
Planta Med. 70:598–602. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pardridge WM: Neurotrophins,
neuroprotection and the blood-brain barrier. Curr Opin Investig
Drugs. 3:1753–1757. 2002.
|
|
5
|
Lee YK, Choi IS, Kim YH, Kim KH, Nam SY,
Yun YW, Lee MS, Oh KW and Hong JT: Neurite outgrowth effect of
4-O-methylhonokiol by induction of neurotrophic factors through ERK
activation. Neurochem Res. 34:2251–2260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee MM, Hseih MT, Kuo JS, Yeh FT and Huang
HM: Magnolol protects cortical neuronal cells from chemical hypoxia
in rats. Neuroreport. 9:3451–3456. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang CP, Hsu YC and Lin MT: Magnolol
protects against cerebral ischaemic injury of rat heatstroke. Clin
Exp Pharmacol Physiol. 30:387–392. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moriyama M, Huang JM, Yang CS, Kubo M,
Harada K, Hioki H and Fukuyama Y: Two new sesquiterpenoids and two
new prenylated phenylpropanoids from Illicium fargesii and
neuroprotective activity of macranthol. Chem Pharm Bull (Tokyo).
56:1201–1204. 2008. View Article : Google Scholar
|
|
9
|
Jia HX, Jiang Y, Ruan Y, Zhang Y, Ma X,
Zhang J, Beyreuther K, Tu P and Zhang D: Tenuigenin treatment
decreases secretion of the Alzheimer's disease amyloid beta-protein
in cultured cells. Neuroscience Lett. 367:123–128. 2004. View Article : Google Scholar
|
|
10
|
Sun GB, Deng XC and Li CH: The protective
effects of tenuigenin on the PC12 cells injury induced by H2O2.
Journal of Chinese Medicinal Materials. 30:991–993. 2007.In
Chinese.
|
|
11
|
Park C, Choi SH, Koo JW, Seo JH, Kim HS,
Jeong SJ and Suh YH: Novel cognitive improving and neuroprotective
activities of Polygala tenuifolia Willdenow extract, BT-11. J
Neurosci Res. 70:484–492. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pi T, Xue XY, Lin LF and Luo HM:
Neurotrophic effects of senegenin on newborn rat cultured cortical
neurons. Chin J Pharmacol Toxicol. 25:40–44. 2011.
|
|
13
|
Pi T, Xue XY and Luo HM: Neurotrophic
effects of senegenin. Journal of Chinese Medicinal Materials.
9:1477–1480. 2013.In Chinese.
|
|
14
|
Lin LF, Xue XY, Liao MJ, Xiao F, Lv RH and
Luo HM: Neurotrophic effects of magnesium fructose 1,6-diphosphate
on cortical neurons. Int J Neurosci. 122:248–254. 2012. View Article : Google Scholar
|
|
15
|
Matsuda S, Saito H and Nishiyama N: Effect
of basic fibroblast growth-factor on neuron cultured from various
regions of postnatal rat-brain. Brain Res. 520:310–316. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tassi E, Walter S, Aigner A, Cabal-Manzano
RH, Ray R, Reier PJ and Wellstein A: Effects on neurite outgrowth
and cell survival of a secreted fibroblast growth factor binding
protein upregulated during spinal cord injury. Am J Physiol Regul
Integr Comp Physiol. 293:R775–R783. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee FS and Chao MV: Activation of Trk
neurotrophin receptors in the absence of neurotrophins. Proc Natl
Acad Sci USA. 98:3555–3560. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiao F, Lin LF, Cheng X, Gao Q and Luo HM:
Nogo-66 receptor activation inhibits neurite outgrowth and
increases β-amyloid protein secretion of cortical neurons. Mol Med
Rep. 5:619–624. 2012.
|
|
19
|
Serres and Carney SL: Nicotine regulates
SH-SY5Y neuroblastoma cell proliferation through the release of
brain-derived neurotrophic factor. Brain Res. 1101:36–42. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Orike N, Thrasivoulou C, Wrigley A and
Cowen T: Differential regulation of survival and growth in adult
sympathetic neurons: An in vitro study of neurotrophin
responsiveness. J Neurobiol. 47:295–305. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rajagopal R, Chen ZY, Lee FS and Chao MV:
Transactivation of Trk neurotrophin receptors by G-protein-coupled
receptor ligands occurs on intracellular membranes. J Neurosci.
24:6650–6658. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee FS and Chao MV: Activation of Trk
neurotrophin receptors in the absence of neurotrophins. Proc Natl
Acad Sci USA. 98:3555–3560. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rosin DL, Robeva A, Woodard RL, Guyenet PG
and Linden J: Immunohistochemical localization of adenosine A2A
receptors in the rat central nervous system. J Comp Neuro.
401:163–186. 1998. View Article : Google Scholar
|
|
24
|
Kaplan DR and Miller FD: Neurotrophin
signal transduction in the nervous system. Curr Opin Neurobiol.
10:381–391. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ditlevsen DK, Køhler LB, Pedersen MV,
Risell M, Kolkova K, Meyer M, Berezin V and Bock E: The role of
phosphatidylinositol 3-kinase in neural cell adhesion
molecule-mediated neuronal differentiation and survival. J
Neurochem. 84:546–556. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
López-Carballo G, Moreno L, Masiá S, Pérez
P and Barettino D: Activation of the phosphatidylinositol
3-kinase/Akt signaling pathway by retinoic acid is required for
neural differentiation of SH-SY5Y human neuroblastoma cells. J Biol
Chem. 277:25297–25304. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ohta H, Arai S, Akita K, Ohta T and Fukuda
S: Neurotrophic effects of a cyanine dye via the PI3K-Akt pathway:
Attenuation of motor discoordination and neurodegeneration in an
ataxic animal model. Plos One. 6:e171372011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liot G, Gabriel C, Cacquevel M, Ali C,
MacKenzie ET, Buisson A and Vivien D: Neurotrophin-3-induced PI-3
kinase/Akt signaling rescues cortical neurons from apoptosis. Exp
Neurol. 187:38–46. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ashcroft M, Stephens RM, Hallberg B,
Downward J and Kaplan DR: The selective and inducible activation of
endogenous PI3-kinase in PC12 cells results in efficient
NGF-mediated survival but defective neurite outgrowth. Oncogene.
18:4586–4597. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
López-Carballo G, Moreno L, Masiá S, Pérez
P and Barettino D: Activation of the phosphatidylinositol
3-kinase/Akt signaling pathway by retinoic acid Is required for
neural differentiation of SH-SY5Y human neuroblastoma cells. J Biol
Chem. 277:25297–25304. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Z, Cai L, Zhou X, Su C, Xiao F, Gao
Q and Luo HM: Methyl 3,4-dihydroxybenzoate promote rat cortical
neurons survival and neurite outgrowth through the adenosine A2a
receptor/PI3K/Akt signaling pathway. Neuroreport. 26:367–373. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chang CP, Hsu YC and Lin MT: Magnolol
protects against cerebral ischaemic injury of rat heatstroke. Clin
Exp Pharmacol Physiol. 30:387–392. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liou KT, Shen YC, Chen CF, Tsao CM and
Tsai SK: Honokiol protects rat brain from focal cerebral
ischemia-reperfusion injury by inhibiting neutrophil infiltration
and reactive oxygen species production. Brain Res. 992:159–166.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou XW, Zhang Z, Su CF, Lv RH, Zhou X,
Cai L, Wang CY, Yan L, Zhang W and Luo HM: Methyl
3,4-dihydroxybenzoate protects primary cortical neurons against
Aβ25–35 -induced neurotoxicity through mitochondria
pathway. J Neurosci Res. 91:1215–1225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chui DH, Dobo E, Makifuchi T, Akiyama H,
Kawakatsu S, Petit A, Checler F, Araki W, Takahashi K and Tabira T:
Apoptotic neurons in Alzheimer's disease frequently show
intracellular Abeta42 labeling. J Alzheimers Dis. 3:231–239.
2001.
|
|
36
|
Qian YH, Xiao Q and Xu J: The protective
effects of tanshinone IIA on β-amyloid protein (1–42)-induced
cytotoxicity via activation of the Bcl-xL pathway in neuron. Brain
Res Bull. 88:354–358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Christensen DZ, Bayer TA and Wirths O:
Intracellular Aβ triggers neuron loss in the cholinergic system of
the APP/PS1KI mouse model of Alzheimer's disease. Neurobiol Aging.
31:1153–1163. 2010. View Article : Google Scholar
|