Introduction
Hepatocellular carcinoma (HCC) causes high rates of
mortality worldwide and is increasing in incidence (1). Epidemiological investigation
demonstrates that the incidence of HCC is associated with the
hepatitis B virus (HBV) x antigen (HBxAg) (2). The risk of developing HCC in
HBxAg-positive individuals is higher compared with that in the
HBxAg-negative populations (1,3).
Currently, the predominant clinical treatment for HCC is surgical
resection and liver transplantation, however, the majority of
patients lose surgical opportunity (4). Therefore, identifying a safe and
efficient therapeutic strategy for HCC is of great global
significance.
Immunotherapy, which stimulates tumor-specific
immune responses has become one of the most promising emerging
treatments (5). Previous studies
have demonstrated the development of dendritic cell (DC)
vaccination. DCs exhibit properties associated with innate and
adaptive protective immune responses, induce tumor-specific
effector T cells, and specifically decrease tumor mass and tumor
relapse (6–8).
Heat shock proteins (HSPs), also termed stress
proteins, are essential in the regulation of protein synthesis and
vesicular trafficking, and have been demonstrated as potent
adjuvants in cancer immunotherapy (9). Certain previous results suggest that
the preparation of HSPs from various types of tumor can elicit
different cytotoxic antitumor immune responses, and induce the
development of distant metastases (10). HSPs can also bind and present
tumor-associated antigens to antigen-presenting cells through major
histocompatibility complex (MHC) class I and II molecules, leading
to the activation of antitumor CD8+ and CD4+
T cells (11).
However, whether using DCs co-pulsed with
Hsp70/HBxAg complexes activates the cytotoxic antitumor
immunoresponse in the HCC cells of HBxAg-positive individuals
remains to be elucidated. The present study used DCs pulsed with
Hsp70-peptide and/or HBxAg complexes to activate autologous T
cells. The present study aimed to use pulsed DCs to develop a novel
therapeutic strategy for the treatment of HCC, and also strived to
provide a novel tumor vaccine approach against human HCC.
Materials and methods
Cell lines and regents
The human LO2 hepatic cell line, human SMMC-7721 HCC
cell line and human K562 natural killer cell sensitive cell line
were purchased from the American Type Culture Collection
(Rockville, MD, USA). The human HepG2 HCC cell line
(HBxAg+), was provided by Professor Xiaodong Zhang
(College of Life Sciences, Nankai University, Tianjin, China). The
cells were cultured in RPMI-1640 medium, containing 10% fetal
bovine serum (HyClone Laboratories Inc., Logan, UT, USA), 100 U/ml
penicillin G and 100 µg/ml streptomycin (Gibco Life
Technologies, Carlsbad, CA, USA), at 37°C in a humidified
atmosphere of 95% air and 5% CO2. The present study was
approved ethics committee of the Second Affiliated Hospital of
Soochow University (Suzhou, China).
HBxAg was purchased from MyBioSource, Inc. (San
Diego, CA, USA). Hsp70 peptide was purified by Enzo Life Sciences,
Inc. (Farmingdale, NY, USA).
Preparation of DCs
DCs were generated, as described previously
(12). Briefly, peripheral blood
mononuclear cells (PBMCs) were isolated from healthy donors (Suzhou
Blood Center, Jiangsu, China), using Ficoll-Hypaque (TBD Science,
Tianjin, China) density gradient centrifugation and cultured in
RPMI-1640 medium, containing 10% FBS for 2 h. The non-adherent
cells were removed for the isolation of T cells and the adherent
cells were cultured for 7 days in RPMI-1640 medium, containing 10%
FCS, 100 ng/ml human granulocyte macrophage colony-stimulating
factor (GM-CSF; Amoytop, Xiamen, China) and 10 ng/ml human
interleukin (IL)-4 (Amoytop). The culture medium and cytokines were
refreshed every 2 days. DCs were harvested on the 7th day.
Flow cytometric assay
The DCs were pulsed with Hsp70-peptide (ProSpec,
Rehovot, Israel) and/or HBxAg (ProSpec) at 37°C for 4 h, and were
cultured for another 48 h. The DCs were washed with cold
phosphate-buffered saline (PBS), and incubated with murine
monoclonal anti-human HLA-DR (cat. no. 560943), CD11c (cat. no.
560999), CD80 (cat. no. 560926), CD86 (cat. no. 560958) and CD83
(cat. no. 560929; BD Pharmingen, San Diego, CA, USA) for 1 h in
ice. Following incubation, the cells were washed with cold PBS and
were incubated with fluorescein isothiocyanate-conjugated with goat
anti-mouse IgG (BD Pharmingen) for 30 min on ice. The cells were
subsequently washed with cold PBS and fixed with 2%
paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA). The
fluorescence intensity was analyzed using an Epics XL FACS Calibur
(Beckman Coulter, Inc., Fullerton, CA, USA) and EXPO32 ADC
CellQuest analysis software (Beckman Coulter, Inc.) (13).
IL-12 release enzyme-linked assay
Following treatment with different antigens, DCs
were cultured in 6-well plates for 24 h. Cytokine release in the
supernatants was subsequently assessed by an ELISA using an IL-12
ELISA detection kit (R&D Systems, Shanghai, China).
Mixed lymphocyte reaction
The mixed lymphocyte reaction was performed, as
described previously (14).
Briefly, 1×105 CD4+ T cells were mixed with
antigen-pulsed DCs and normal control DCs (R/S ratio, 10:1, 20:1
and 50:1). They were seeded into flat-bottomed 96-well plates in
200 ml RPMI-1640 medium, containing 40 ng/ml IL-2, and were
cultured at 37°C for 5 days. The cells were harvested onto glass
fiber filters with a 96-well plate cell harvester (PLT 96WL;
Corning Incorporated, Corning, NY, USA). Cell-associated
radioactivity was determined using a cell counting kit-8 assay kit
(Shanghai Qcbio Science & Technologies Co., Ltd., Shanghai,
China). The results of triplicate assays are expressed as the mean
counts per minute ± standard deviation.
Cytotoxicity assay
The autologous CD8+ T cells were
incubated with stimulators (PBS-DCs, Hsp70-DCs, HBxAg and
Hsp70/HBxAg-DCs) at a ratio of 20:1 in 96-well culture plates in
RPMI-1640 medium, containing 40 ng/ml IL-2 for 5 days at 37°C with
5% CO2. The cytotoxicity analysis was performed using a
lactate dehydrogenase (LDH) release assay. Briefly, the effector T
cells were harvested and incubated with HepG2 cells at ratios of
5:1, 10:1 and 20:1 in 96-microwell plates at 37°C and 5%
CO2 for 4 h. The plates were subsequently centrifuged
for 5 min at 250 × g, at room temperature. The supernatants from
each well (100 µl) were transferred into 96-well
flat-bottomed microwell plates and 100 ml LDH substrate mixture was
added. Following incubation for 15 min, the absorbance was measured
at 490 nm on an ELISA 550 Microplate reader (Bio-Rad Laboratories,
Inc. Hercules, CA, USA). The CTL-mediated cytotoxicity was
calculated using the following formula: Cytotoxicity-[1-(effector
target-effector)/target] × 100.
Different cell types, HepG2
(HLA-A2+/HBxAg+), SMMC-7721
(HLA-A2+/HBxAg−), K562
(HLA-A2−/HBxAg−) and LO2-DCs human hepatic
cells, were used as target cells. The cytotoxicity was subsequently
detected by the LDH assay kit (Promega Corporation, Madison, WI,
USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA). The total RNA
(2.5 µg) was reverse transcribed using a Superscript™ III
kit (Invitrogen Life Technologies), according to the manufacturer's
instructions. PCR amplification was performed in a 50 µl PCR
reaction mixture, containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2
mM MgCl2, 20 pmol each primer set, 2 units Taq DNA
polymerase (Beyotime Institute of Biotechnology, Jiangsu, China),
0.2 mM dNTPs and 2 ml cDNA. PCR was performed for 40 cycles at 95°C
for 10 min, 95°C for 15 sec and 60°C for 1 min in a 7900HT Fast
Real-Time PCR System (ABI, Palo Alto, CA, USA), and analysis of the
dissociation curve was performed at 95°C for 15 sec, 60°C for 1
min, 95°C for 15 sec and 60°C for 15 sec. The nucleotide sequences
of the oligonucleotide primers for HBxAg were as follows: Forward:
5′-ACCGACCTTGAGGCCTACTT-3′ and reverse: 5′-GCTTGGCAGAGGTGAAAAAG-3′
(Sangon Biotech Co., Ltd., Shanghai, China).
Immunoblot analysis
The total cellular proteins were extracted by lysing
cells in a sodium dodecyl sulfate (SDS) sample buffer, containing
62.5 mM Tris-HCl (pH 6.8), 2% SDS, 1 mM phenylmethylsulfonyl
fluoride, 10 mg/ml pepstatin, 12.5 mg/ml leupeptin, 2 mg/ml
aprotinin, 1 mM sodium orthovanadate and 1 mM sodium molybdate. The
method of cell extraction for western blotting was performed, as
described previously (15). The
HBxAg used for immunoblot analysis was purchased from MyBioSource,
Inc. (San Diego, CA, USA).
Adaptive immunotherapy in the xenograft
model of nude mice
Female severe combined immunodeficiency (SCID) mice
(n=10; age, 4 weeks; weight, 9.7±0.51 g) were purchased from
(Shanghai Laboratory Animal Center, Shanghai, China). The SCID mice
were inoculated subcutaneously with 7.5×106 HepG2 tumor
cells in 150 ml Matrigel (Becton Dickinson, Bedford, MD, USA) and
50 ml sterile PBS. Following tumor inoculation for 7 days, the SCID
mice were randomized into three groups and treated with T cells
(2.5×106) stimulated with the following: i) Fusion cells
(cells expressing HBxAg and Hsp70); ii) DCs; or iii) PBS. The T
cells were injected into tumors every 4 days and the tumors were
measured each time using vernier calipers. Tumor volume was
calculated as follows: 1/2 × (length × width2).
Statistical analysis
The experiments and in vitro assays were
performed at least three times. The differences between the mean
values were assessed by Student's t-test. Statistical analysis was
conducted using Prism 4 (GraphPad Software, Inc., La Jolla, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
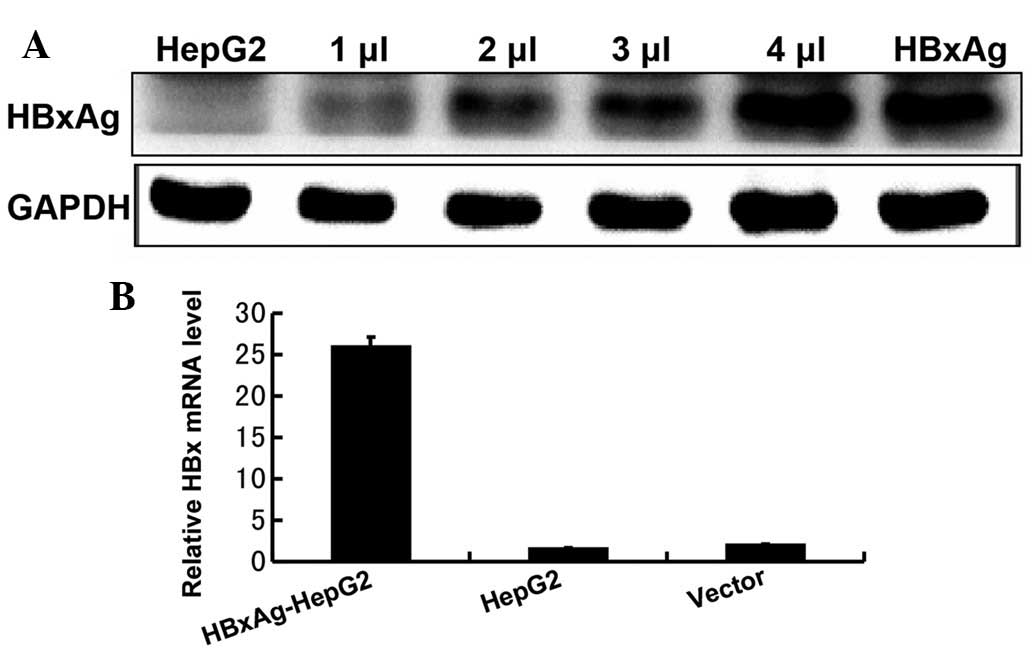
HepG2 cells stably express HBxAg
In order to determine the role of HbxAg in human
hepatic cell lines, it was first confirmed that the HepG2 cell line
stably expressed the HbxAg following transfection. Western blotting
and RT-qPCR were used to detect the expression levels of HbxAg in
the HepG2 cells. As shown in Fig.
1, the results revealed that the HepG2 cell line exhibited a
high expression level of HbxAg, following transfection.
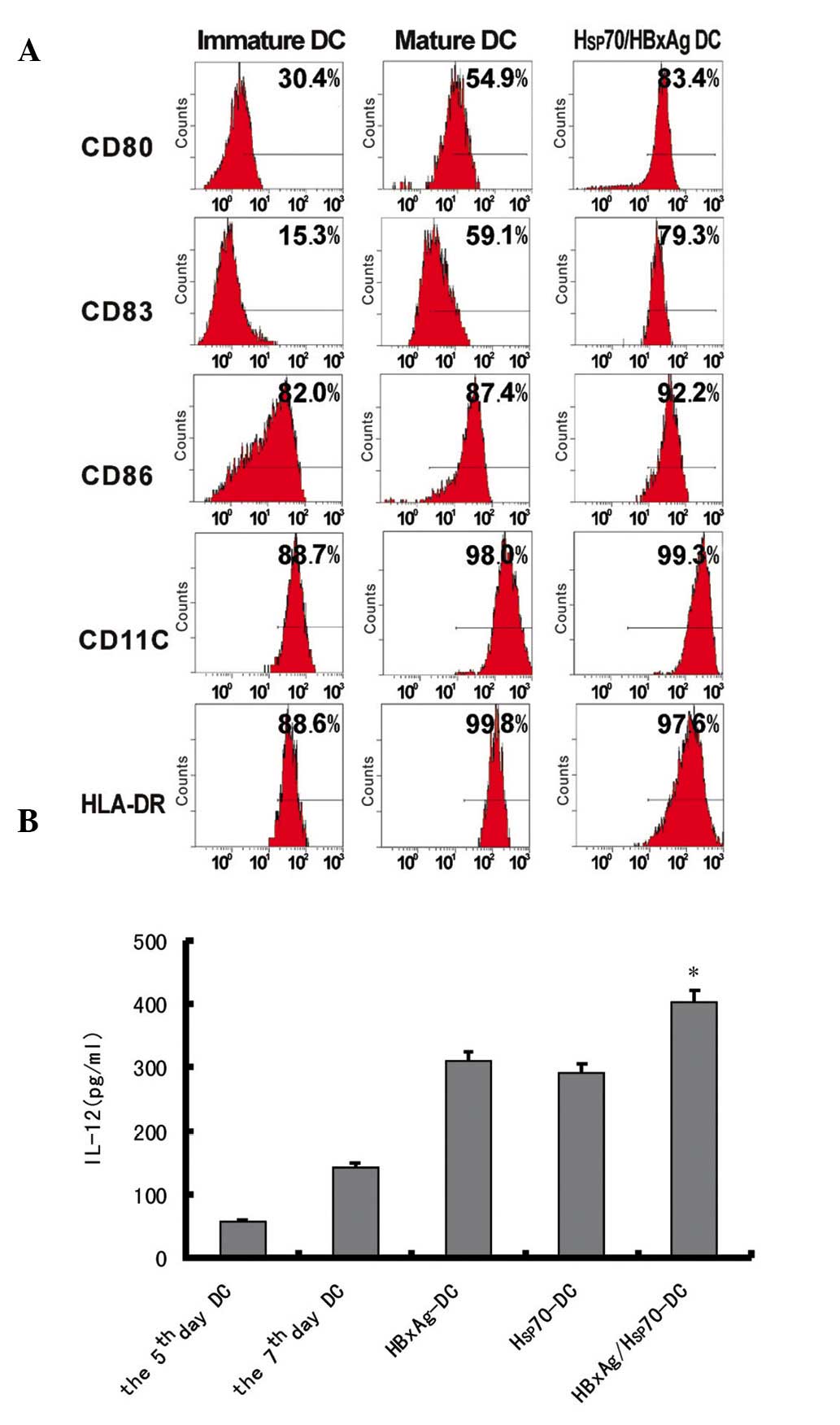
Maturation of human DCs pulsed with
different antigen complexes
To investigate the effects of antigen complexes
derived from HepG2 cells on DCs, immature DCs were generated by
culturing human PBMCs in the presence of human GM-CSF and IL-4 for
7 days. The immature DCs were incubated with antigen complexes at
37°C and were subsequently cultured for 48 h. The expression levels
of HLA-DR, CD86, CD11c, CD80 and CD83 were determined by flow
cytometry. The expression levels of HLA-DR, CD86 co-stimulation
molecule, CD83 maturation marker, CD11c and CD80 were significantly
upregulated (Fig. 2A). These
results indicated that antigen complexes induced the maturation of
DCs, suggesting that antigen complexes effectively activated
DCs.
Cytokine release of DCs pulsed with
different antigens
The IL-12 release in the supernatants of DCs either
pulsed or non-pulsed with different antigens was assessed by ELISA.
The results revealed that the levels of IL-12 were significantly
upregulated following infection for 24 h. However, when the DCs
were pulsed with Hsp70/HBxAg, the level of IL-12 was higher
compared with that of DCs pulsed with PBS, HBxAg and Hsp70
(Fig. 2B). The results
demonstrated that the antigens activated CD4+ and
CD8+ T cells.
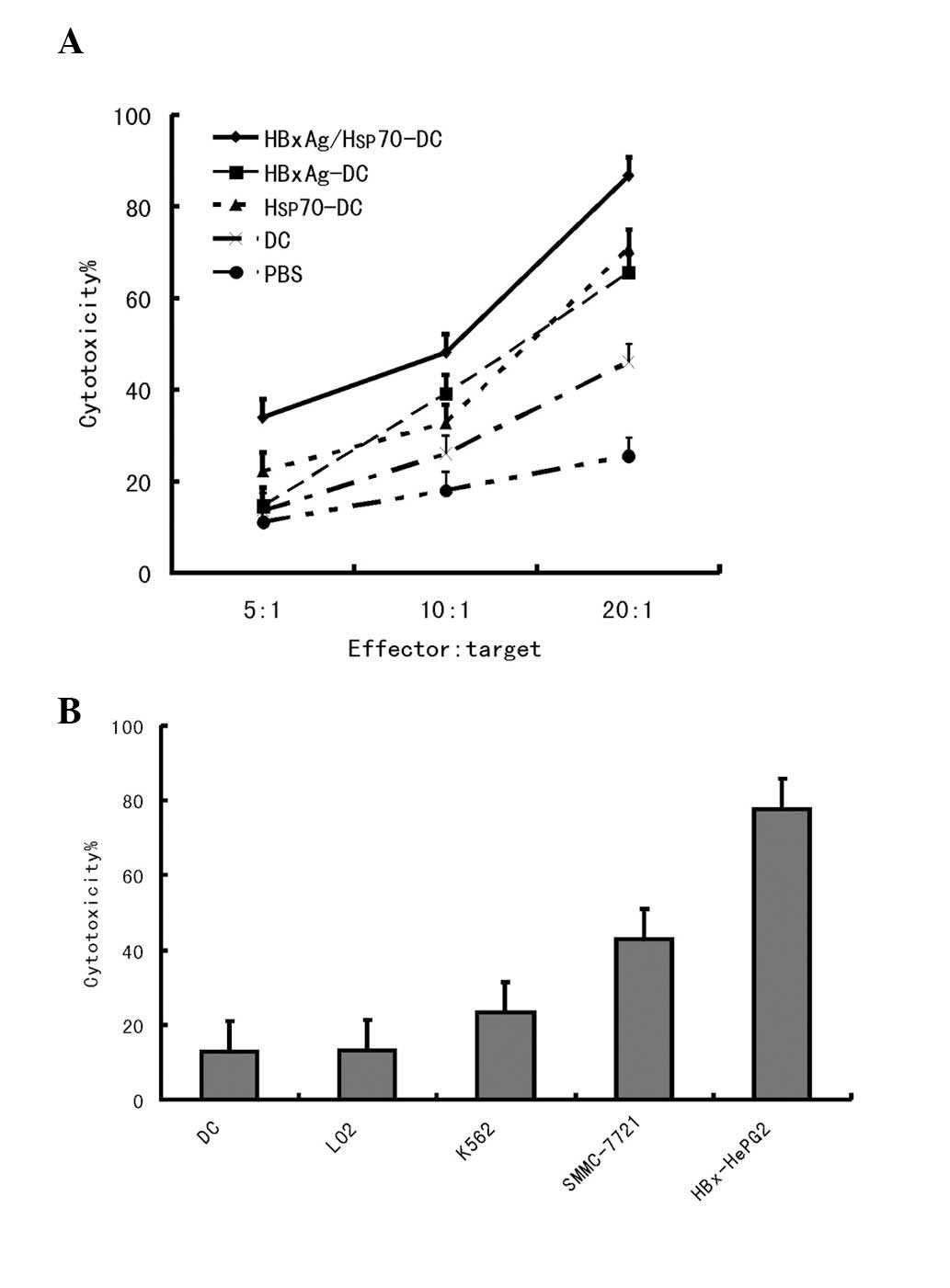
Induction of specific CTLs against HepG2
by DCs pulsed with Hsp70/HBxAg complexes
The functional capability of the CTLs responding to
antigen-pulsed DCs was assessed by determining whether it
specifically killed tumor cells. CD8+ T cells were
plated into 96-well plates in a medium containing IL-2. DCs were
added at a 1:20 ratio and cocultured at 37°C in 5% CO2.
As shown in Fig. 3, the DCs pulsed
with antigens significantly induced T-cell proliferation. The
highest level of T-cell proliferation was observed when the DCs
were co-pulsed with HBxAg/Hsp70.
The cytotoxic activity against HCC cells was also
assessed. Target cells were composed of HepG2
(HLA-A2+/HBxAg+), SMMC-7721
(HLA-A2+/HBxAg−), K562
(HLA-A2−/HBxAg−) and LO2 cells. A small
number of CTLs were induced by the HBxAg-DC vaccine; however, no
significant CTL induction was observed by PBS-DCs. The results
indicated that Hsp70/HBxAg-DCs specifically induced high CTL
activity against HBxAg-expressing HepG2 cells. In the
Hsp70/HBxAg-positive group, the CTL response was markedly higher
compared with that observed in the Hsp70/HBxAg-negative group,
indicating that the CTL response is antigen dependent (Figs. 3 and 4).
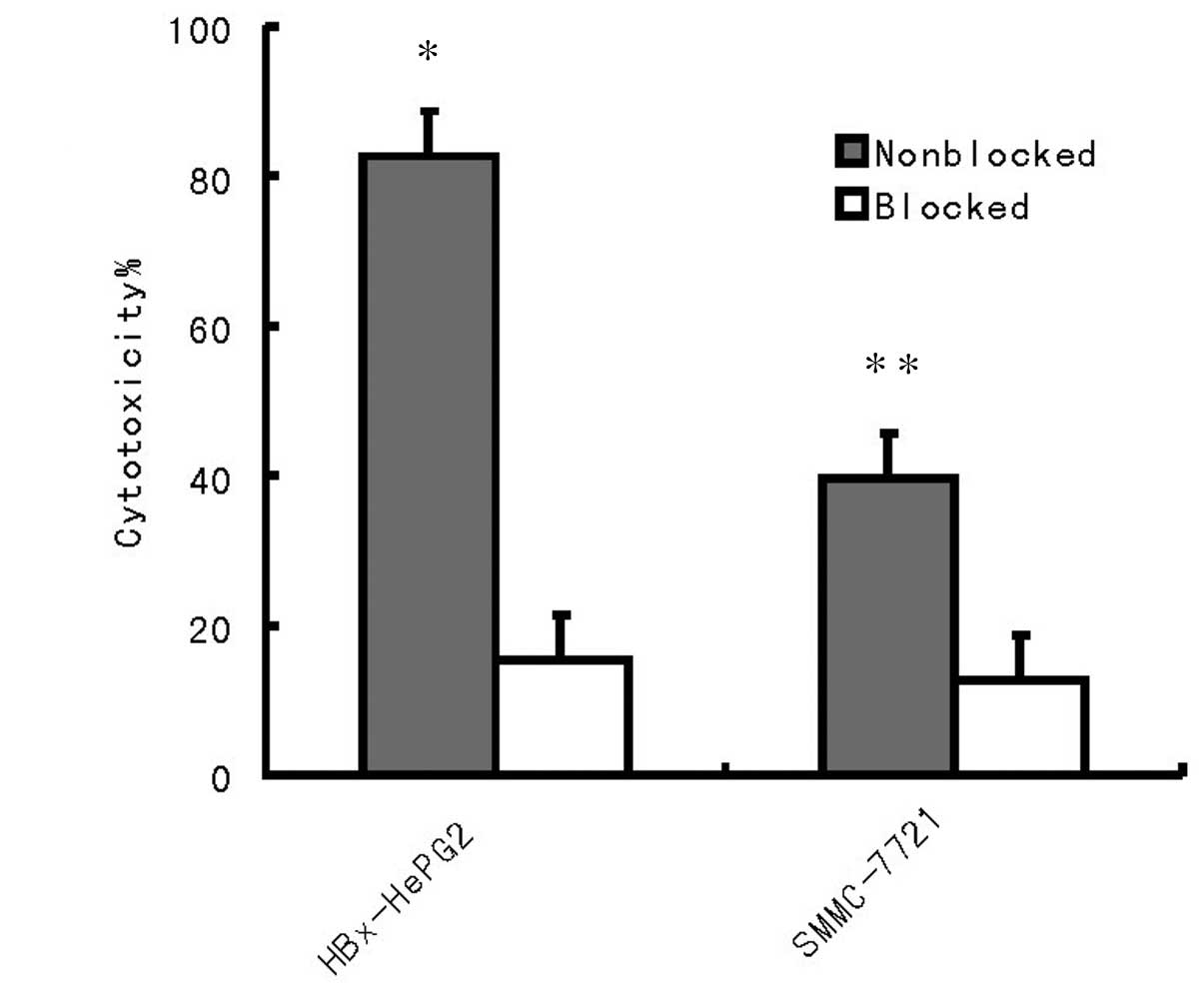
To further confirm whether the cytotoxicity in
tumors is independent of the MHC class I immunoresponse, two HCC
cell lines HepG2 and SMMC-7721 were used for detection (Fig. 5). The results revealed that the
cytotoxicity against HepG2 and SMMC-7721 cells was independent of
MHC class I.
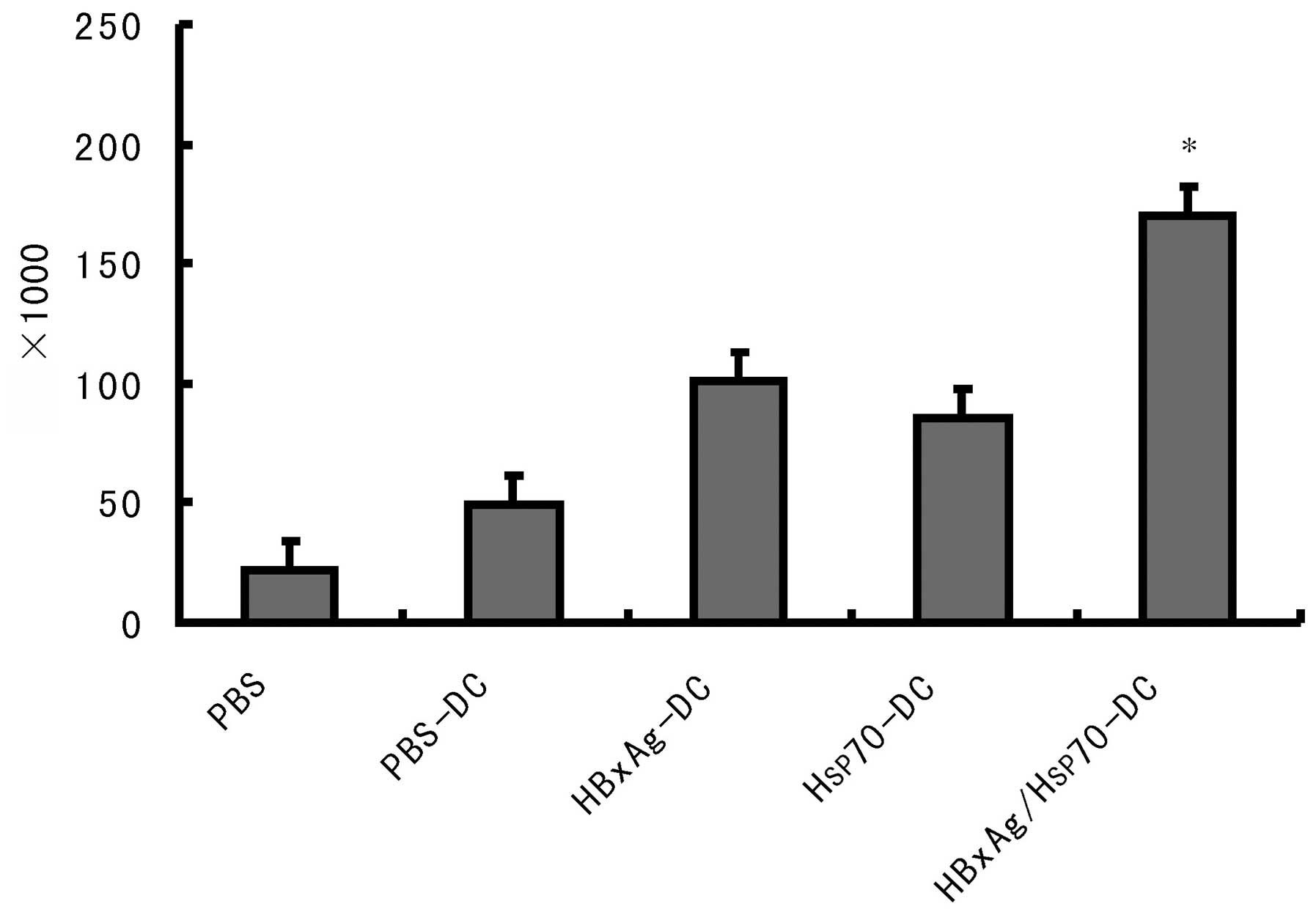
Establishment of Hsp70/HBxAg-DCs in the
SCID mouse model
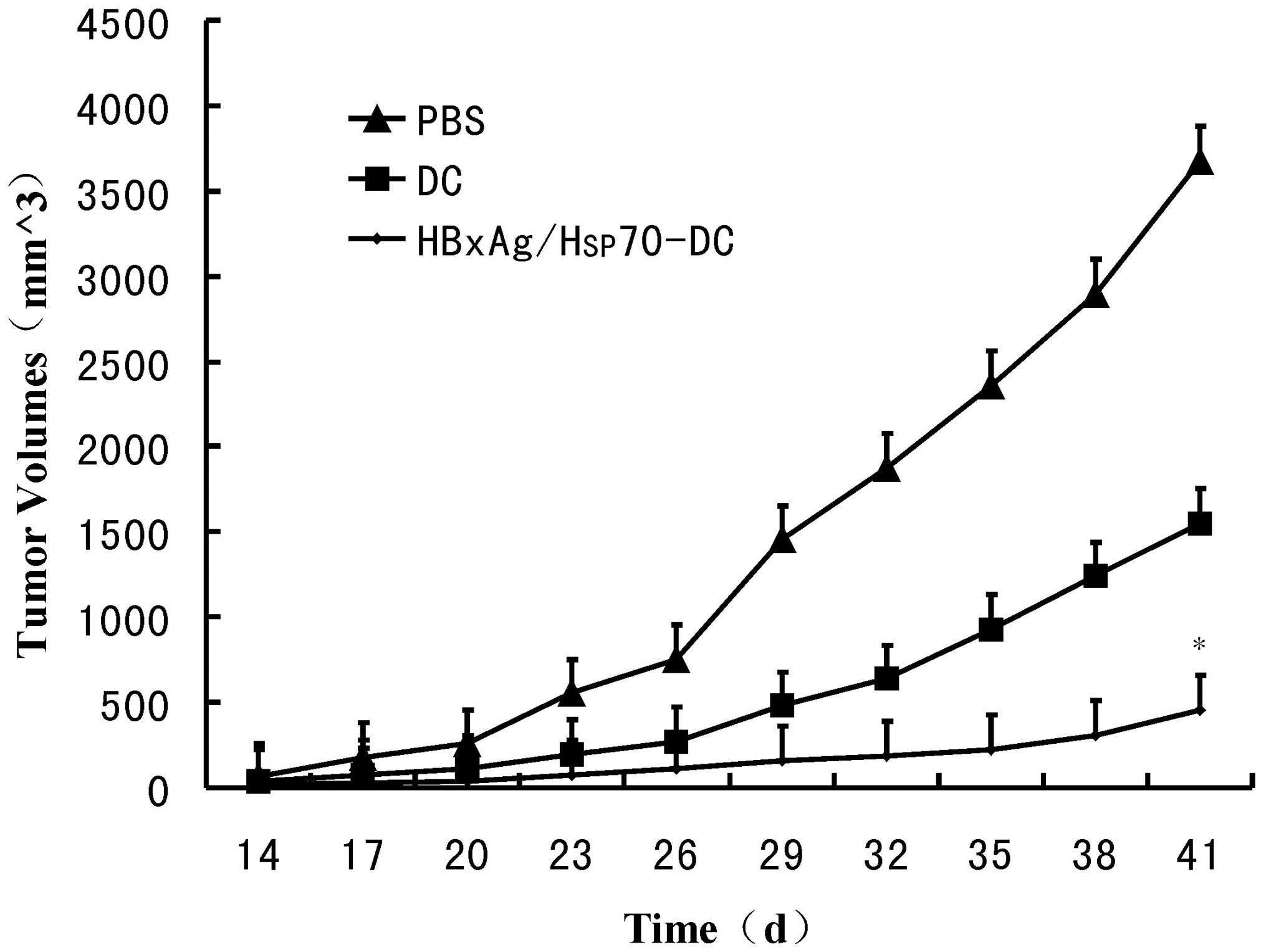
To determine the efficacy of CTLs stimulated by
Hsp70/HBxAg-DCs, SCID mice were inoculated with HepG2 cells.
Following inoculation for 7 days, the mice were treated with CTLs
induced by fusion cells, DCs or PBS. Following treatment for 5
weeks, the fusion cell (Hsp70/HBxAg-DCs)-stimulated T cells
significantly reduced the tumor volumes (Fig. 6). The results indicated the
possible therapeutic potency of T cells stimulated by
Hsp70/HBxAg-DCs.
Discussion
DCs are efficient antigen-presenting stimulators of
B and T lymphocytes, which have potent immunostimulatory properties
(16,17). Several immunotherapies regulate DCs
to stimulate the immune response (18). Previous studies demonstrated that
DC-based vaccine therapy effectively stimulates T-cell immunity and
kills tumor cell lines, including malignant melanoma (19), breast cancer (20) and ovarian cancer (21) cells.
A previous study suggested that HSPs, including
Hsp70 and Hsp90, are potent tumor-antigen sources for pulsing DCs
to activate macrophages and release specific and non-specific
effecter molecules, which can increase the effect of the
macrophages (22). Inducible Hsp70
may function as a crosslink between the innate and adaptive immune
response (23,24).
HBxAg is associated with several advanced liver
diseases, and HBxAg and other etiological factors have been
implicated in hepatocarcinogenesis (25). HBxAg can increase the risk of HCC
in patients with chronic HBV infection. Previous studies have
revealed that a vaccination composed of particulate hepatitis B
antigen induces a specific immune response and significant
antitumor effects in vivo (26) and in vitro (27).
However, whether the Hsp70/HBxAg complex enhanced
the immune response in the HepG2 cells expressed by HBxAg remains
to be elucidated. Therefore, the present study predominantly used
HepG2 (HBxAg+) (Fig. 1A and
B) and SMMC-7721 (HBxAg−) cells to confirm this
hypothesis. The functional maturation and proliferation of DCs is a
critical step in the stimulation of tumor-specific CTLs (28). In addition, the results
consistently indicated the detection of different markers, such as
the CD86 costimulatory molecule, CD83 maturation marker, and other
markers including CD80 and CD11c (Fig.
2A), thereby demonstrating that different antigens induce the
maturation of allogeneic DCs by upregulating.
CD4+ T cells have an important role in
the induction and maintenance of the CTL response (29). The results from the present study
demonstrated that DCs pulsed with Hsp70 complexes stimulated
CD4+ T cell proliferation and unpulsed DCs exhibited few
stimulatory effects. DCs pulsed with Hsp70/HBxAg also activated a
more marked increase in CD4+ T cell proliferation
compared with the DCs pulsed with only Hsp70 or HBxAg alone
(Fig. 2B).
A previous study revealed that tumor cytotoxicity is
mediated by lymphocytes, notably by CTLs (30). Therefore, in the process of tumor
immunotherapy, activating tumor-specific CTLs to kill tumor cells
is considered an important event (31). The present study revealed that
Hsp70-DCs and HBxAg-DCs were able to induce CTL activity against
HCC cells with high efficiency, consistent with this previous
observation (Fig. 3). However, the
data demonstrated that Hsp70- or HBxAg-DCs elicited limited CTL
responses against the HCC tumor cell. Notably, after they were
co-pulsed with Hsp70 and HBxAg, the Hsp70/HBxAg-DCs were able to
stimulate CD4+ T cells, which proliferated more
effectively compared with those activated by DCs pulsed with HBxAg
or Hsp70 alone (Fig. 3).
To further confirm that CTLs killed the HepG2 cells,
and to define the specificity of the CTLs generated by the
coculture of T cells with autologous DCs pulsed with Hsp70-peptide
and/or HBxAg complexes, the cytotoxicity of the T-cell against
HepG2 (HLA-A2+/HBxAg+), SMMC-7721
(HLA-A2+/HBxAg−) cells and another human
cancer cell, K562 (HLA-A2−/HBxAg−), was
compared. Notably, the results demonstrated that the cytotoxicity
against HepG2 and SMMC-7721 cells was caused by the presence of
CTLs, not by natural killer cells. The reason is that the
cytotoxicity against the natural killer cell-sensitive cell line,
K562, is slightly lower compared with SMMC-7721 and HepG2 cells.
K562 cells are HLA-A2−/HbxAg−, SMMC-7721
cells are HLA-A2+/HBxAg− and HepG2 cells are
HLA-A2+/HbxAg+. Thus, in conclusion
cytotoxicity occurred in an antigen-specific manner (Fig. 4B).
Numerous co-stimulatory molecules, including CD80 or
CD86, can induce tumor-specific T lymphocytes. The cytotoxicity
induced by the antigens can be blocked by anti-MHC class I antibody
(12). The present study used two
HCC cell lines, HepG2 and SMMC-7721, for detection. As shown in
Fig. 5, pre-incubation of the
HepG2 and SMMC-7721 cells with anti-MHC class I antibody resulted
in the abrogation of tumor cell lysis. Therefore, cytotoxicity
against HepG2 and SMMC-7721 cells was MHC class I restricted
(Fig. 4). In vivo results
also confirmed that the Hsp70/HBxAg complexes significantly
inhibited tumor growth in the SCID mouse model compared with the
control group (Fig. 6).
In conclusion, the present study is the first, to
the best of our knowledge, to demonstrate that
Hsp70/HBxAg-co-pulsed with DCs elicited a marked and specific
antitumor immune response. These results indicated that an
Hsp70/HBxAg-co-pulsed DC-based cancer vaccine may be a useful
application for tumor immunotherapy and may reveal promise in the
development of HCC immunotherapy.
Acknowledgments
The current study was supported by grants from the
Bureau of Science and Technology of Suzhou, Jiangsu Province, China
(grant no. SYS201129) and the Bureau of Public Health of Jiangsu
Province, China (grant no. H201413).
References
|
1
|
Arzumanyan A, Reis HM and Feitelson MA:
Pathogenic mechanisms in HBV- and HCV-associated hepatocellular
carcinoma. Nat Rev Cancer. 13:123–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu XH, Lin J, Zhang SH, Zhang SM,
Feitelson MA, Gao HJ and Zhu MH: COOH-terminal deletion of HBx gene
is a frequent event in HBV-associated hepatocellular carcinoma.
World J Gastroenterol. 14:1346–1352. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lehman EM and Wilson ML: Epidemiology of
hepatitis viruses among hepatocellular carcinoma cases and healthy
people in Egypt: A systematic review and meta-analysis. Int J
Cancer. 124:690–697. 2009. View Article : Google Scholar
|
|
4
|
Wang Z, Zhang G, Wu J and Jia M: Adjuvant
therapy for hepatocellular carcinoma: Current situation and
prospect. Drug Discov Ther. 7:137–143. 2013.PubMed/NCBI
|
|
5
|
Ibrahim SM, Lewandowski RJ, Sato KT, Gates
VL, Kulik L, Mulcahy MF, Ryu RK, Omary RA and Salem R:
Radioembolization for the treatment of unresectable hepatocellular
carcinoma: A clinical review. World J Gastroenterol. 14:1664–1669.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Palucka K and Banchereau J: Cancer
immunotherapy via dendritic cells. Nat Rev Cancer. 12:265–277.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhan HL, Gao X, Pu XY, Li W, Li ZJ, Zhou
XF and Qiu JG: A randomized controlled trial of postoperative tumor
lysate-pulsed dendritic cells and cytokine-induced killer cells
immunotherapy in patients with localized and locally advanced renal
cell carcinoma. Chin Med J (Engl). 125:3771–3777. 2012.
|
|
8
|
Berntsen A, Trepiakas R, Wenandy L,
Geertsen PF, thor Straten P, Andersen MH, Pedersen AE, Claesson MH,
Lorentzen T, Johansen JS and Svane IM: Therapeutic dendritic cell
vaccination of patients with metastatic renal cell carcinoma: A
clinical phase 1/2 trial. J Immunother. 31:771–780. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murphy ME: The HSP70 family and cancer.
Carcinogenesis. 34:1181–1188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Multhoff G, Pockley AG, Streffer C and
Gaipl US: Dual role of heat shock proteins (HSPs) in anti-tumor
immunity. Curr Mol Med. 12:1174–1182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ciocca DR, Cayado-Gutierrez N, Maccioni M
and Cuello-Carrion FD: Heat shock proteins (HSPs) based anti-cancer
vaccines. Curr Mol Med. 12:1183–1197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang XH, Qin Y, Hu MH and Xie Y: Dendritic
cells pulsed with hsp70-peptide complexes derived from human
hepatocellular carcinoma induce specific anti-tumor immune
responses. World J Gastroenterol. 11:5614–5620. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li QL, Bu N, Yu YC, Hua W and Xin XY: Ex
vivo experiments of human ovarian cancer ascites-derived exosomes
presented by dendritic cells derived from umbilical cord blood for
immunotherapy treatment. Clin Med Oncol. 2:461–467. 2008.
|
|
14
|
Yang JY, Cao DY, Xue Y, Yu ZC and Liu WC:
Improvement of dendritic-based vaccine efficacy against hepatitis B
virus-related hepatocellular carcinoma by two tumor-associated
antigen gene-infected dendritic cells. Hum Immunol. 71:255–262.
2010. View Article : Google Scholar
|
|
15
|
Zhang Z, Du X, Zhao C, Cao B, Zhao Y and
Mao X: The antidepressant amitriptyline shows potent therapeutic
activity against multiple myeloma. Anticancer Drugs. 24:792–798.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Steinman RM: Dendritic cells and the
control of immunity: Enhancing the efficiency of antigen
presentation. Mt Sinai J Med. 68:160–166. 2001.PubMed/NCBI
|
|
17
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ovali E, Dikmen T, Sonmez M, Yilmaz M,
Unal A, Dalbasti T, Kuzeyli K, Erturk M and Omay SB: Active
immunotherapy for cancer patients using tumor lysate pulsed
dendritic cell vaccine: A safety study. J Exp Clin Cancer Res.
26:209–214. 2007.PubMed/NCBI
|
|
19
|
Dannull J, Haley NR, Archer G, Nair S,
Boczkowski D, Harper M, De Rosa N, Pickett N, Mosca PJ, Burchette
J, et al: Melanoma immunotherapy using mature DCs expressing the
constitutive proteasome. J Clin Invest. 123:3135–3145. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abdul Hafid SR, Chakravarthi S, Nesaretnam
K and Radhakrishnan AK: Tocotrienol-adjuvanted dendritic cells
inhibit tumor growth and metastasis: A murine model of breast
cancer. PLoS One. 8:e747532013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kandalaft LE, Powell DJ Jr, Chiang CL,
Tanyi J, Kim S, Bosch M, Montone K, Mick R, Levine BL, Torigian DA,
et al: Autologous lysate-pulsed dendritic cell vaccination followed
by adoptive transfer of vaccine-primed ex vivo co-stimulated T
cells in recurrent ovarian cancer. Oncoimmunology. 2:e226642013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gautam PK, Kumar S, Deepak P and Acharya
A: Morphological effects of autologous hsp70 on peritoneal
macrophages in a murine T cell lymphoma. Tumour Biol. 34:3407–3415.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vrieling M, Santema W, Vordermeier M,
Rutten V and Koets A: Hsp70 vaccination-induced primary immune
responses in efferent lymph of the draining lymph node. Vaccine.
31:4720–4727. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khanna R: Tumour surveillance: Missing
peptides and MHC molecules. Immunol Cell Biol. 76:20–26. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu JW, Yang WY, Tsai SM, Lin YM, Chang PH,
Chen JR, Wang HD, Wu JL, Jin SL, Yuh CH, et al: Liver-specific
expressions of HBx and src in the p53 mutant trigger
hepatocarcinogenesis in zebrafish. PLoS One. 8:e769512013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Buchmann P, Dembek C, Kuklick L, Jäger C,
Tedjokusumo R, von Freyend MJ, Drebber U, Janowicz Z, Melber K,
Protzer U, et al: A novel therapeutic hepatitis B vaccine induces
cellular and humoral immune responses and breaks tolerance in
hepatitis B virus (HBV) transgenic mice. Vaccine. 31:1197–1203.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi M, Qian S, Chen WW, Zhang H, Zhang B,
Tang ZR, Zhang Z and Wang FS: Hepatitis B virus (HBV)
antigen-pulsed monocyte-derived dendritic cells from HBV-associated
hepatocellular carcinoma patients significantly enhance specific T
cell responses in vitro. Clin Exp Immunol. 147:277–286. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cassel JA, Ilyin S, McDonnell ME and Reitz
AB: Novel inhibitors of heat shock protein Hsp70-mediated
luciferase refolding that bind to DnaJ. Bioorg Med Chem.
20:3609–3614. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bajana S, Herrera-González N, Narváez J,
Torres-Aguilar H, Rivas-Carvalho A, Aguilar SR and Sánchez-Torres
C: Differential CD4(+) T-cell memory responses induced by two
subsets of human monocyte-derived dendritic cells. Immunology.
122:381–393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Harty JT, Tvinnereim AR and White DW: CD8+
T cell effector mechanisms in resistance to infection. Annu Rev
Immunol. 18:275–308. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hanson HL, Donermeyer DL, Ikeda H, White
JM, Shankaran V, Old LJ, Shiku H, Schreiber RD and Allen PM:
Eradication of established tumors by CD8+ T cell
adoptive immunotherapy. Immunity. 13:265–276. 2000. View Article : Google Scholar : PubMed/NCBI
|