Introduction
Prostate cancer (PCa) is one of the most frequently
diagnosed and mortality-associated cancer types in men,
particularly in western countries (1). The morbidity and mortality of PCa are
markedly increasing in China in previous years (2). Although patients with localized PCa
are reported to have a survival rate of >5 years (3,4), it
is hypothesized that few effective therapeutic options are
effective for advanced PCa, even if applying advanced therapeutic
strategies. A previous study demonstrated that in the USA, there
were an estimated 240,000 new cases and 33,000 deaths in 2011,
predominantly due to its high potential for tumor progression
(5). Molecular strategies for the
diagnosis and treatment of cancer have been developing (6,7).
Therefore, the detection of efficient diagnostic and prognostic
biomarkers for PCa is becoming extremely necessary for the
improvement of the clinical outcome of patients with this disease.
Micro (mi)RNAs are a class of short (19–22 nucleotides), non-coding
RNA sequences, which negatively regulate their target genes
post-transcriptionally (8). There
are two ways in which miRNAs regulate their target genes, by either
promoting mRNA degradation or repressing protein translation
through sequence-specific interaction with the 3′-untranslated
region of targeted mRNA (9,10).
Previous studies have shown that deregulation of miRNAs is closely
associated with the process of tumor progression, including
inhibiting apoptosis (11),
promoting proliferation (12),
invasion (13) and angiogenesis
(14). In PCa, it is reported that
deregulation of miRNAs are vital in the initiation and progression
of PCa, including miR-361-5p (15)
and miR-26a (16). To date,
miR-126 has been demonstrated to be downregulated in several cancer
types, including cervical (17)
and colon (18). A previous study
revealed that the expression level of miR-126 is significantly
decreased in PCa tissues and the expression of miR-126 is an
independent prognostic factor of PCa following radical
prostatectomy (19). In the
present study, the underlying mechanism of miR-126 in mediating PCa
progression was the primary focus. In vitro investigations
showed that miR-126 markedly suppressed the ability of PCa cells to
invade extracellular matrix gel and proliferate. Furthermore, the
data indicated that pik3r2 was a direct target of miR-126 in PCa
cells. In clinical tissues, it was also confirmed that the
expression of miR-126 and pik3r2 were inversely correlated.
Additionally, in vivo experiments revealed that miR-126 had
an obvious antiproliferative effect. These findings highlighted the
important functions of miR-126 in PCa initiation and progression,
and therefore, miR-126 may be a promising therapeutic molecular
target for PCa.
Materials and methods
Patients and samples
PCa tissues enrolled in the present study were
obtained from 20 patients who underwent radical prostatectomy and
had not received any previous treatment in our center (The Second
Xiangya Hospital of Central South University, Changsha, China). For
frozen specimens, surgical pathologists examined the
clinicopathological features of freshly frozen sections following
staining with hematoxylin and eosin and subsequently the samples
were snap frozen in liquid nitrogen. Only those samples with
>70% tumor content were included in the present study. The
present study was approved by the Ethics Committee of The Second
Xiangya Hospital of Central South University (no. S048, 2013) and
were performed after obtaining written informed consent from each
of the patients.
RNA preparation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The total RNA was extracted from tissues or cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), according to the manufacturer's protocol. A
total of 2 µg RNA was transcribed into cDNA using MMLV
reverse transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.)
for mRNA and an RT-PCR kit (RR716, Takara Bio Inc., Otsu, Japan)
for miRNA. All qPCR reactions were performed with an ABI7500fast
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
primers for miR-126 and RNU6B were purchased from Takara Bio Inc.
The sequences of the other primers were as follows: Pik3r2,
forward: 5′-ATGGCACCTTCCTAGTCCGAGA-3′ and reverse:
5′-CTCTGAGAAGCCATAGTGCCCA-3′; GAPDH, forward:
5′-GTCTCCTCTGACTTCAACAGCG-3′ and reverse:
5′-ACCACCCTGTTGCTGTAGCCAA-3′; and TGCGGGTGCTCGTTCGGCAGC and the
Universal Primer (Takara Bio, Inc.) for RNU6B. GAPDH and RNU6B were
used as internal controls.
Cell culture
Human DU145, PC-3 and 293T PCa cell lines were
purchased from the Shanghai Institute of Cell Biology (Shanghai,
China). The DU-145 and PC-3 cells were cultured in RPMI-1640 medium
(Hyclone, Logan, UT, USA) and the 293T cells were cultured in
Dulbecco's modified Eagle's medium (Hyclone). Each culture medium
was supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.) and the cells were maintained at 37°C in a
humidified environment, containing 5% CO2.
Oligonucleotides and cell
transfection
miR-126 mimics and its negative control (NC) were
designed and chemically synthesized by GenePharma (Shanghai,
China). For transfection, the cells were plated into 6-well plates
with 2.5×104 cells/well. Once the cells were 30–50%
confluent, miR-126 mimics or NC, accompanied with Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) were transfected
into the PCa cells. The medium was changed following a 24 h
incubation.
Cell proliferation assay
The DU145 and PC-3 cells were seeded and transfected
in 6-well plates with 2.5×104 cells/well, and were
subsequently transferred into 96-well plates with 3,000 cells/well
24 h following the transfection. The proliferation of the cells was
detected using a Cell Counting kit-8 reagent (Dojindo Laboratories,
Kyushu Island, Japan), according to the manufacturer's protocol.
The relative numbers of viable cells were represented by the
absorbance at a wavelength of 450 nm following 24, 48 and 72 h
incubation in 96-well plates (UV-6100; Shanghai Mapada Instruments,
Co., Ltd., Shanghai, China). The experiments were performed in
triplicate.
Migration and invasion assays of PCa
cells
The migration and invasion of PCa cells were
analyzed in 24-well Boyden chambers with 8 µm pore size
polycarbonate membranes (Corning, Inc., Corning, NY, USA). A total
of 4×104 transfected cells were suspended in the upper
chamber with serum-free medium, while the lower chamber was filled
with 10% FBS-containing medium. In the invasion assays, the
membranes were covered with Matrigel (BD Biosciences, San Diego,
CA, USA) to form matrix barriers. Following incubation for 30
(migration assay) or 48 h (invasion assay), the cells on the upper
surface were removed by wiping with a cotton swab and the cells on
the lower surface of the membrane were fixed in methanol, stained
with 0.1% crystal violet (Sigma-Aldrich, St. Louis, MO, USA) and
counted. The experiments were repeated independently three
times.
Plasmid construction and luciferase
reporter assay
The binding site of miR-126 and pik3r2 was predicted
by TargetScan (www.targetscan.org) and miRanda (www.microrna.org). To form a wild-type luciferase
reporter plasmid of pik3r2 (Guangzhou RiboBio Co., Ltd., Guangzhou,
China), the binding segment was chemosynthesized, amplified and
cloned into the firefly luciferase reporter plasmid. The binding
site was subsequently altered to form a mutant type luciferase
reporter plasmid of pik3r2.
For luciferase assay, the 293T cells were seeded
into 24-well plates and were co-transfected with miR-126 or NC, and
wild-type or mutant-type pik3r2 plasmid. Following incubation for
48 h, the luciferase activity was measured by dual-luciferase
assays (E2920; Promega, Madison, WI, USA).
Immunohistochemistry and western
blotting
The paraffin-embedded tissues were assessed by
immunohistochemistry, or the proteins of cells were extracted using
radioimmunoprecipitation buffer for western blotting (Beyotime
Institute of Biotechnology, Haimen, China). The detailed process of
these two protocols was described previously (20). Protein lysates (30 µg) were
loaded into 10% SDS-PAGE gels (Thermo Fisher Scientific, Inc.) and
run for 20 min at 80 and then 120 V, and then transferred to
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). Following blocking with nonfat milk powder, the membranes
were incubated with primary antibodies overnight, followed by 1 h
incubation with the secondary antibody and then were subjected to a
chemiluminescence kit (EZ-ECL kit; Biological Industries, Beit
Haemek, Israel) For immunohistochemistry, 4 µm sections
underwent for antigen retrieval by microwave for 25 min following
deparaffinization and hydration. The sections were blocked by 5%
FBS for 1 h prior to incubation with primary antibody overnight.
The sections were then washed with phosphate-buffered saline
(Thermo Fisher Scientific, Inc.) and incubated with secondary
antibodies prior to treatment with 3,3′-diaminobenzidine staining
(ZSGB-BIO, Beijing, China). The primary antibodies used in the
present study were as follows: Rabbit polyclonal anti-pik3r2
(1:500; cat. no. ab131067; Abcam, Cambridge, UK), rabbit monoclonal
anti-phosphorylated (p-)AKT (1:2,000; cat. no. 2118-1; Epitomics,
Burlingame, CA, USA), and rabbit monoclonal anti-GAPDH (1:5,000;
cat. no. 5632-1; Epitomics). The secondary antibodies were
horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (heavy
+ light; 1:3,000; cat. no. SA00001-1; Guangzhou Biological
Technology, Co., Ltd., Guangzhou, China) and HRP-conjugated goat
anti-rabbit IgG (heavy + light; 1:3,000; cat. no. SA00001-2;
Guangzhou Biological Technology, Co., Ltd.)
The results of the immunohistochemistry were scored
in a semi-quantitative manner. The final score was a composite
score obtained by multiplying the values of staining intensity and
the percentage of positive cells. Briefly, the staining intensity
was categorized as follows: 0, negative; 1, weak; 2, moderate; 3,
strong. The percentage of positive cells was recorded as 0–100% and
the final score, representing the level of pik3r2, ranged between 0
and 3.
Stable overexpression of miR-126 in PC-3
cells and nude mice xenograft assay
The oligonucleotide sequence of miR-126
(5′-UCGUACCGUGAGUAAUAAUGCG-3′) was synthesized, amplified and
cloned into a lentiviral vector. For lentiviral infection, PC-3
cells without transfection were eliminated by consistent puromycin
incubation and the overexpression of miR-126 in puromycin-resistant
PC-3 cells was verified by RT-qPCR.
Female BALB/c athymic mice (Shanghai Laboratory
Animal Research Centre, Shanghai, China; age, 4–6 weeks; weight,
15–18 g) were used and raised under standard animal care conditions
with five rats per cage and free access to food and water under a
16-h/8-h light/dark cycle. All animal experiments were performed in
accordance with the National Institute of Health Guide for the Care
and Use of Laboratory Animals, and were approved by the Ethics
Committee of The Second Xiangya Hospital of Central South
University. PC-3 cells (1×106), stably overexpressing
miR-126 or NC, were suspended in 100 µl phosphate-buffered
saline and were subsequently injected subcutaneously into the
posterior flank of the mice. Tumor volumes in the mice were
measured with a slide caliper weekly, until the mice were
sacrificed at the 6th week. A total of 12 mice were
included in the total. The results are presented as the mean ±
standard deviation.
Statistical analysis
Each value in the present study was obtained from at
least three independent experiments and the data are expressed as
the mean ± standard deviation. Comparisons between two groups were
analyzed by Student's t-test. Statistical analysis was performed
using SPSS 15.0 software (SPSS, Inc., Chicago, IL, USA) and
GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA,
USA. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-126 is significantly downregulated in
PCa tissues
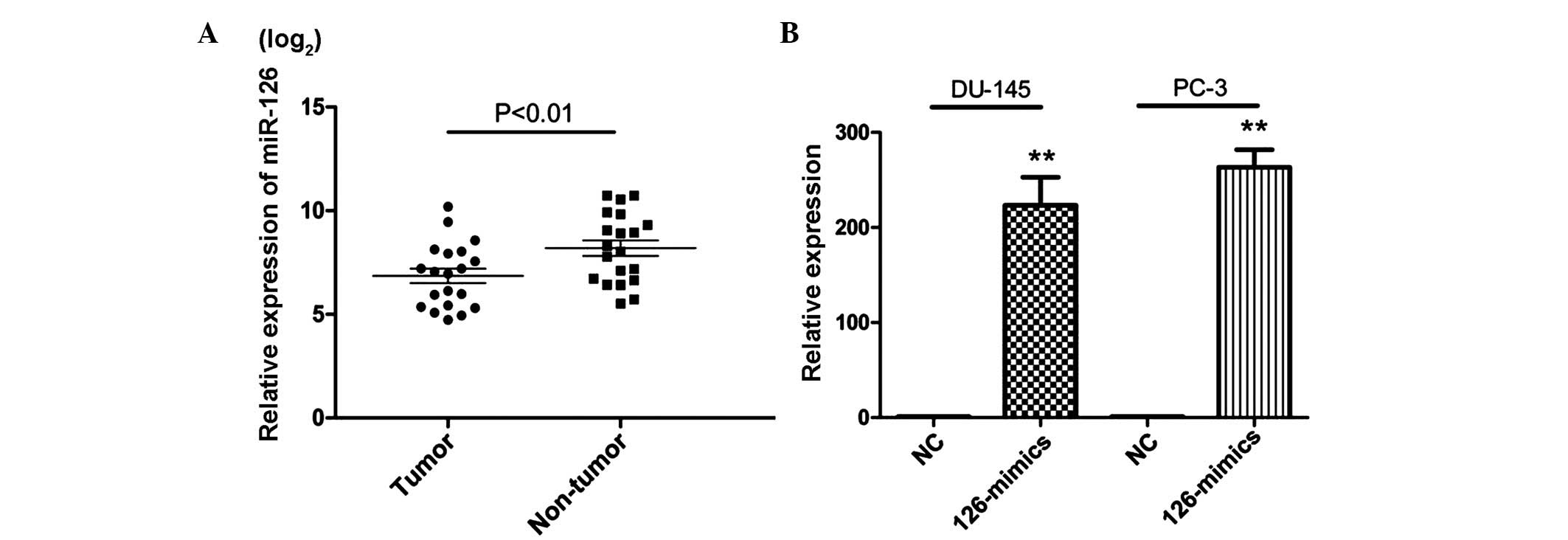
To assess the expression of miR-126 in PCa, RT-qPCR
was performed in 20 pairs of PCa tissue. The results showed that 18
PCa tissue samples revealed a lower expression level of miR-126
compared with their corresponding normal tissues and a significant
difference was observed between the two groups (Fig. 1A). This suggested that miR-126 may
exert an important role in the progression of PCa.
Enhanced expression of miR-126
significantly suppresses the proliferation of PCa in vitro and in
vivo
To assess the potential role of miR-126 in PCa,
miR-126 mimics were used to overexpress the level of miR-126 in
DU-145 and PC-3 cells (Fig. 1B). A
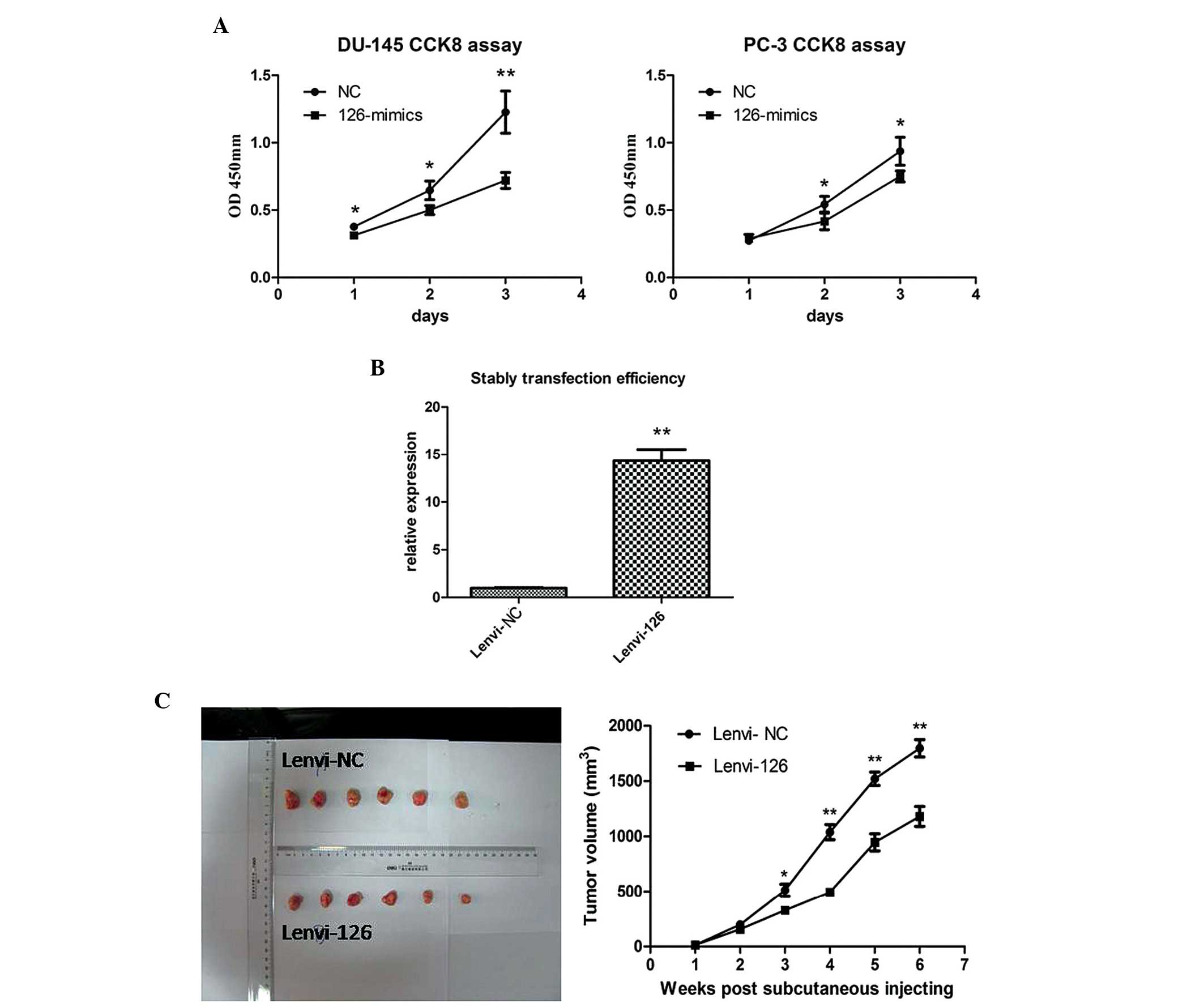
CCK-8 assay was subsequently used to evaluate the proliferation
effects of miR-126 in PCa cells. As shown in Fig. 2A, overexpression of miR-126
significantly inhibited the growth rate in the PCa cell lines.
To further confirm the antiproliferative role of
miR-126 in PCa, in vivo proliferation assays were preformed
using a nude mice xenograft model. miR-126 or NC were stably
transfected into PC-3 cells (Fig.
2B). Following subcutaneous injection for 6 weeks, the tumor
volume of the lenvi-126 group revealed a slower proliferation trend
compared with the lenvi-NC group (Fig.
2C). The data suggested that overexpression of miR-126
significantly inhibited the proliferation in vitro and in
vivo.
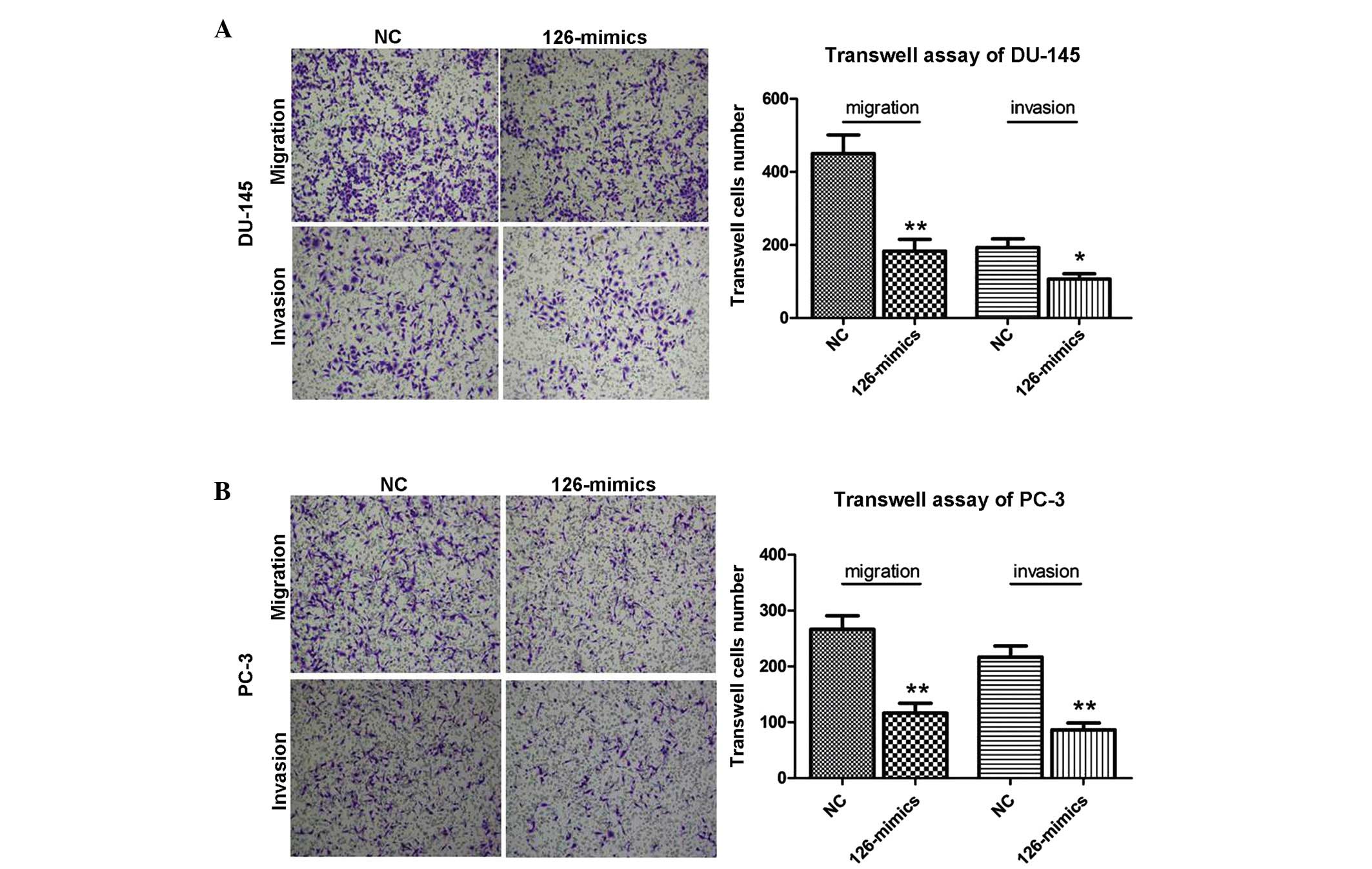
miR-126 inhibits metastasis in DU-145 and
PC-3 cells
Transwell assays were performed to examine the
metastasis potential of miR-126. As shown in Fig. 3, the invasion and migration
activities were markedly reduced following the overexpression of
miR-126 in DU-145 and PC-3 cells. The results indicated the
suppressive role of miR-126 in metastasis in PCa cells.
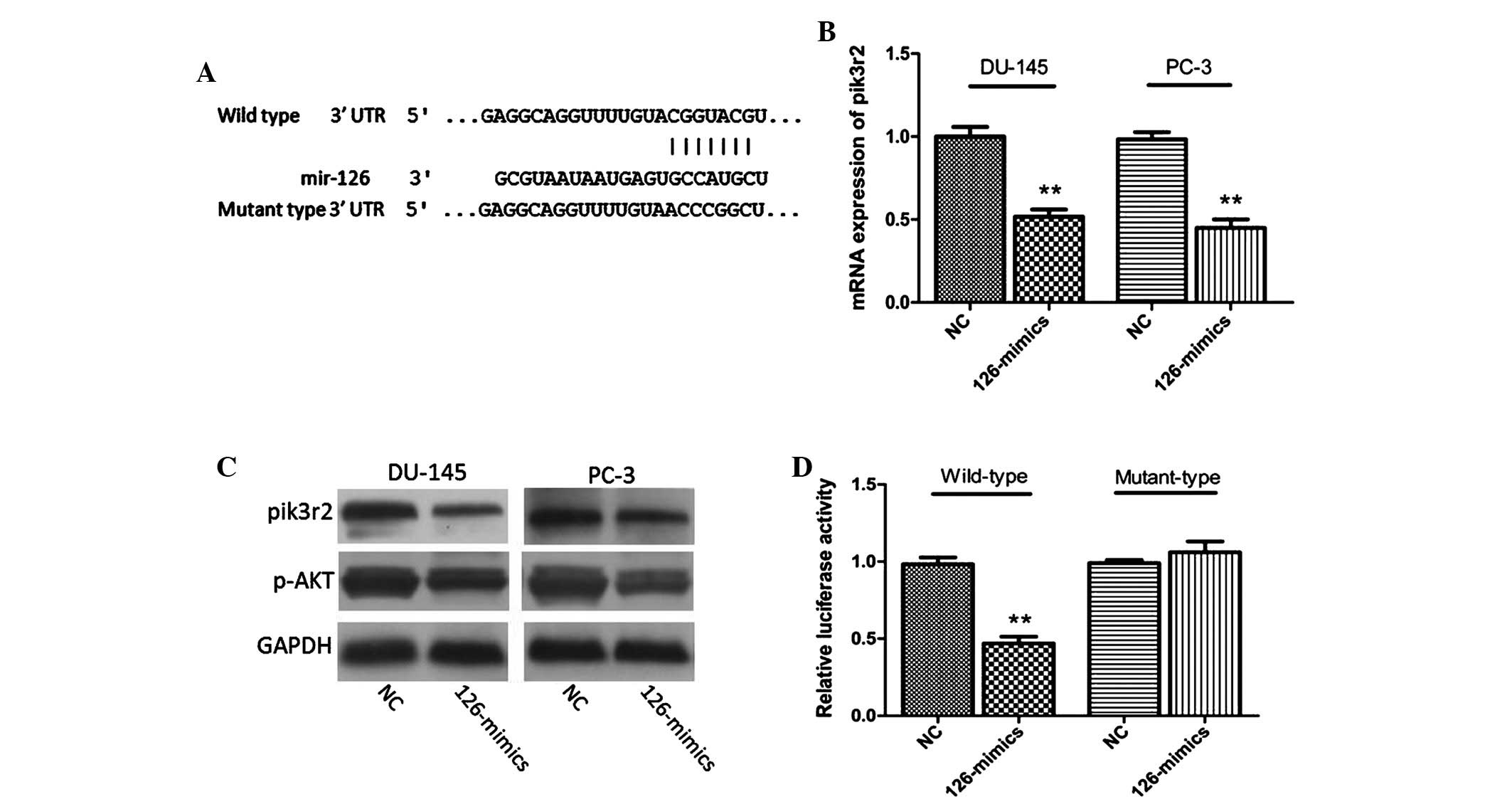
Pik3r2 is a direct target of miR-126 in
PCa cells and tissues
As the functions of miRNAs are mediated by
regulating target genes (21), the
present study sought to identify a potential target of miR-126 in
PCa. By comprehensive analysis of the bioinformatics websites,
TargertScan and miRanda, the gene, pik3r2, was identified (Fig. 4A). This gene is a well-known
component of the PI3K/p-AKT pathway (22). As shown in Fig. 4B and C, the mRNA and protein
expression levels of pik3r2 in DU-145 and PC-3 cells were markedly
decreased following treatment with miR-126 mimics. In addition,
western blotting indicated that the protein expression of p-AKT was
also reduced, accompanied by the decrease in pik3r2 (Fig. 4C). A luciferase reporter assay was
subsequently performed in 293T cells to further confirm the direct
interaction of miR-126 and pik3r2. MiR-126 overexpression decreased
the luciferase activity of the wild type pik3r2 plasmid, while
there were no effects of miR-126 mimics when the binding sites of
pik3r2 plasmid were mutated (Fig.
4D).
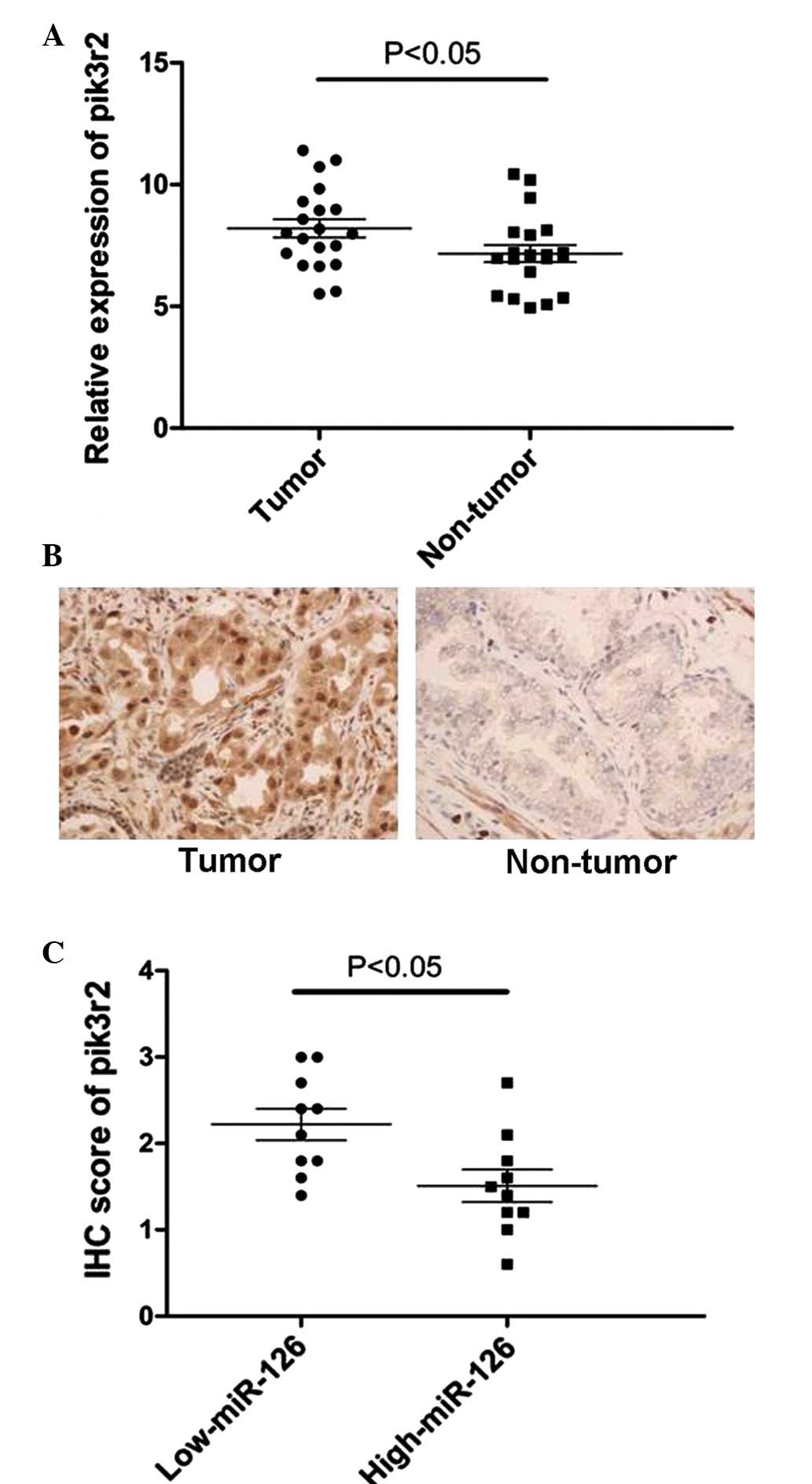
Based on the observation that the expression level
of miR-126 was markedly downregulated in 20 PCa tissues, RT-qPCR
and immunohistochemistry assays of pik3r2 were performed in these
20 tissue samples. By contrast, the expression of pik3r2 was
significantly upregulated in PCa tissues compared with their
corresponding normal tissues (Fig. 5A
and B). These PCa tissues were subsequently divided into two
groups by selecting the median of miR-126 expression of all 20 PCa
tissues as the cut-off. It was then revealed that the expression of
miR-126 inversely correlated with the expression of pik3r2 in these
20 PCa tissues (Fig. 5C). Taken
together, the results demonstrated that pik3r2 is a direct target
of miR-126 in vitro and in vivo in PCa.
Discussion
PCa is one of most frequent and malignant types of
cancer in men, which owns an increasing morbidity and mortality
rate. Over past decades, researchers have investigated the
underlying molecular mechanism involved in the initiation and
progression of PCa. miRNAs are hypothesized to be a highly involved
in mediating malignant biological functions in cancer development
(23,24). In PCa, several miRNAs are
demonstrated to have a vital role in the process of antiapoptosis
(25), pro-proliferation (26) and pro-metastasis (27).
To date, the role of miR-126 in PCa, particularly
its functional role, remains to be elucidated. In the present
study, it was demonstrated that the expression of miR-126 was
significantly lower in the 20 PCa cases compared with their
corresponding normal tissues, which is consistent with a previous
report (19). It was subsequently
demonstrated that overexpression of miR-126 suppressed the
proliferation and inhibited the metastasis in two PCa cell lines,
DU-145 and PC-3. Using nude mice xenograft models, it was also
revealed that miR-126 markedly suppressed the growth of
subcutaneous tumors. Additionally, it was confirmed that pik3r2 was
a direct target gene of miR-126 in vitro and in vivo.
This is the first attempt, to the best of our knowledge, to
illuminate the role of miR-126 deregulation in the proliferation
and metastasis of PCa, using both in vitro and in
vivo models.
A high frequency of proliferation is a basic
characteristic of cancer, and metastasis is the major cause of
cancer mortality, while angiogenesis is essential for tumor growth
and metastasis (28). Therefore,
the identification of novel therapeutics targeting proliferation
and metastasis may be beneficial for clinical applications. miR-126
has been shown to affect numerous cellular processes, particularly
inhibiting angiogenesis and metastasis in cancer (29,30),
which suggests that it is important in cancer progression. However,
its potential role in PCa remains to be elucidated. The present
data revealed a decreased expression of miR-126 in PCa tissues,
suggesting a potential tumor suppressor role in PCa. Subsequent
functional assays revealed the antiproliferative and antimetastatic
effects of miR-126 in PCa cells, which were in agreement with the
role of miR-126 in other cancer types.
Pik3r2 is a regulatory component of PI3K, which is
located on the upstream of AKT (31). The PI3K/p-AKT pathway is well-known
to be responsible for regulating cell growth, proliferation,
survival and angiogenesis in the development of cancer. Therefore,
molecules altering the expression of pik3r2 may be an effective way
to lower the malignancy of cancer types. Several previous reports
have demonstrated that miR-126 reduces the expression of pik3r2 by
directly targeting its 3′-untranslated region (32,33).
Similarly, the present data revealed that pik3r2 is a target gene
of miR-126 in PCa, based on the following evidence: i) The mRNA and
protein expression levels of pik3r2 were significantly decreased
following the overexpression of miR-126 in PCa cells; ii) The
expression levels of miR-126 and pik3r2 were inversely correlated
in PCa tissues. In the present study, it was also demonstrated that
the expression of p-AKT was markedly decreased, accompanied by
downregulation of pik3r2 following the overexpression of miR-126.
Since the activation of p-AKT is an important mechanism to maintain
proliferation and metastasis in the initiation and progression of
cancer development (34,35), it was concluded that the biological
mechanism of miR-126 in PCa is potentially mediated by the
PI3K/p-AKT pathway.
In conclusion, the present study suggested that
downregulation of miR-126 is a frequent event in PCa and
restoration of miR-126 significantly inhibited the proliferation
and metastasis of PCa via the PI3K/p-AKT pathway. Therefore,
miR-126 may be a potential target for PCa therapy.
Acknowledgments
The authors would like to thank all doctors and
staff who contributed to the sample recruitment and information
collection.
References
|
1
|
Yamamoto S, Kawakami S, Yonese J, Fujii Y,
Urakami S, Masuda H, Numao N, Ishikawa Y, Kohno A and Fukui I:
Long-term oncological outcome and risk stratification in men with
high-risk prostate cancer treated with radical prostatectomy. Jpn J
Clin Oncol. 42:541–547. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peyromaure EM, Mao K, Sun Y, Xia S, Jiang
N, Zhang S, Wang G, Liu Z and Debré B: A comparative study of
prostate cancer detection and management in China and in France.
Can J Urol. 16:4472–4477. 2009.PubMed/NCBI
|
|
3
|
Klotz L: Active surveillance for prostate
cancer: For whom? J Clin Oncol. 23:8165–8169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walz J, Gallina A, Saad F, Montorsi F,
Perrotte P, Shariat SF, Jeldres C, Graefen M, Bénard F, McCormack
M, et al: A nomogram predicting 10-year life expectancy in
candidates for radical prostatectomy or radiotherapy for prostate
cancer. J Clin Oncol. 25:3576–3581. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vaiopoulos AG, Athanasoula KCh and
Papavassiliou AG: Epigenetic modifications in colorectal cancer:
Molecular insights and therapeutic challenges. Biochim Biophys
Acta. 1842:971–980. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Romero Otero J, Garcia Gomez B, Campos
Juanatey F and Touijer KA: Prostate cancer biomarkers: An update.
Urol Oncol. 32:252–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Petri A, Lindow M and Kauppinen S:
MicroRNA silencing in primates: Towards development of novel
therapeutics. Cancer Res. 69:393–395. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang J, Zhao H, Xin Y and Fan L:
MicroRNA-198 inhibits proliferation and induces apoptosis of lung
cancer cells via targeting FGFR1. J Cell Biochem. 115:987–995.
2014. View Article : Google Scholar
|
|
12
|
Nassirpour R, Mehta PP and Yin MJ: miR-122
regulates tumorigenesis in hepatocellular carcinoma by targeting
AKT3. PLoS One. 8:e796552013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ouyang H, Gore J, Deitz S and Korc M:
microRNA-10b enhances pancreatic cancer cell invasion by
suppressing TIP30 expression and promoting EGF and TGF-β actions.
Oncogene. 33:4664–4674. 2014. View Article : Google Scholar :
|
|
14
|
Fang JH, Zhou HC, Zeng C, Yang J, Liu Y,
Huang X, Zhang JP, Guan XY and Zhuang SM: MicroRNA-29b suppresses
tumor angiogenesis, invasion and metastasis by regulating matrix
metal-loproteinase 2 expression. Hepatology. 54:1729–1740. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu D, Tao T, Xu B, Chen S, Liu C, Zhang
L, Lu K, Huang Y, Jiang L, Zhang X, et al: MiR-361-5p acts as a
tumor suppressor in prostate cancer by targeting signal transducer
and activator of transcription-6 (STAT6). Biochem Biophys Res
Commun. 445:151–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu X, Meng Z, Liang W, Tian Y, Wang X, Han
W, Lou G, Wang X, Lou F, Yen Y, et al: miR-26a enhances miRNA
biogenesis by targeting Lin28B and Zcchc11 to suppress tumor growth
and metastasis. Oncogene. 33:4296–4206. 2014. View Article : Google Scholar
|
|
17
|
Huang TH and Chu TY: Repression of miR-126
and upregulation of adrenomedullin in the stromal endothelium by
cancer-stromal cross talks confers angiogenesis of cervical cancer.
Oncogene. 33:3636–3647. 2014. View Article : Google Scholar
|
|
18
|
Li Z, Li N, Wu M, Li X, Luo Z and Wang X:
Expression of miR-126 suppresses migration and invasion of colon
cancer cells by targeting CXCR4. Mol Cell Biochem. 381:233–242.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun X, Liu Z, Yang Z, Xiao L, Wang F, He
Y, Su P, Wang J and Jing B: Association of microRNA-126 expression
with clini-copathological features and the risk of biochemical
recurrence in prostate cancer patients undergoing radical
prostatectomy. Diagn Pathol. 8:2082013. View Article : Google Scholar
|
|
20
|
Cheng J, Xie HY, Xu X, Wu J, Wei X, Su R,
Zhang W, Lv Z, Zheng S and Zhou L: NDRG1 as a biomarker for
metastasis, recurrence and of poor prognosis in hepatocellular
carcinoma. Cancer Lett. 310:35–45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bagchi P, Nandi S, Nayak MK and
Chawla-Sarkar M: Molecular mechanism behind rotavirus NSP1-mediated
PI3 kinase activation: Interaction between NSP1 and the p85 subunit
of PI3 kinase. J Virol. 87:2358–2362. 2013. View Article : Google Scholar :
|
|
23
|
Mulrane L, McGee SF, Gallagher WM and
O'Connor DP: miRNA dysregulation in breast cancer. Cancer Res.
73:6554–6562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai J, Yang C, Yang Q, Ding H, Jia J, Guo
J, Wang J and Wang Z: Deregulation of let-7e in epithelial ovarian
cancer promotes the development of resistance to cisplatin.
Oncogenesis. 2:e752013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li X, Chen YT, Josson S, Mukhopadhyay NK,
Kim J, Freeman MR and Huang WC: MicroRNA-185 and 342 inhibit
tumorigenicity and induce apoptosis through blockade of the SREBP
metabolic pathway in prostate cancer cells. PLoS One. 8:e709872013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tian L, Fang YX, Xue JL and Chen JZ: Four
microRNAs promote prostate cell proliferation with regulation of
PTEN and its downstream signals in vitro. PLoS One. 8:e758852013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kao CJ, Martiniez A, Shi XB, Yang J, Evans
CP, Dobi A, de Vere White RW and Kung HJ: miR-30 as a tumor
suppressor connects EGF/Src signal to ERG and EMT. Oncogene.
33:2495–2503. 2014. View Article : Google Scholar
|
|
28
|
Kerbel RS: Tumor angiogenesis. N Engl J
Med. 358:2039–2049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou Y, Feng X, Liu YL, Ye SC, Wang H, Tan
WK, Tian T, Qiu YM and Luo HS: Down-regulation of miR-126 is
associated with colorectal cancer cells proliferation, migration
and invasion by targeting IRS-1 via the AKT and ERK1/2 signaling
pathways. PLoS One. 8:e812032013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de Giorgio A, Castellano L, Krell J and
Stebbing J: Crosstalk-induced loss of miR-126 promotes
angiogenesis. Oncogene. 33:3634–3635. 2014. View Article : Google Scholar
|
|
31
|
Coutte L, Dreyer C, Sablin MP, Faivre S
and Raymond E: PI3K-AKT-mTOR pathway and cancer. Bull Cancer.
99:173–180. 2012.In French.
|
|
32
|
Fish JE, Santoro MM, Morton SU, Yu S, Yeh
RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY and Srivastava D:
miR-126 regulates angiogenic signaling and vascular integrity. Dev
Cell. 15:272–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang J, Zhang Z, Zhang DY, Zhu J, Zhang T
and Wang C: microRNA 126 inhibits the transition of endothelial
progenitor cells to mesenchymal cells via the PIK3R2-PI3K/Akt
signalling pathway. PLoS One. 8:e832942013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Skeen JE, Bhaskar PT, Chen CC, Chen WS,
Peng XD, Nogueira V, Hahn-Windgassen A, Kiyokawa H and Hay N: Akt
deficiency impairs normal cell proliferation and suppresses
oncogenesis in a p53-independent and mTORC1-dependent manner.
Cancer Cell. 10:269–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah
G, Riveros M, Perruzzi C, Sun J, Monahan-Earley RA, Shiojima I,
Nagy JA, et al: Pathological angiogenesis is induced by sustained
Akt signaling and inhibited by rapamycin. Cancer Cell. 10:159–167.
2006. View Article : Google Scholar : PubMed/NCBI
|